
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Athina Angelopoulou | -- | 19311 | 2024-02-05 14:35:22 | | | |
| 2 | Athina Angelopoulou | -13880 word(s) | 5431 | 2024-02-06 14:37:12 | | | | |
| 3 | Peter Tang | -2 word(s) | 5429 | 2024-02-07 04:43:08 | | | | |
| 4 | Peter Tang | Meta information modification | 5429 | 2024-02-07 04:44:26 | | | | |
| 5 | Athina Angelopoulou | -666 word(s) | 4763 | 2024-02-08 11:13:29 | | | | |
| 6 | Peter Tang | Meta information modification | 4763 | 2024-02-17 03:30:59 | | |
Video Upload Options
Solid tumors are composed of a highly complex and heterogenic microenvironment, with increasing metabolic status. This environment plays a crucial role in the clinical therapeutic outcome of conventional treatments and innovative antitumor nanomedicines. Scientists have devoted great efforts to conquering the challenges of the tumor microenvironment (TME), in respect of effective drug accumulation and activity at the tumor site. The main focus is to overcome the obstacles of abnormal vasculature, dense stroma, extracellular matrix, hypoxia, and pH gradient acidosis. In this endeavor, nanomedicines that are targeting distinct features of TME have flourished; these aim to increase site specificity and achieve deep tumor penetration. The development of such systems has significantly advanced the application of biomaterials in combinational therapies and in immunotherapies for improved anticancer effectiveness.
1. Introduction
Cancer incidence and mortality has increased dramatically, with female breast cancer being the most commonly diagnosed, surpassing lung cancer [1][2]. It represents a major public health issue in emerging and developing countries, and poses great socioeconomic and psychological challenges. According to recent world statistics, cancer is the first or second leading cause of death; there are nearly 20 million new cases and almost 10 million deaths [1]. Global cancer statistics have estimated that near to 30 million new cases should be expected by 2040, and this is expected to affect more developed than emerging economies. Due to migration and demographic changes, the rate of cancer incidence is seriously affected by everyday risk factors, such as tobacco and alcohol use, unhealthy diet, and anxiety [1][2]. Despite the disappointing statistics, however, there was a decline in the overall cancer death rate of about 33% between 1991 and 2020. Furthermore, an estimated 4 million deaths were prevented. Moreover, a decline of 65% in cervical cancer incidence among women in their 20s during the period 2012 to 2019 was observed. This is due to the preventive effect of the human papillomavirus vaccine [1][2]. The decline in rates of cancer mortality can, undoubtedly, be related to increased research efforts in the field of cancer vaccines (RNA technologies) and personalized nanomedicines [3]. The greatest challenges for scientists are to ensure early diagnosis and prevention of cancer, which could effectively reduce cancer mortality.
Early diagnosis is crucial due to the high differentiation rate of tumor cells that promote the development of highly aggressive cancerous cells associated with multidrug resistance (MDR), stemness [4], and invasion [5]. A critical stimulus of MDR and invasion is tumor microenvironment (TME) (Scheme 1). This is a complicated interpenetrating network of varied cancerous and stromal cell types, extracellular matrix (ECM), and interstitial fluid (IF) [6][7][8]. TME is hostile to normal cells while being hospitable to stromal cells that are the nonmalignant components of solid tumors. These include endothelial cells (ECs), fibroblasts (FCs), immune cells (lymphocytes, macrophages, dendritic cells), and perivascular cells (PCs). These cells are interconnected in a protein-rich matrix that promotes angiogenesis and neovascularization [9][10]. Within the heterogenic TME, vasculature abnormalities are related to variations in oxygenation. Furthermore, the elevated presence of reactive oxygen species (ROS), glutathione (GSH), enzymes, and adenosine triphosphate (ATP) further promote a hypoxic status with acidic pH levels (pH 5.5–6.2). These features—in combination with secreted growth factors, cytokines, chemokines, and macromolecules such as proteases and proteins in the surrounding stroma—regulate the stimulation of cancer-associated fibroblasts (CAFs), and play key role in metastatic potency [11][12][13]. The stroma in combination with the highly dynamic ECM act as supportive reservoirs that directly or indirectly interconnect TME with capillary and vascular system cells, and immune system cells. This provide the essential nutrition components of oxygen, gas exchange, and metabolites withdrawal, to support tumorigenesis and continuous neovascularization [14][15][16].
