Solid tumors are composed of a highly complex and heterogenic microenvironment, with increasing metabolic status. This environment plays a crucial role in the clinical therapeutic outcome of conventional treatments and innovative antitumor nanomedicines. Scientists have devoted great efforts to conquering the challenges of the tumor microenvironment (TME), in respect of effective drug accumulation and activity at the tumor site. The main focus is to overcome the obstacles of abnormal vasculature, dense stroma, extracellular matrix, hypoxia, and pH gradient acidosis. In this endeavor, nanomedicines that are targeting distinct features of TME have flourished; these aim to increase site specificity and achieve deep tumor penetration. Recently, research efforts have focused on the immune reprograming of TME in order to promote suppression of cancer stem cells and prevention of metastasis. Thereby, several nanomedicine therapeutics which have shown promise in preclinical studies have entered clinical trials or are already in clinical practice. Various novel strategies were employed in preclinical studies and clinical trials. Among them, nanomedicines based on biomaterials show great promise in improving the therapeutic efficacy, reducing side effects, and promoting synergistic activity for TME responsive targeting. In this review, we focused on the targeting mechanisms of nanomedicines in response to the microenvironment of solid tumors. We describe responsive nanomedicines which take advantage of biomaterials’ properties to exploit the features of TME or overcome the obstacles posed by TME. The development of such systems has significantly advanced the application of biomaterials in combinational therapies and in immunotherapies for improved anticancer effectiveness.
- biomaterials
- tumor microenvironment
- nanomedicine
- hypoxia
- acidosis
- resistance
- tumor vasculature
- targeting
- stimuli-responsiveness
1. Introduction
Cancer incidence and mortality has increased dramatically, with female breast cancer being the most commonly diagnosed, surpassing lung cancer [1,2]. It represents a major public health issue in emerging and developing countries, and poses great socioeconomic and psychological challenges. According to recent world statistics, cancer is the first or second leading cause of death; there are nearly 20 million new cases and almost 10 million deaths [1]. Global cancer statistics have estimated that near to 30 million new cases should be expected by 2040, and this is expected to affect more developed than emerging economies. Due to migration and demographic changes, the rate of cancer incidence is seriously affected by everyday risk factors, such as tobacco and alcohol use, unhealthy diet, and anxiety [1,2]. Despite the disappointing statistics, however, there was a decline in the overall cancer death rate of about 33% between 1991 and 2020. Furthermore, an estimated 4 million deaths were prevented. Moreover, a decline of 65% in cervical cancer incidence among women in their 20s during the period 2012 to 2019 was observed. This is due to the preventive effect of the human papillomavirus vaccine [1,2]. The decline in rates of cancer mortality can, undoubtedly, be related to increased research efforts in the field of cancer vaccines (RNA technologies) and personalized nanomedicines [3]. The greatest challenges for scientists are to ensure early diagnosis and prevention of cancer, which could effectively reduce cancer mortality.
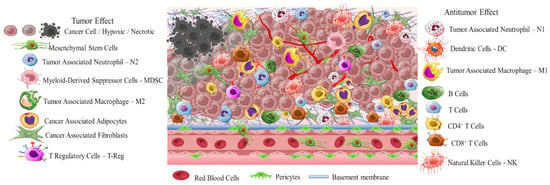
Early diagnosis is crucial due to the high differentiation rate of tumor cells that promote the development of highly aggressive cancerous cells associated with multidrug resistance (MDR), stemness [4], and invasion [5]. A critical stimulus of MDR and invasion is tumor microenvironment (TME) (Scheme 1). This is a complicated interpenetrating network of varied cancerous and stromal cell types, extracellular matrix (ECM), and interstitial fluid (IF) [6–8]. TME is hostile to normal cells while being hospitable to stromal cells that are the nonmalignant components of solid tumors. These include endothelial cells (ECs), fibroblasts (FCs), immune cells (lymphocytes, macrophages, dendritic cells), and perivascular cells (PCs). These cells are interconnected in a protein-rich matrix that promotes angiogenesis and neovascularization [9,10]. Within the heterogenic TME, vasculature abnormalities are related to variations in oxygenation. Furthermore, the elevated presence of reactive oxygen species (ROS), glutathione (GSH), enzymes, and adenosine triphosphate (ATP) further promote a hypoxic status with acidic pH levels (pH 5.5–6.2). These features—in combination with secreted growth factors, cytokines, chemokines, and macromolecules such as proteases and proteins in the surrounding stroma—regulate the stimulation of cancer-associated fibroblasts (CAFs), and play key role in metastatic potency [11–13]. The stroma in combination with the highly dynamic ECM act as supportive reservoirs that directly or indirectly interconnect TME with capillary and vascular system cells, and immune system cells. This provide the essential nutrition components of oxygen, gas exchange, and metabolites withdrawal, to support tumorigenesis and continuous neovascularization [14–16].
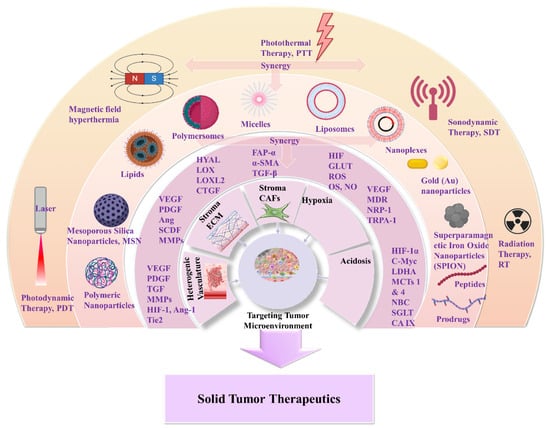
The greatest difficulty posed by the TME of solid tumors is MDR. This results in reduced therapeutic efficiency of traditional interventions such as chemo- and radiotherapy. The backbone of traditional therapeutic approaches is surgical ablation followed by chemo- and radiotherapy or a combination of both, depending on tumor severity. Chemo- and radiotherapy cause serious side effects for the patients within the therapeutic window of the administered doses [17]. Great progress has been achieved with advanced investigation of new therapeutic agents including peptides, antibodies, and prodrugs [18–22]. However, the success of these compounds is compromised by the limitations of abnormal vasculature, heterogenic basement membranes, and poor blood supply. These are all inherited by TME, and are the causes of therapeutic failure [23–25]. Nanomedicine represents an important strategy to improve the delivery of therapeutic agents such as drugs, peptides, antibodies, proteins, genes, and immunotherapeutic agents in a selective and controlled manner for efficient accumulation and stimuli responsiveness [26–34]. Although great progress has been achieved in this field, the clinical translation of nanomedicines is still limited. In this review, we aim to present a discussion on the field of responsive nanomedicines. This will emphasize the application of biomaterials including natural polymers such as polysaccharides, biodegradable polymers, and metal oxides, in targeting the TME of solid tumors. Biomaterials represent a field of distinct research interest, due to their unique inherent properties. Their structure allows for effective functionalization for the co-delivery of multiple compounds and for effective responsiveness to an internal (chemical and/or biological) or external physical stimulus (magnetic field, light, radiation, ultrasound). Biomaterials have proved to be great supporters of theranostic applications in cancer treatment [35,36]. Overall, in this review we will discuss the role of biomaterial-based nanomedicines in targeting the TME. This includes heterogenic vasculature, tumor stroma ECM, CAFs, tumor hypoxia, and acidosis. We will examine the most recent advances in therapeutic nanomedicine for solid tumors; these have the potential to improve clinical outcomes. Finally, we will summarize the challenges and future outlook for the application of nanomedicines in tumor immunotherapy and combinational therapy to overcome limitations and improve the therapeutic outcome.
2. Solid Tumor Nanomedicine: Distribution in Tumor Microenvironment
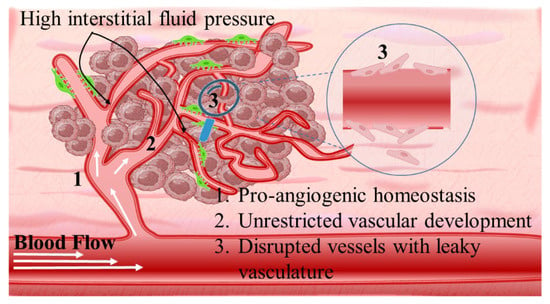
Intriguingly, solid tumors are pathological organ-like tissues with heterogenic TME and increased metabolic status, which promote and support processes mimicking normal tissues, as angiogenesis [37–39]. Due to the elevated dysregulation of angiogenetic factors, abnormal and destabilized blood and lymphatic vessels are developed. These have major variations in diameter, density, shape (spiral-like) and overall distribution within TME. Additionally, a simultaneous discontinuation of endothelium with leaking cell gaps, irregularly thick or thin basement membranes and disruption of blood flow cause excessive spatial stress and increased interstitial fluid pressure (IFP) [40–42]. These features promote the transport of nutrients, oxygen, and blood away from the central region of solid tumors. This stimulates ATP regulation, hypoxia, and acidosis. The same features prohibit the transfer of therapeutic drugs, and this results in an inferior targeting effect and heterogenic tumor distribution of drugs [43]. Nanomedicines improve targeting effectiveness and selectivity [44–46]. This is especially the case when they are supported by an enhanced permeability and retention (EPR) effect, which promotes extravasation and effectual intratumor localization [47] (Scheme 2). Despite progress being made, moderate clinical success has been achieved so far. This is because nanomedicine applications have encountered severe obstacles related to avascular tumor sites due to the TME’s characteristics restricting nanomedicines’ access only to highly vascular regions with increased perfusion [47,48]. These limitations in the therapeutic efficacy of nanomedicines have been tackled recently by exploiting the complex mechanisms and associated properties of TME. This improves intratumoral localization. Furthermore, specially designed nanomedicines exhibit stimuli-responsiveness in TME features, such as hypoxia and acidity, by combining ligand-mediated active targeting of selective receptors with growth factors, inhibitors, enzymes, and peptides. Combining the benefits of external stimuli-responsiveness has, beneficially, increased the targeting efficiency of nanomedicines and amplified the therapeutic activity [49–52].
Nanomedicines that target solid tumors traverse a highly variable environment. This emphasizes the importance of adaptability and spatiotemporal pharmacological activity. The current treatment strategies that aim to overcome MDR and the multi-factorial TME rely on a combination of chemo-, radio-, and immunotherapies (Scheme 3). Approved nanomedicines are co-administered for improved synergistic therapeutic effect. In this way, biomaterials have greatly assisted in orchestrating the pharmacokinetics and targeting potential of nanomedicines. This is due to their inherent properties of biodegradability and ease of surface modification which offer effective sites for receptor-mediated ligand targeting (peptides, antibodies, nucleic acids, small organic molecules). Biomaterials also make possible theranostic applications of nanomedicines for real-time therapy and monitoring of tumor tissues. Moreover, biomaterials can be appropriately functionalized to obtain stimuli-responsiveness and manipulate TME hypoxia and spatial pH distribution (acidic extracellularly vs. basic intracellularly). In addition, the effectual combination of biomaterials with acquired responsiveness on varied external stimuli (hyperthermia, photodynamic, sonodynamic) has highly improved tumor-specific accumulation. This modulates cellular apoptotic cascades and promotes cellular death by associated gene regulation [53–55]. In this respect, biomaterials are important for TME-responsive applications, and they demonstrate promising results in combinational therapies and immunotherapies. Some of these are FDA-approved or under clinical trials for specific tumor types (Table 1) [56–59]. The clinical trials and applications of nanomedicines have mostly relied on improving drugs’ activity, as well as reducing side effects, and on enhancing biodistribution of drugs and agents within solid tumor tissues. For this, strategies have been developed to overcome physical, biological, and chemical obstacles in the TME, and achieve efficient specificity on molecular targets (such as cellular receptors, CAFs, CSC) and physiological factors (such as ECM, angiogenesis, IFP) [50,55,60]. The innate biological properties of biomaterials against opsonization have offered great support, because they provide longer and efficient systemic circulation time of the nanomedicines [61]. Natural polymers and synthetic biodegradable polymers which have been extensively reviewed and discussed for their application in the pharmaceutical and biomedical fields include: (i) polysaccharides such as alginic acid (alginate), dextran, agarose, hyaluronic acid, carrageenan, chitosan, and cyclodextrin: (ii) protein-based polymers such as gelatin, albumin, soy, and collagen; and (iii) synthetic polymers such as polyesters, polyamides, polyanhydrides, phosphorous based, and polyurethanes [62–67]. In solid tumor therapeutics, the benefits of novel theranostic and multifunctional nanomedicines are a niche research area that aims to overcome the limitations of TME and design novel therapies [67] (Scheme 1). In the following, we discuss novel therapeutic and targeting concepts of biomaterial-based nanomedicines which exploit TME specificity and responsiveness.