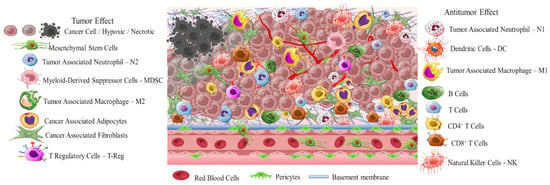
The greatest difficulty posed by the TME of solid tumors is MDR. This results in reduced therapeutic efficiency of traditional interventions such as chemo- and radiotherapy. The backbone of traditional therapeutic approaches is surgical ablation followed by chemo- and radiotherapy or a combination of both, depending on tumor severity. Chemo- and radiotherapy cause serious side effects for the patients within the therapeutic window of the administered doses [17]. Great progress has been achieved with advanced investigation of new therapeutic agents including peptides, antibodies, and prodrugs [18][19][20][21][22]. However, the success of these compounds is compromised by the limitations of abnormal vasculature, heterogenic basement membranes, and poor blood supply. These are all inherited by TME, and are the causes of therapeutic failure [23][24][25]. Nanomedicine represents an important strategy to improve the delivery of therapeutic agents such as drugs, peptides, antibodies, proteins, genes, and immunotherapeutic agents in a selective and controlled manner for efficient accumulation and stimuli responsiveness [26][27][28][29][30][31][32][33][34]. Although great progress has been achieved in this field, the clinical translation of nanomedicines is still limited. In this research, the researchers aim to present a discussion on the field of responsive nanomedicines. This will emphasize the application of biomaterials including natural polymers such as polysaccharides, biodegradable polymers, and metal oxides, in targeting the TME of solid tumors. Biomaterials represent a field of distinct research interest, due to their unique inherent properties. Their structure allows for effective functionalization for the co-delivery of multiple compounds and for effective responsiveness to an internal (chemical and/or biological) or external physical stimulus (magnetic field, light, radiation, ultrasound). Biomaterials have proved to be great supporters of theranostic applications in cancer treatment [35][36]. Overall, in this research the researchers will discuss the role of biomaterial-based nanomedicines in targeting the TME. This includes heterogenic vasculature, tumor stroma ECM, CAFs, tumor hypoxia, and acidosis. The researchers will examine the most recent advances in therapeutic nanomedicine for solid tumors; these have the potential to improve clinical outcomes. Finally, the researchers will summarize the challenges and future outlook for the application of nanomedicines in tumor immunotherapy and combinational therapy to overcome limitations and improve the therapeutic outcome.
2. Solid Tumor Nanomedicine: Distribution in Tumor Microenvironment
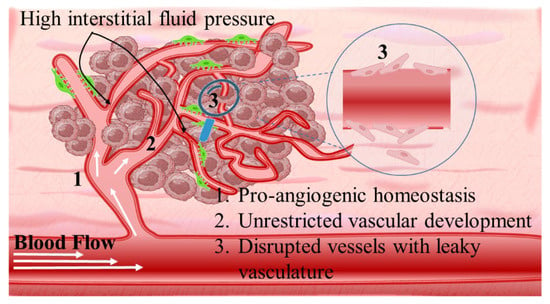
Intriguingly, solid tumors are pathological organ-like tissues with heterogenic TME and increased metabolic status, which promote and support processes mimicking normal tissues, as angiogenesis [37][38][39]. Due to the elevated dysregulation of angiogenetic factors, abnormal and destabilized blood and lymphatic vessels are developed. These have major variations in diameter, density, shape (spiral-like) and overall distribution within TME. Additionally, a simultaneous discontinuation of endothelium with leaking cell gaps, irregularly thick or thin basement membranes and disruption of blood flow cause excessive spatial stress and increased interstitial fluid pressure (IFP) [40][41][42]. These features promote the transport of nutrients, oxygen, and blood away from the central region of solid tumors. This stimulates ATP regulation, hypoxia, and acidosis. The same features prohibit the transfer of therapeutic drugs, and this results in an inferior targeting effect and heterogenic tumor distribution of drugs [43]. Nanomedicines improve targeting effectiveness and selectivity [44][45][46]. This is especially the case when they are supported by an enhanced permeability and retention (EPR) effect, which promotes extravasation and effectual intratumor localization [47] (Scheme 2). Despite progress being made, moderate clinical success has been achieved so far. This is because nanomedicine applications have encountered severe obstacles related to avascular tumor sites due to the TME’s characteristics restricting nanomedicines’ access only to highly vascular regions with increased perfusion [47][48]. These limitations in the therapeutic efficacy of nanomedicines have been tackled recently by exploiting the complex mechanisms and associated properties of TME. This improves intratumoral localization. Furthermore, specially designed nanomedicines exhibit stimuli-responsiveness in TME features, such as hypoxia and acidity, by combining ligand-mediated active targeting of selective receptors with growth factors, inhibitors, enzymes, and peptides. Combining the benefits of external stimuli-responsiveness has, beneficially, increased the targeting efficiency of nanomedicines and amplified the therapeutic activity [49][50][51][52].
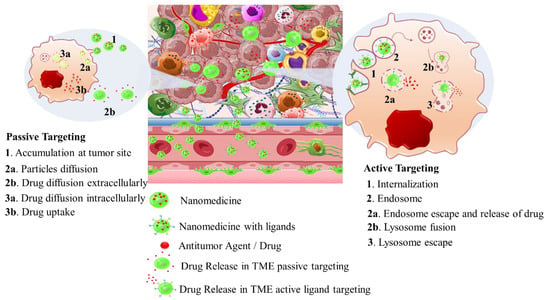
Table 1. Therapeutic nanomedicines approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
|
Carrier Type |
Product Name |
Therapeutic Agent |
Cancer Type |
Stage |
Ref. |
|---|---|---|---|---|---|
|
Liposomes |
Zolsketil® |
Doxorubicin |
Metastatic breast cancer, advanced ovarian cancer, multiple myeloma, AIDS-related Kaposi’s sarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/zolsketil-pegylated-liposomal (accessed on 15 January 2024) |
Approved (EMA, 2022) |
|
|
Vyxeos® |
Cytarabine: daunorubicin |
Newly diagnosed therapy-related acute myeloid leukemia, acute myeloid leukemia with myelodysplasia related changes (https://www.ema.europa.eu/en/medicines/human/EPAR/vyxeos-liposomal-previously-known-vyxeos, accessed on 15 January 2024) |
Approved (EMA, 2018) (FDA, 2017) |
||
|
Onivyde®/CPX-351 |
Irinotecan |
Pancreatic cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/onivyde-pegylated-liposomal-previously-known-onivyde, accessed on 15 January 2024) |
Approved (EMA, 2016) (FDA, 2015) |
[56][57] | |
|
Mepact® |
Mifamurtide |
Osteosarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/mepact, accessed on 15 January 2024) |
Approved (EMA, 2009) |
||
|
Ameluz® |
5-aminolevulinic acid |
Superficial and/or nodular basal cell carcinoma (https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz, accessed on 15 January 2024) |
Approved (EMA, 2011) |
||
|
DaunoXome® |
Daunorubicin |
Kaposi’s sarcoma |
Approved (FDA 1996) Discontinued (FDA, 2021) |
||
|
Iron Oxide nanoparticles |
NanoTherm® |
Fe2O3 |
Glioblastoma, prostate, and pancreatic cancer (https://www.eib.org/en/stories/new-cancer-treatments, accessed on 15 January 2024) |
Approved (EMA, 2013) |
[57] |
|
Albumin nanoparticles |
Abraxane® |
Paclitaxel |
Metastatic breast cancer, locally advanced or metastatic non-small cell lung cancer, metastatic adenocarcinoma of the pancreas (https://www.ema.europa.eu/en/medicines/human/EPAR/abraxane, accessed on 15 January 2024) |
Approved (EMA 2008) (FDA 2005) |
|
|
Pazenir® |
Paclitaxel |
Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, non-small cell lung cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/pazenir, accessed on 15 January 2024) |
Approved (EMA 2019) |
[58] |
|
|
Vaccines |
Adstiladrin® |
Adenoviral vector-based gene therapy |
Bacillus Calmette–Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-adstiladrin-nadofaragene-firadenovec-vncg-patients-high-risk, accessed on 15 January 2024) |
Approved (FDA 2022) |
[59] |
|
Provenge® |
Autologous peripheral-blood mononuclear cells |