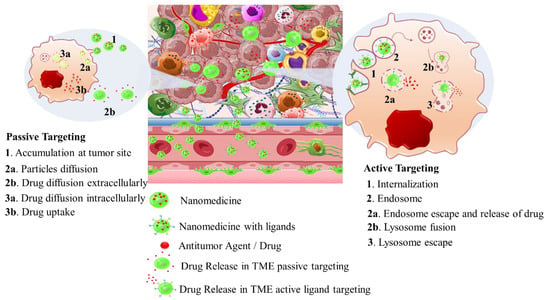
Table 1. Therapeutic nanomedicines approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
|
Carrier Type |
Product Name |
Therapeutic Agent |
Cancer Type |
Stage |
Ref. |
|---|---|---|---|---|---|
|
Liposomes |
Zolsketil® |
Doxorubicin |
Metastatic breast cancer, advanced ovarian cancer, multiple myeloma, AIDS-related Kaposi’s sarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/zolsketil-pegylated-liposomal (accessed on 15 January 2024) |
Approved (EMA, 2022) |
[56,57] |
|
Vyxeos® |
Cytarabine: daunorubicin |
Newly diagnosed therapy-related acute myeloid leukemia, acute myeloid leukemia with myelodysplasia related changes (https://www.ema.europa.eu/en/medicines/human/EPAR/vyxeos-liposomal-previously-known-vyxeos, accessed on 15 January 2024) |
Approved (EMA, 2018) (FDA, 2017) |
[56,57] |
|
|
Onivyde®/CPX-351 |
Irinotecan |
Pancreatic cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/onivyde-pegylated-liposomal-previously-known-onivyde, accessed on 15 January 2024) |
Approved (EMA, 2016) (FDA, 2015) |
[56,57] |
|
|
Mepact® |
Mifamurtide |
Osteosarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/mepact, accessed on 15 January 2024) |
Approved (EMA, 2009) |
[56,57] |
|
|
Ameluz® |
5-aminolevulinic acid |
Superficial and/or nodular basal cell carcinoma (https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz, accessed on 15 January 2024) |
Approved (EMA, 2011) |
[56,57] |
|
|
DaunoXome® |
Daunorubicin |
Kaposi’s sarcoma |
Approved (FDA 1996) Discontinued (FDA, 2021) |
[56,57] |
|
|
Iron Oxide nanoparticles |
NanoTherm® |
Fe2O3 |
Glioblastoma, prostate, and pancreatic cancer (https://www.eib.org/en/stories/new-cancer-treatments, accessed on 15 January 2024) |
Approved (EMA, 2013) |
[57] |
|
Albumin nanoparticles |
Abraxane® |
Paclitaxel |
Metastatic breast cancer, locally advanced or metastatic non-small cell lung cancer, metastatic adenocarcinoma of the pancreas (https://www.ema.europa.eu/en/medicines/human/EPAR/abraxane, accessed on 15 January 2024) |
Approved (EMA 2008) (FDA 2005) |
[57,58] |
|
Pazenir® |
Paclitaxel |
Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, non-small cell lung cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/pazenir, accessed on 15 January 2024) |
Approved (EMA 2019) |
[58] |
|
|
Vaccines |
Adstiladrin® |
Adenoviral vector-based gene therapy |
Bacillus Calmette–Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-adstiladrin-nadofaragene-firadenovec-vncg-patients-high-risk, accessed on 15 January 2024) |
Approved (FDA 2022) |
[59] |
|
Provenge® |
Autologous peripheral-blood mononuclear cells |
Metastatic castration-resistant prostate cancer (mCRPC) (https://www.drugs.com/history/provenge.html, accessed on 15 January 2024) |
Approved (EMA 2013) (FDA 2010) Discontinued (EMA, 2015) |
[59] |
3. Nanomedicines for Targeting TME: Application of Natural and Synthetic Biomaterials
3.1. The Heterogenic Vasculature
Angiogenetic mechanism is divided into two phases: the avascular, wherein tumor progression is suppressed due to controlled homeostasis of pro- and anti-angiogenetic factors; and the vascular, wherein tumor development is promoted by a switched homeostasis favoring a pro-angiogenetic environment. For solid tumors, in order to progress and develop, a de facto ultimate need is presented for blood, oxygen, and nutrient supply. This is supported by the continuously evolving tumor vasculature (Scheme 4) [68–70]. Thus, regulating angiogenesis is a key step in tackling TME abnormal vasculature that is being targeted by nanomedicines through angiogenetic inhibitors. The intention is to promote tumor suppression mechanisms by limiting the unrestricted vascular development [71]. The main target of angiogenetic therapeutics (Table 2) is the inhibition of growth factors of the pro-angiogenetic domain that present elevated affinity with surface receptors of ECs. These include: (i) soluble factors such as vascular endothelial growth factor (VEGF family factors comprising A to F members), platelet-derived growth factor (PDGF), beta and alpha transforming growth factors (TGF-α, -β), angiopoietins (Ang); and (ii) insoluble membrane-bound proteins such as ephrins, integrins, cadherins, matrix metalloproteinases (MMPs), and hypoxia-inducible factor-1 (HIF-1) (Scheme 5) [72].