Metastatic castration-resistant prostate cancer (mCRPC) (https://www.drugs.com/history/provenge.html, accessed on 15 January 2024) |
Approved (EMA 2013) (FDA 2010) Discontinued (EMA, 2015) |
[59] |
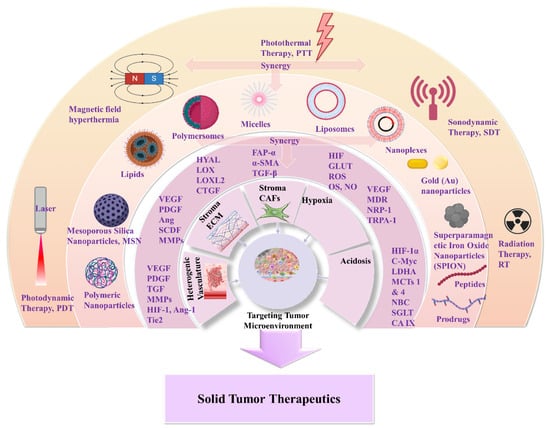
3. Nanomedicines for Targeting TME: Application of Natural and Synthetic Biomaterials
3.1. The Heterogenic Vasculature
3.2. The Tumor Stroma Extracellular Matrix
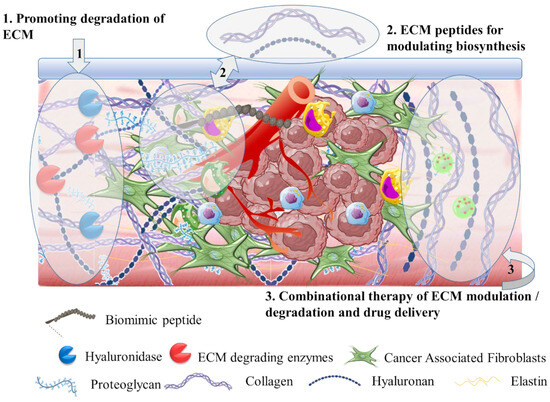
3.3. The Tumor Stroma Cancer Associated Fibroblasts
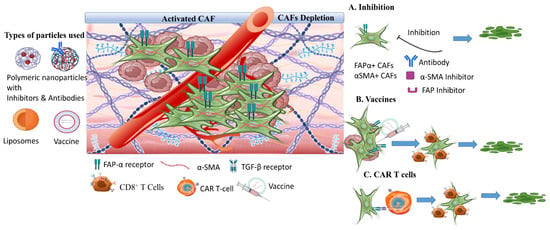
3.4. The Tumor Hypoxia
Another therapeutic target of responsive nanomedicine is the TME hypoxia. This is a direct consequence of heterogenic vasculature and fluctuating blood flow, and results in insufficient oxygen diffusion and perfusion within the tumor environment. The rapid proliferation rate of tumor and stromal cells creates excessive consumption of supplied oxygen, nutrients, and energy [87]. Imbalance in the diffusion mechanisms of oxygen supply is observed at depths after 70–150 μm from peripheral tumor blood vessels. This results in gas oxygen (gas-O2) levels falling below 1–2% in hypoxic solid tumors. There are two types of hypoxia: the chronic, wherein oxygen’s concentration is characterized by a longitudinal gradient drop for a prolonged time period of several hours; and the acute, in which tumor ECs and stromal cells are attached to vasculature with deprived oxygen perfusion [88]. Extensive research has resulted in the understanding of hypoxia mechanisms and their effects on tumor biology by participating in the regulation of angiogenesis, metastasis, and multidrug resistance [89][90]. Hypoxia-regulated genes are expressed among various tumor types with high hypoxic gene expressions such as the squamous cell carcinoma (SCC) of the head and neck, lung, and cervix tumors; these have also been investigated [91].
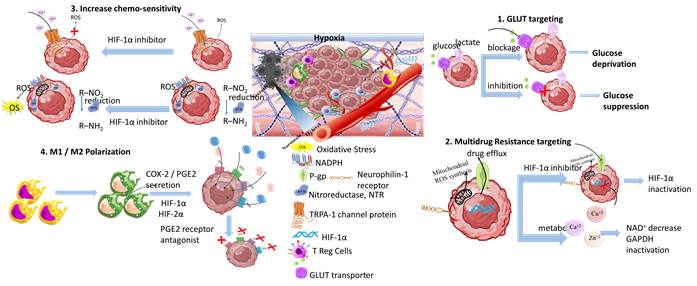
Scheme 7. Tumor hypoxia represents a significant target for responsive nanomedicines. Important axes of nanomedicine research are GLUT targeting, multidrug resistance targeting, increase of chemo-sensitivity, M1/M2 macrophage polarization, increase of tumor oxygenation, and antioxidants. Biomaterials used for hypoxia targeting nanomedicines include polysaccharides, polymers, hybrid nanoparticles, metal oxides and hybrid metal–polymer nanoparticles, polymersomes, nanogels, and liposomes in combination with chemotherapeutic drug targeting (CDT), magnetic targeting, and PDT/PTT therapy. (Created with the assistance of BioRender.com https://app.biorender.com/user/signin?illustrationId=6156d45891063d00af8af51d (accessed on 1 September 2023 up to 31 November 2023), and Microsoft ppt).