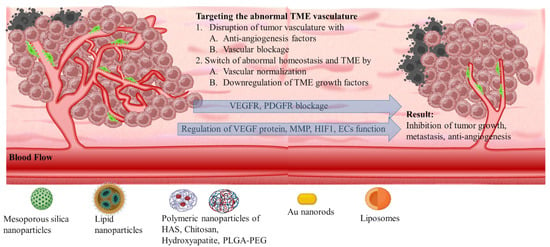
3.1.1. VEGF Therapeutic Targeting
3.1.2. Targeting Molecular Markers for Vasculature Regulation
3.1.3. Targeting Formulation Based on FDA-Approved Drugs
3.1.4. Responsive Targeting and Combinational Therapies
|
Targeting Effects |
Carrier Type |
Therapeutic Agent |
Characteristics |
Ref. |
|
VEGF |
Hydroxyapatite (HA) |
Sulfated s-HA |
Non-selective binding of VEGF165a |
[75] |
|
Chitosan (CS) |
Sulfated s-CS |
Inhibition of VEGF/VEGFR2 signaling pathway |
[76] |
|
|
CS/siRNA nanoplexes |
siRNA |
Silencing effect of siVEGF-A, siVEGFR-1, siVEGFR-2, and NRP-1 inhibiting proliferation with improved immune functions |
[77] |
|
|
Carboxymethyl chitosan (CMCS) |
CMCS |
Regulate dexpression of VEGF levels, MMP-1, and CD34, and promoted inhibition of angiogenesis |
[78] |
|
|
Endothelial Cell Regulation |
HA-P123/F127 Polymeric nanoparticles |
Thymoquinone |
Modulating expression of miR-362/Rac1/RhoA and miR-361/VEGF-A pathways for inhibiting angiogenesis |
[85] |
|
CS nanoplexes |
siRNA |
Targeting PDGF-D and PDGFR-β expressions |
[86] |
|
|
Hydroxyapatite nanoparticles (HANP) |
HANP |
Regulating ECs function by the PI3K/Akt/eNOS signaling pathway |
[87] |
|
|
Hydroxyapatite nanoparticles |
p53 plasmid and candesartan |
Downregulation of VEGF protein secretion and functional microvessel density |
[88] |
|
|
PLGA nanoparticles |
P28 peptide and gefitinib |
Inhibit tumor angiogenesis, primary tumor growth, and metastasis |
[89] |
|
|
FDA-Approved Drugs |
Mesoporous silica nanoparticles (PEG-MSNs) |
Sunitinib (anti-VEGFR) |
Increased VEGFR targeting specificity, efficient inhibition of angiogenesis |
[93] |
|
Lipid-chitosan nanoparticles |
Bevacizumab (VEGF-A antibody) |
Suppressing proliferation and endothelial cells angiogenesis |
[94] |
|
|
PLGA-PEG nanoparticles |
Bevacizumab |
Higher internalization and bevacizumab delivery into CD44v6+ ECs |
[95] |
|
|
Human serum albumin nanoparticles |
Bevacizumab |
Decreased glycolysis and metabolic tumor volume, inhibition of tumor growth |
[96] |
|
|
Chitosan nanoparticles |
Sorafenib (Tie2 inhibitor) |
Superior antitumor activity |
[97] |
|
|
Combinational Therapies |
PEG-PCL-PAEA-SA nanoparticles |
Gambogenic acid/charge-reversible effect |
Suppressed tumor angiogenesis, very little to no vascular tubes inside tumor models |
[99] |
|
PLGA nanoparticles |
Sorafenib/Sunitinib/siRNA |
Synergistic effect inhibiting cell proliferation |
[101] |
|
|
PLGA-PEG nanoparticles |
Anlotinib/pH-sensitivity |
Inhibited tumor growth and metastasis suppressing lymphangiogenesis |
[102] |
|
|
Polycation liposomes |
siRNA/calcium phosphate particles |
Suppressed tumor growth and angiogenesis |
[103] |
|
|
PEG-liposomes |
Doxorubicin/curcumin |
Suppressed tumor growth, invasion, and metastasis |
[104] |
|
|
Au nanorods |
NRP-1 peptide/PDT |
Inhibition of angiogenesis |
[108] |
3.2. The Tumor Stroma Extracellular Matrix
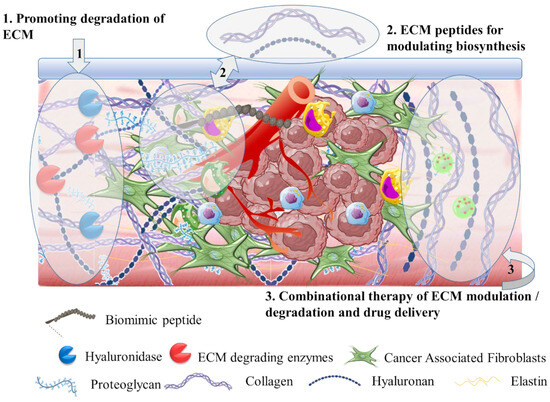
3.2.1. Hyaluronidase for ECM Targeting
3.2.2. Extracellular Matrix Degradation
3.2.3. Targeting Biomolecules for Extracellular Matrix
|
Targeting Effects |
Carrier Type |
Therapeutic Agent |
Characteristics |
Ref. |
|
Hyaluronidase |
PEGPH20 |
PEGPH20/gemcitabine/nab-paclitaxel |
Phase III trial (HALO-109-301) |
[117] |
|
PLGA-PEG nanoparticles |
rHUPH20/doxorubicin |
Effective tumor accumulation enhanced antitumor effect |
[118] |
|
|
ECM Degradation |
Doxorubicin liposome (Doxosome) |
Bromelain/Hyaluronic acid linked collagen type IV-binding peptide |
Decayed the density of collagen fibers and advanced the tumor distribution |
[119] |
|
PLGA-polydopamine-PEG nanoparticles |
Collagenase I/Doxorubicin |
Degradation, enhanced the intratumoral distribution, and enhanced antitumor immunity |
[120] |
|
|
LOXL2 antibody |
LOXL2 antibody |
Control of collagen assembly in ECM, potentially control tumor progression |
[123] |
|
|
PLGA-PEG-PLGA thermosensitive hydrogel |
Trastuzumab (Herceptin)/collagenase |
Degradation of intratumoral collagen promoting the antibody effect |
[124] |
|
|
mPEG-PLGA nanoparticles |
LOL2 and DDR1 inhibitors/Nab-paclitaxel |
Enhanced penetration and accumulation in tumor |
[125] |
|
|
Ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles |
MMP9-sensitive peptide/Doxorubicin |
Effective bioimaging and synergistic chemo-photothermal antitumor effect |
[126] |
|
|
ECM Biomolecules |
Peptide nanoparticles |
Laminin (LN) mimic peptide |
Increased distribution in the tumor site and simultaneous transformation into nanofibers surrounding the tumor site |
[128] |
|
Hyaluronic acid mesoporous silica nanoparticles |
siRNA suppressing CTGF expression/Doxorubicin |
Inhibition of multidrug resistance and increased susceptibility of tumor cells to drug-induced apoptosis |
[131] |
3.3. The Tumor Stroma Cancer Associated Fibroblasts
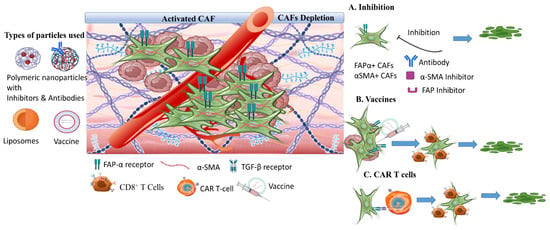
3.3.1. Targeting Nanomedicine for CAFs Depletion
3.3.2. Synergistic CAFs Inactivation with Antitumor Targeting
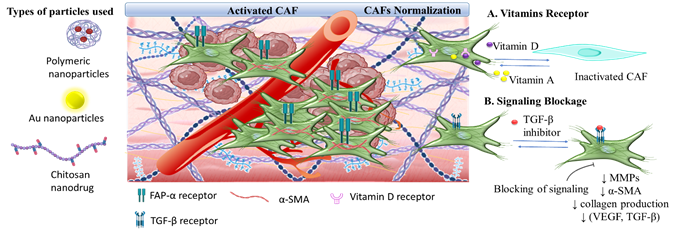
|
Targeting Effects |
Carrier Type |
Therapeutic Agent |
Characteristics |
Ref. |
|
CAFs depletion |
Anti-FAP-IL liposomes |
Single-chain Fv fragments against FAP (scFv’FAP) |
Specifically and efficiently respond to FAP-α cell surface biomarker |
[139] |
|
Cleavable amphiphilic peptide (CAP) nanoparticles |
Doxorubicin/CAP |
Disturbed the stromal barrier and increased drug intratumoral accumulation |
[140] |
|
|
Thermosensitive liposomes (CAP-TSL) |
IR-780 photothermal agent/paclitaxel/human serum albumin |
Increased cells apoptosis, expanded tumor interstitial space, promoted deep tumor penetration |
[141] |
|
|
Poly(amidoamine) (PAMAM) hyaluronic acid nanoparticles |
Doxorubicin/CAP peptide |
Deep intratumoral penetration, suppression of TGF-β, α-SMA, and FAP-α, degradation of tumor fibrotic stroma |
[143] |
|
|
Vaccines |
FAP targeting |
Synergistic antitumor immunity effect |
[136,144,145] |
|
|
Synergistic inactivation |
Glycol chitosan–DEAP nanodrug |
Methotrexate/quercetin |
Inhibition of pre-metastatic initiation, downregulation of metastasis promoting factors inactivation of CAFs |
[146] |
|
Hydroxyethyl starch PLA nanoparticles |
Doxorubicin/TGF-β receptor inhibitor |
Suppression of tumor growth and metastasis |
[147] |
|
|
Au nanoparticles |
Photodynamic therapy |
Inhibit the expression of pro-fibrotic signaling via Akt pathway |
[154] |
3.4. The Tumor Hypoxia
Another therapeutic target of responsive nanomedicine is the TME hypoxia (Scheme 9). This is a direct consequence of heterogenic vasculature and fluctuating blood flow, and results in insufficient oxygen diffusion and perfusion within the tumor environment. The rapid proliferation rate of tumor and stromal cells creates excessive consumption of supplied oxygen, nutrients, and energy [155]. Imbalance in the diffusion mechanisms of oxygen supply is observed at depths after 70–150 μm from peripheral tumor blood vessels. This results in gas oxygen (gas-O2) levels falling below 1–2% in hypoxic solid tumors. There are two types of hypoxia: the chronic, wherein oxygen’s concentration is characterized by a longitudinal gradient drop for a prolonged time period of several hours; and the acute, in which tumor ECs and stromal cells are attached to vasculature with deprived oxygen perfusion [156]. Extensive research has resulted in the understanding of hypoxia mechanisms and their effects on tumor biology by participating in the regulation of angiogenesis, metastasis, and multidrug resistance (Table 5) [157,158]. Hypoxia-regulated genes are expressed among various tumor types with high hypoxic gene expressions such as the squamous cell carcinoma (SCC) of the head and neck, lung, and cervix tumors; these have also been investigated [159].