3.5. The Tumor Acidosis
3.6. Tumor Immunotherapies
4. Conclusions
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- World Health Organization. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2022; p. 23.
- Peng, S.; Xiao, F.; Chen, M.; Gao, H. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy. Adv. Sci. 2022, 9, 2103836.
- Miao, L.; Lin, C.M.; Huang, L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J. Control. Release 2015, 219, 192–204.
- Liu, J.; Chen, Q.; Feng, L.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 2018, 21, 55–73.
- Yang, S.; Gao, H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017, 126, 97–108.
- Das, M.; Law, S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int. J. Biochem. Cell Biol. 2018, 103, 115–124.
- Ribeiro Franco, P.I.; Perillo Rodrigues, A.; Borges de Menezes, L.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729.
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796.
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626.
- Martin, J.D.; Miyazaki, T.; Cabral, H. Remodeling tumor microenvironment with nanomedicines. WIREs Nanomed. Nanobiotechnol. 2021, 13, 1730.
- Saini, H.; Eliato, K.R.; Veldhuizen, J.; Zare, A.; Allam, M.; Silva, C.; Kratz, A.; Truong, D.; Mouneimne, G.; LaBaer, J.; et al. The role of tumor-stroma interactions on desmoplasia and tumorigenicity within a microengineered 3D platform. Biomaterials 2020, 247, 119975.
- Klemm, F.; Joyce, J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015, 25, 198–213.
- Deasy, S.K.; Erez, N. A glitch in the matrix: Organ-specific matrisomes in metastatic niches. Trends Cell Biol. 2022, 32, 110–123.
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406.
- Anand, U.; Dey, A.; Singh Chandel, A.K.; Sanyal, R.; Mishra, A.; Kumar Pandey, D.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401.
- Kifle, Z.D.; Tadele, M.; Alemu, E.; Gedamu, T.; Ayele, A.G. A recent development of new therapeutic agents and novel drug targets for cancer treatment. SAGE Open Med. 2021, 23, 1–12.
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The Use of Therapeutic Peptides to Target and to Kill Cancer Cells. Curr. Med. Chem. 2012, 19, 3794.
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-drug conjugates: A comprehensive review. Mol. Cancer Res. 2020, 18, 3–19.
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608.
- Muthiah, G.; Jaiswal, A. Can the Union of Prodrug Therapy and Nanomedicine Lead to Better Cancer Management? Adv. NanoBiomed Res. 2022, 2, 2100074.
- Kashkooli, F.M.; Soltani, M.; Hamedi, M.-H. Drug delivery to solid tumors with heterogeneous microvascular networks: Novel insights from image-based numerical modeling. Eur. J. Pharm. Sci. 2020, 151, 105399.
- Wu, M.; Frieboes, H.B.; Chaplain, M.A.J.; McDougall, S.R.; Cristini, V.; Lowengrub, J.S. The effect of interstitial pressure on therapeutic agent transport: Coupling with the tumor blood and lymphatic vascular systems. J. Theor. Biol. 2014, 355, 194–207.
- Lim, Z.-F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134.
- Sarkar, S.; Levi-Polyachenko, N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv. Drug Deliv. Rev. 2020, 163–164, 40–64.
- Liao, J.; Jia, Y.; Wu, Y.; Shi, K.; Yang, D.; Li, P.; Qian, Z. Physical-, chemical-, and biological-responsive nanomedicine for cancer therapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1581.
- Granja, A.; Pinheiro, M.; Sousa, C.T.; Reis, S. Gold nanostructures as mediators of hyperthermia therapies in breast cancer. Biochem. Pharmacol. 2021, 190, 114639.
- Angelopoulou, A.; Voulgari, E.; Kolokithas-Ntoukas, A.; Bakandritsos, A.; Avgoustakis, K. Magnetic Nanoparticles for the Delivery of Dapagliflozin to Hypoxic Tumors: Physicochemical Characterization and Cell Studies. AAPS PharmSciTech 2018, 19, 2.
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144.
- Majumder, N.; Das, N.G.; Das, S.K. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635.
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79.
- Klein, O.; Kee, D.; Markman, B.; Carlino, M.S.; Underhill, C.; Palmer, J.; Power, D.; Cebon, J.; Behren, A. Evaluation of TMB as a predictive biomarker in patients with solid cancers treated with anti-PD-1/CTLA-4 combination immunotherapy. Cancer Cell 2021, 39, 592–593.
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81.
- Kirtane, K.; Elmariah, H.; Chung, C.H.; Abate-Daga, D. Adoptive cellular therapy in solid tumor malignancies: Review of the literature and challenges ahead. J. ImmunoTher. Cancer. 2021, 9, e002723.
- Zhang, X.; Zhang, Y.; Zheng, H.; He, Y.; Jia, H.; Zhang, L.; Lin, C.; Chen, S.; Zheng, J.; Yang, Q.; et al. In Situ biomimetic Nanoformulation for metastatic cancer immunotherapy. Acta Biomater. 2021, 134, 633–648.
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23P, 522–532.
- Ribatti, D.; Pezzella, F. Overview on the Different Patterns of Tumor Vascularization. Cells 2021, 10, 639.
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as organs: Complex tissues that interface with the entire organism. Dev. Cell 2010, 18, 884–901.
- Siemann, D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74.
- Nagl, L.; Horvath, L.; Pircher, A.; Wolf, D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment—New Findings and Future Perspectives. Front. Cell Dev. Biol. 2020, 8, 766.
- Salavati, H.; Debbaut, C.; Pullens, P.; Ceelen, W. Interstitial fluid pressure as an emerging biomarker in solid tumors. BBA–Rev. Cancer 2022, 1877, 188792.
- Jiao, D.; Cai, Z.; Choksi, S.; Ma, D.; Choe, M.; Kwon, H.-J.; Baik, J.Y.; Rowan, B.G.; Liu, C.; Liu, Z.-G. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018, 28, 868–870.
- Seok Youn, Y.; Han Bae, Y. Perspectives on the past, present, and future of cancer nanomedicine. Adv. Drug Deliv. Rev. 2018, 130, 3–11.
- Al-Abd, A.M.; Aljehani, Z.K.; Gazzaz, R.W.; Fakhri, S.H.; Jabbad, A.H.; Alahdal, A.M.; Torchilin, V.P. Pharmacokinetic strategies to improve drug penetration and entrapment within solid tumors. J. Control. Release 2015, 219, 269–277.
- Lammers, T.; Kiessling, F.; Ashford, M.; Hennink, W.; Crommelin, D.; Storm, G. Cancer nanomedicine: Is targeting our target? Nat. Rev. Mater. 2016, 1, 16069.
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771.
- Nicolas-Boluda, A.; Silva, A.K.A.; Fournei, S.; Gazeau, F. Physical oncology: New targets for nanomedicine. Biomaterials 2018, 150, 87–99.
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091–8108.
- Dai, W.; Wang, X.; Song, G.; Liu, T.; He, B.; Zhang, H.; Wang, X.; Zhang, Q. Combination antitumor therapy with targeted dual-nanomedicines. Adv. Drug Deliv. Rev. 2017, 115, 23–45.
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121.
- Khot, V.M.; Salunkhe, A.B.; Pricl, S.; Bauer, J.; Thorat, N.D.; Townley, H. Nanomedicine-driven molecular targeting, drug delivery, and therapeutic approaches to cancer chemoresistance. Drug Discov. Today 2021, 26, 724–739.
- Ang, M.J.Y.; Chan, S.Y.; Goh, Y.-Y.; Luo, Z.; Lau, J.W.; Liu, X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv. Drug Deliv. Rev. 2021, 178, 113907.
- Anjali Das, C.G.; Ganesh Kumar, V.; Stalin Dhas, T.; Karthick, V.; Vineeth Kumar, C.M. Nanomaterials in anticancer applications and their mechanism of action—A review. Nanomed. Nanotechnol. Biol. Med. 2023, 47, 102613.
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246.
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615.
- Rodriguez, F.; Caruana, P.; De la Fuente, N.; Espanol, P.; Gamez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784.
- Choo, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728.
- Pallerla, S.; Abdul, A.R.M.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779.
- Micale, N.; Molonia, M.S.; Citarella, A.; Cimino, F.; Saija, A.; Cristami, M.; Speciale, A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules 2021, 26, 4665.
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264.
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501.
- Van Vierken, L.E.; Amiji, M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006, 3, 205.
- Kuang, Y.; Zhai, J.; Xiao, Q.; Zhao, S.; Li, C. Polysaccharide/mesoporous silica nanoparticle-based drug delivery systems: A review. Int. J. Biol. Macromol. 2021, 193, 457.
- Wu, P.; Han, J.; Gong, Y.; Liu, C.; Yu, H.; Xie, N. Nanoparticle-Based Drug Delivery Systems Targeting Tumor Microenvironment for Cancer Immunotherapy Resistance: Current Advances and Applications. Pharmaceutics 2022, 14, 1990.