3.4.1. GLUT Targeting Nanomedicines
A central factor in targeting TME hypoxia is effective exploitation of transcription factors related to various regulatory pathways of glycolysis, oxygen homeostasis, MDR, and resistance to apoptosis. Such factors within TME include the carbonic anhydrase IX (CA-IX), the glucose transporter-1 and 4 (GLUT-1, 4), and the hypoxia inducible factors (HIF) family (including HIF-1, HIF-2, HIF-3). These act as oxygen regulators to stabilize hypoxic conditions and promote tumor cell survival and angiogenesis through PDGF secretion [160]. Specifically, the blockage of GLUT-1 and GLUT-4 transporters with glucose-modified PLGA and chitosan nanoparticles was selected as an active targeting strategy to limit nutrient supply to tumor cells by Abolhasani et al. [161]. They found that this glucose-modified nanoparticle effectively inhibited GLUTs function and stimulated glucose deprivation and increased apoptotic enzyme expression. In a recent study by Sun et al. [162], the ATRP copolymerization of glucose-containing methacrylate (GluMA) and OEGMA was used for the conjugation of interferon-α (IFN-α) for effective GLUTs targeting. The study outlined the importance of optimizing glucose content. This is because excessive glucose concentration resulted in inhibition of the antitumor activity, while the optimal system showed enhanced tumor targeting and antitumor immunity; this was expressed by the secretion levels of TNF-α and IL-2 cytokines. In general, glycosylation of nanoparticles has been exploited for its increased GLUT-targeting ability, and it promotes metabolic changes and immune responses. This has been reviewed by Torres-Perez et al. [163]. Moreover, glycolysis is the main source of energy production in hypoxic and aerobic tumor sites (the Warburg effect). This demonstrates the importance of nanomedicines targeting the glycolytic mechanisms. In a recent paper by Geng et al. [164], the targeting of glycolytic enzymes and transporters, and combinational strategies based on nanomedicines were reviewed. Also, glucose metabolism in hypoxic tumor sites highly activates cancer stem cell (CSCs) phenotypes, and this promotes the activation of a stem-like polarization that is mainly regulated by the transcription factor HIF-1α and the Notch pathways [165]. Shibuya et al. [166] demonstrated the importance of modulating GLUT-1 transporter in regulating the stem-like phenotype in pancreatic, ovarian, and glioblastoma CSCs. By specifically inhibiting GLUT-1 with WZB117, the self-renewal and tumor-initiating activities of the CSCs were effectively suppressed in animal models upon implantation of CSCs.
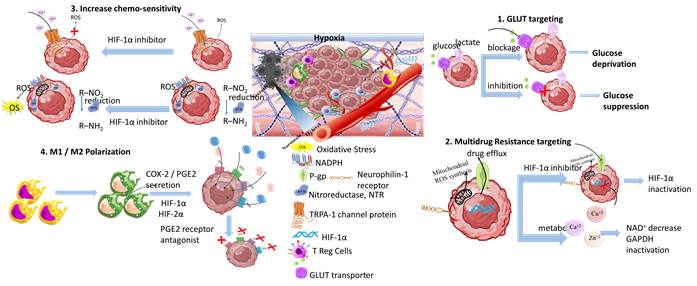
Scheme 9. Tumor hypoxia represents a significant target for responsive nanomedicines. Important axes of nanomedicine research are GLUT targeting, multidrug resistance targeting, increase of chemo-sensitivity, M1/M2 macrophage polarization, increase of tumor oxygenation, and antioxidants. Biomaterials used for hypoxia targeting nanomedicines include polysaccharides, polymers, hybrid nanoparticles, metal oxides and hybrid metal–polymer nanoparticles, polymersomes, nanogels, and liposomes in combination with chemotherapeutic drug targeting (CDT), magnetic targeting, and PDT/PTT therapy. (Created with the assistance of BioRender.com https://app.biorender.com/user/signin?illustrationId=6156d45891063d00af8af51d (accessed on 1 September 2023 up to 31 November 2023), and Microsoft ppt).
Moreover, the energy supply blockage promoted by specific starvation therapeutics is highly associated with hypoxia-targeting nanomedicines. This is caused by exploitation of biological metal ions (e.g., Ca2+, Zn2+, Cu2+) in tumor starvation mechanisms. Among them, Cu2+ and Zn2+ are the most prevalent components of enzymes, and they play a crucial role in energy metabolism, gene expression, and genomic stability. Yang et al. [167] studied the effect of copper-based ultra-small nanoparticles (Cu2−xSe) modified with HIF-1α inhibitor and coated with tumor cell membrane. The nanoparticles were effectively transported through the blood–brain barrier upon focused ultrasound application, and accumulated at glioblastoma tumor sites where they exhibited significant antitumor activity by inhibiting HIF-1α expression and enhancing tumor sensitivity to disulfiram. In another study, Wu et al. [168] examined the effect of zinc imidazole metal-organic particles (ZIF-8) modified with hyaluronic acid for systemic glycolytic energy deprivation. The nanoparticles expressed CD44-targeting mechanism. This led to effective tumor accumulation and cellular endocytosis that promoted the disaggregation of zinc core by hyaluronidase. The latter further triggered a decrease of NAD+ and inactivation of GAPDH, and obtained strong glycolysis inhibition. Additionally, it suppressed GLUT1 regulation which promoted energy starvation in B16-F10 tumor-bearing C57BL/6 mice. The energy demand of tumor cells is critically supported by increased glucose production that leads to increased expression levels of GLUT1 and GLUT2, and abundant glycolytic enzymes. The phenotyping of tumor cells with elevated glucose expression levels represents a negative prognostic factor that can be exploited in diagnosis for the effective design of personalized nanomedicines. In this respect, glucose nanosensors have been investigated for application in diagnosis by Nascimento et al. [169]. They studied the effectiveness of nanopipette-based glucose sensors functionalized with glucose oxidase (GOx) to quantify single cell intracellular glucose levels.