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191.
- Fathi, M.; Abdolahinia, E.D.; Bara, J.; Omidi, Y. Smart stimuli-responsive biopolymeric nanomedicines for targeted therapy of solid tumors. Nanomedicine 2020, 15, 2171–2200.
- Salavati, H.; Soltani, M.; Amanpour, S. The pivotal role of angiogenesis in a multi-scale modeling of tumor growth exhibiting the avascular and vascular phases. Microvasc. Res. 2018, 119, 105–116.
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399.
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422.
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2020, 9, 84.
- Fu, L.-Q.; Du, W.-L.; Cai, M.-H.; Yao, J.-Y.; Zhao, Y.-Y.; Mou, X.-Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immun. 2020, 353, 104119.
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 637675.
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol. Cancer 2022, 21, 208.
- Zhao, X.; He, Y.; Chen, H. Autophagic tumor stroma: Mechanisms and roles in tumor growth and progression. Int. J. Cancer. 2013, 132, 1–8.
- Simsek, H.; Klotzsch, E. The solid tumor microenvironment Breaking the barrier for T cells How the solid tumor microenvironment influences T cells. BioEssays 2022, 44, 2100285.
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. Mechanobiology of solid tumors. BBA–Mol. Basis Dis. 2022, 1868, 166555.
- Rianna, C.; Kumar, P.; Radmacher, M. The role of the microenvironment in the biophysics of cancer. Semin. Cell Dev. Biol. 2018, 73, 107–114.
- Abyaneh, H.S.; Regenold, M.; McKee, T.D.; Allen, C.; Gauthier, M.A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics 2020, 10, 1960–1980.
- Cheng, B.; Yu, Q.; Wang, W. Intimate communications within the tumor microenvironment: Stromal factors function as an orchestra. J. Biomed. Sci. 2023, 30, 1.
- Li, Z.; Zheng, Z.; Li, C.; Li, Z.; Wu, J.; Zhang, B. Therapeutic drugs and drug delivery systems targeting stromal cells for cancer therapy: A review. J. Drug Deliv. 2020, 28, 714–726.
- Hurtado, P.; Martinez-Pena, I.; Pineiro, R. Dangerous Liaisons: Circulating Tumor Cells (CTCs) and Cancer-Associated Fibroblasts (CAFs). Cancers 2020, 12, 2861.
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer- associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115.
- Shahvali, S.; Rahiman, N.; Jaafari, M.R.; Arabi, L. Targeting fibroblast activation protein (FAP): Advances in CAR-T cell, antibody, and vaccine in cancer immunotherapy. Drug Deliv. Transl. Res. 2023, 13, 2041–2056.
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315.
- Li, F.; Zhao, S.; Wei, C.; Hu, Y.; Xu, T.; Xin, X.; Zhu, T.; Shang, L.; Ke, S.; Zhou, J.; et al. Development of Nectin4/FAP-targeted CAR-T cells secreting IL-7, CCL19, and IL-12 for malignant solid tumors. Front. Immunol. 2022, 13, 958082.
- Garcia-Bermudez, J.; Williams, R.T.; Guarecuco, R.; Birsoy, K. Targeting extracellular nutrient dependencies of cancer cells. Mol. Metab. 2020, 33, 67–82.
- Horsman, M.R.; Sorensen, B.S.; Busk, M.; Siemann, D.W. Therapeutic Modification of Hypoxia. Clin. Oncol. 2021, 33, e492–e509.
- McAleese, C.E.; Choudhury, C.; Butcher, N.J.; Minchin, R.F. Hypoxia-mediated drug resistance in breast cancers. Cancer Lett. 2021, 502, 189–199.
- Khoshinani, H.M.; Afshar, S.; Najafi, R. Hypoxia: A Double-Edged Sword in Cancer Therapy. Cancer Investig. 2016, 34, 536–545.
- Wiechec, E.; Matic, N.; Ali, A.; Roberg, K. Hypoxia induces radioresistance, epithelial-mesenchymal transition, cancer stem cell-like phenotype and changes in genes possessing multiple biological functions in head and neck squamous cell carcinoma. Oncol. Rep. 2022, 47, 58.
- Razi, S.; Haghparast, A.; Khameneh, S.C.; Sadrabadi, A.E.; Aziziyan, F.; Bakhtiyari, M.; Nabi-Afjadi, M.; Tarhriz, V.; Jalili, A.; Zalpoor, H. The role of tumor microenvironment on cancer stem cell fate in solid tumors. Cell Commun. Signal. 2023, 21, 143.