3.4.2. Multidrug Resistance Targeting Therapeutics
The targeting of HIF transcription factors will not be broadly discussed here. This is because its role in hypoxia mechanisms and in responsive therapeutic nanomedicine has been extensively reviewed [170–172]. A comprehensive mini-review on engineered nanomedicines summarizing recent progress on HIF-1 targeting was presented by Zhang et al. [173]. HIF is highly associated with multidrug resistance in solid tumors. It develops as tumor cells develop a defense mechanism against drugs, and this results in drug efflux and decrease of intracellular drug concentration. MDR is an ultimately complex mechanism related to gene mutations, increased DNA repair ability, and epigenetic alterations involved in the regulation of gene expression such as silencing of tumor suppressor genes and overexpression of oncogenes. In MDR, drug efflux from tumor cells is mediated by efflux transmembrane pumps such as permeability-glycoprotein (P-gp) (also known as multidrug resistance protein 1 (MDR1)), and important transmembrane proteins that are ATP-dependent and regulated by HIF proteins [174]. Certain chemotherapeutic agents that act through mitochondrial ROS formation, e.g., cisplatin (CSP or DDP), can affect MDR either through inducing ROS-mediated cancer cell death by activating apoptotic signaling pathways or through promoting drug resistance and activation of HIF-1 cascade due to elevated ROS production. However, ROS-induced HIF-1 activation also leads to severe damage to the surrounding normal tissues. In a recent study, Zhang et al. [175] investigated the effect of cisplatin on inhibiting ROS induced HIF-1 activation and acquired resistance using chitosan-coated selenium/cisplatin nanoparticles. Chitosan provided long circulation and effective nanoparticles accumulation at the tumor site in cisplatin-resistant A549 tumor-bearing mice. At the tumor, cisplatin promoted HIF-1 expression in an ROS dependent function while the selenium antioxidant effect suppressed ROS formation and inhibited HIF-1 activation. The inhibitory effect of selenium nanoparticles was also confirmed by the downregulation of GCLM, P-gp, MDR2, and HIF-1α protein expression levels. The acquired drug resistance promoted by cisplatin application was examined by Zhang et al. [176] in organosilica-coated cisplatin nanoparticles that co-deliver the HIF-1 inhibitor acriflavine (ACF). The nanoparticles were efficiently accumulated at the tumor site, being susceptible to glutathione triggered biodegradation, and released ACF for inhibiting HIF-1 activity by preventing the formation of HIF-1α/β dimers via HIF-1α binding. The synergistic release of cisplatin resulted in a highly effective system suppressing tumor growth and metastasis. Reversing MDR was also studied by Li et al. [177] through the combined delivery of doxorubicin and the HIF inhibitor PX478 by silk fibroin nanoparticles. The nanoparticles were functionalized with folic acid (FA) for cancer cells targeting. The multi-responsive nanoparticles inhibited HIF gene expression by the synergistic action of FA receptor-mediated endocytosis and PX478 inhibition that effectively downregulated MDR1 expression levels, thus eliminating DOX efflux. Neuropilin-1 (NRP1) is another receptor involved in cellular processes related to MDR in tumor cells. The role of NRP-1 on the loss of therapeutic efficacy of lenvatinib was evaluated by Fernandez-Palanca et al. [178] in relation to hypoxia and modulation of HIF-1α. Mamnoon et al. [179] studied NRP-1 as a molecular target for the delivery of iRGD peptide and doxorubicin by hypoxia-responsive PLA-diazobenzene-PEG polymersomes. Evaluation in tumor-bearing animal models demonstrated the accumulation of the polymersomes at the tumor site and the antitumor effect of their drug cargo.
3.4.3. Increasing Chemo-Sensitivity
On the basis of chemotherapy and radiotherapy resistance, the researched solutions focused on sensitizers and in enhancing TME oxygenation. The sensitizers are, in general, chemical compounds (originally nitro-aromatic ring compounds) that act by elevating the tumor cellular sensitivity to ionizing radiation, and produce free radicals and promote DNA damage [180]. A great drawback of the application of chemical sensitizers was the dose-dependent toxicity and adverse effects related to ROS and RNS expression levels. However, more effective sensitizers with low toxicity were designed by exploiting small molecules (O2, NO), macromolecules (proteins, peptides, miRNA, siRNA), and nanomaterials (noble metals, ferrite, heavy metals) that act without distressing ROS expression levels [181]. In tumors, TRPA-1 is a channel plasma membrane protein that promotes extracellular Ca2+ influx and represents the most abundant redox sensor upregulated in metastatic cells of solid tumors such as human oral squamous cell carcinoma, colorectal cancer adenocarcinoma, and glioblastoma multiform. The TRPA-1 was evaluated as a ROS sensor, since its upregulation-mediated enhanced ROS-induced Ca2+ influx as a result of oxidative stress. This promoted anti-apoptotic signaling pathways [182]. Wang et al. [183] studied TRPA-1 inhibition in order to increase tumor chemosensitivity in tumor-bearing mice. They used hyaluronic acid nanogels functionalized with DSPE-PEG nano-micelles conjugated with a tumor homing penetrating tLyP-1 peptide for co-delivering doxorubicin and TRPA-1 inhibitor (AP-18). The combined effects of HA and penetrating peptide resulted in enhanced intratumoral and intracellular localization becasue the HA nanogels disassembled in irregular fragments releasing peptide conjugated nano-micelles upon hyaluronidase effect in TME. The TRPA-1 inhibitor enhanced tumors sensitivity to DOX by suppressing Ca2+ uptake and AKT phosphorylation, and promoting regulation of EMT-related proteins.
A redox enzyme that is highly associated with tumor hypoxia is nitroreductase (NTR). This catalyzes the reduction of nitro compounds in the mitochondria by using NADPH. Jang et al. [184] examined the effect of NTR in self-assembled nanoparticles of glycol chitosan loaded with doxorubicin, functionalized with folic acid andmodified with 4-nitrobenzyl chloroformate (4NC). The nanoparticles effectively accumulated at the tumor site due to the combined effects of CS and FA targeting, and disassembled due to the reduction of the chitosan bonded 4NC under hypoxic conditions by the NTR/NADPH cascade. This efficiently promoted DOX antitumor activity. Another study exploiting the hypoxia-mediated bond reduction was by Luo et al. [185]. They examined self-assembling thioether linked dihydroartemicin (DHA) prodrug nanoparticles loaded with chlorin e6 and further encapsulated in core-shell amphiphilic carboxymethyl chitosan polymer particles, grafted with maleimide and 2-nitroimidazole (NI) groups. The nanoparticles showed significantly long circulation time and accumulation ability through the combined chitosan and EPR effect. Once PDT was applied, oxygen consumption was increased and the NI groups were reduced. This destabilized the structure of the chitosan particles, and further promoted drug release at the tumor site. The synergistic effect resulted in suppression of HIF-1α and VEGF expression levels, inhibition of tumor metastasis, and an improved therapeutic effect in LLC tumor-bearing rats and mice.