- Vaupel, P.; Multhoff, G. Fatal Alliance of Hypoxia-/HIF-1α-Driven Microenvironmental Traits Promoting Cancer Progression. In Oxygen Transport to Tissue XLI; Advances in Experimental Medicine and, Biology; Ryu, P.D., LaManna, J., Harrison, D., Lee, S.S., Eds.; Springer: Cham, Switzerland, 2020; Volume 1232.
- Kes, M.M.G.; Van den Bossche, J.; Griffioen, A.W.; Huijbers, E.J.M. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. BBA–Rev. Cancer 2020, 1874, 188427.
- Singh, L.; Nair, L.; Kumar, D.; Arora, M.K.; Bajaj, S.; Gadewar, M.; Mishra, S.S.; Rath, S.K.; Dubey, A.K.; Kaithwas, G.; et al. Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival. Front. Oncol. 2023, 13, 1034205.
- Wang, J.; Matisevic, S. Functional and metabolic targeting of natural killer cells to solid tumors. Cell. Oncol. 2020, 43, 577–600.
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Manipulating T-cell metabolism to enhance immunotherapy in solid tumor. Front. Immunol. 2022, 13, 1090429.
- Goswami, K.K.; Banerjee, S.; Bose, A.; Baral, R. Lactic acid in alternative polarization and function of macrophages in tumor microenvironment. Hum. Immunol. 2022, 83, 409–417.
- Koltai, T. The complex relationship between multiple drug resistance and the tumor pH gradient: A review. Cancer Drug Resist. 2022, 5, 277.
- Vaidya, F.U.; Chhipa, A.S.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022, 5, e1291.
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936.
- Zhou, W.; Jia, Y.; Liu, Y.; Chen, Y.; Zhao, P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346.
- Yan, Y.; Ding, H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613.
- Lim, E.-K.; Chung, B.H.; Chung, S. Recent Advances in pH-Sensitive Polymeric Nanoparticles for Smart Drug Delivery in Cancer Therapy. Curr. Drug Targets 2018, 19, 300.
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Kandasamy, R. Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems. Curr. Drug Targets 2021, 22, 947.
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577.
- Wang, C.; Ding, S.; Wang, S.; Shi, Z.; Pandey, N.K.; Chudal, L.; Wang, L.; Zhang, Z.; Wen, Y.; Yao, H.; et al. Endogenous tumor microenvironment-responsive multifunctional nanoplatforms for precision cancer theranostics. Coord. Chem. Rev. 2021, 426, 213529.
- Ruan, S.; Huang, Y.; He, M.; Gao, H. Advanced Biomaterials for Cell-Specific Modulation and Restore of Cancer Immunotherapy. Adv. Sci. 2022, 9, 2200027.
- Cai, L.; Xu, J.; Yang, Z.; Tong, R.; Dong, Z.; Wang, C.; Leong, K.W. Engineered biomaterials for cancer immunotherapy. Med. Comm. 2020, 1, 35.
- Xie, Y.-Q.; Wei, L.; Tang, L. Immunoengineering with biomaterials for enhanced cancer immunotherapy. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1506.
- Bo, Y.; Wang, H. Biomaterial-Based In Situ Cancer Vaccines. Adv. Mater. 2023, 2210452.
- Abdou, P.; Wang, Z.; Chen, Q.; Chan, A.; Zhou, D.R.; Gunadhi, V.; Gu, Z. Advances in engineering local drug delivery systems for cancer immunotherapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1632.
- Hu, L.; Cao, Z.; Ma, L.; Liu, Z.; Liao, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials 2019, 223, 119469.
- Wang, C.; Ye, Y.; Hu, Q.; Bellotti, A.; Gu, Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017, 29, 1606036.
- Rosalia, R.A.; Cruz, L.J.; van Duikeren, S.; Tromp, A.T.; Silva, A.L.; Jiskoot, W.; de Gruijl, T.; Lowik, C.; Oostendorp, J.; van der Burg, A.; et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015, 40, 88.
- Wang, C.; Li, P.; Liu, L.I.; Pan, H.; Li, H.; Cai, L.; Ma, Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials 2016, 79, 88.
- Yan, S.; Luo, Z.; Li, Z.; Wang, Y.; Tao, J.; Gong, C.; Liu, X. Improving Cancer Immunotherapy Outcomes Using Biomaterials. Angew. Chem. 2020, 132, 17484.
- Yang, J.; Zhang, C. Regulation of cancer-immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1612.