3.4.4. Targeting M1/M2 Macrophage Polarization
In TME, hypoxia is responsible for inducing the reprogramming of pro-inflammatory M1 macrophages to take a M2 anti-inflammatory phenotype; this promotes tumor aggressiveness and MDR. M1 macrophages play critical roles in innate host immunity and tumor cell death through the production of ROS/RNS and pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. M2 macrophages polarization induced by Th2 cytokines such as IL-4, IL-10, and IL-13 play a critical immunosuppressive role in immune responses, under the activation of immune complexes (IC) and TLR ligands, producing anti-inflammatory cytokines such as IL-10, IL-13, and TGF-β to promote tumor development. By altering macrophage responses, hypoxia downregulates the expression of antigens and the release of pro-angiogenetic factors. This exerts immunosuppressive effects that promote cell proliferation. While the M1/M2 macrophage states have been identified, the regulation of macrophage phenotypes remains a complex mechanism. The production of distinct functional phenotypes in macrophages (polarization), as a consequence of hypoxia, is essentially regulated by HIF-1α, which plays a key role in the expression of several genes [186]. Among them, the PTGS gene encoding cyclooxygenase-2 (COX-2) is of major importance, since it is related to increased accumulation of pro-inflammatory signals, and represents an indicator associating inflammation with cancer [187]. COX-2 is secreted by CAFs, M2 macrophages and TME cells inducing cancer stem-like cells activity, MDR, and metastasis. COX-2 induced HIF-1α activity is responsible for biosynthesis of prostanoids, like prostaglandin E2 (PGE2); this is overexpressed in a variety of cancers including breast cancer, osteosarcoma, and colorectal cancer. The HIF-1α/COX2 signaling axis is highly related to resistance, invasion, and metastasis [188]. Karpisheh et al. [189] studied the effect of HIF-1α silencing siRNA and PGE2 receptor antagonist (EP4-antagonist) as a combinational therapy with hyperthermia in tumor-bearing BALB/c nude mice. For this purpose, superparamagnetic iron oxide nanoparticles (SPIONs) functionalized with hyaluronic acid and trimethyl chitosan were used for the delivery of siRNA and the EP4 antagonist. The synergistic effect of hyperthermia, CS and HA resulted in increased SPIONs biodistribution at the tumor site, and effectively delivered the siRNA. The combined effect of HIF-1α siRNA and the EP4 antagonist resulted in (i) suppression of proliferation and migration of tumor cells by decreasing the expression levels of ki-67 gene, anti-apoptotic protein Bcl-2, and MMP-2/-9; and (ii) inhibition of VEGF, FGF, and TGF-β. This promoted suppression of angiogenesis and inhibition of tumor growth.
3.4.5. Combinational Targeting for Increasing Tumor Oxygenation
In order to increase tumor oxygenation, various strategies have been investigated. These include: (i) modulation of tumor blood flow with compounds, such as noradrenaline, benzyl nicotinate, nicotinamide, and pentoxifylline; (ii) high oxygen breathing through hyperbaric chambers, and carbogen breathing; (iii) targeting of tumor vasculature with anti-angiogenetic therapies and vascular disruptive agents [190]. Hyperbaric oxygen (HBO) treatment was used to suppress hypoxia by effectively elevating the oxygen concentration in plasma and, subsequently, enhancing oxygen delivery in tumor tissues independently of hemoglobin. This resulted in reduced tumor growth in breast cancer; however, cervical and bladder cancers appear to be insensitive to HBO [191]. In a recent study by Wang et al. [192], HBO was applied in combination with Abraxane and gemcitabine (GEM) to trigger antitumor activity against murine PDAC tumors, expressing inhibitory activity over ECM by decreasing fibril deposition of collagen I and fibronectin. As CAFs are the main cellular components of ECM, HBO significantly inhibited their activity by suppressing tumor hypoxia that, in combination with abraxane and GEM, resulted in inhibition of CSCs. This is evidenced by the decreased expression levels of CD133 and Sox2 CSCs-related biomarkers, and this further promoted the antitumor activity of the drugs in primary and in metastatic PANC02 tumors.
In a recent review by Wu et al. [65], nanoparticle-based systems targeting TME were presented. These include hypoxia-responsive nanomedicines that effectively target hypoxic TME to promote tumor oxygenation. Tumor oxygenation in order to inhibit hypoxia was examined by Song et al. [193], with the application of manganese dioxide nanoparticles (MnO2) coated with hyaluronic acid and modified with mannan-PE ligands in combination with priming of pro-inflammatory M1 macrophages phenotype. The nanoparticles caused elevation of tumor oxygenation. This is shown by the suppression of HIF-1α and VEGF expression levels, and expressed immune-toxicological effects in reprogramming the antitumor M1 phenotype. Furthermore, the nanoparticles had synergistic effect upon administration with doxorubicin in tumor-bearing mice; in which they inhibited tumor growth and cell proliferation. Importantly, the MnO2 nanoparticles express a redox-active catalytic behavior toward hydrogen peroxide (H2O2) to produce oxygen and regulate acidic pH. This is because their Mn2+ decomposing products are excellent T1-shortening MRI agents; this is described by Lin et al. [194] in human serum albumin MnO2 nanoparticles conjugated with Ce6 photosensitizer. The evaluation of the nanoparticles in tumor-bearing mice showed increased tumor-targeting ability and accumulation efficacy, with elevated oxygenation levels. Additionally, treatment with PDT-enhanced cellular apoptosis, resulted in a large tumor necrosis area. Another effective system promoting oxygenation was studied by Jiang et al. [195], who used MnO2 albumin nanoparticles delivering indocyanine green (ICG), a hydrophilic anion drug, for effective PDT of hypoxic tumors. The nanoparticles were evaluated in both CT26 and B16F10 tumor-bearing mice and showed enhanced tumor accumulation efficacy and successful responsive release of ICG in the presence of hydrogen peroxide. The combined effect with MnO2 and PDT resulted in enhanced oxygen production and attenuation of hypoxic TME, while elevated distribution of CD3+ and CD8+ T cells was promoted at tumor sites. In advanced hepatocellular carcinoma (HCC) the anti-angiogenetic agent sorafenib is a first-line treatment. However, the hypoxic TME of advanced HCC regulated anti-apoptotic signaling pathways and promoted immunosuppressive reprogramming, resulting in MDR against sorafenib. Ren et al. [196] studied the synergistic effect of tumor oxygenation and PDT by a strategic hypoxia relieving nanodrug (SHRN). SHRN was based on pegylated liposomes encapsulating MnO2-BSA nanoparticles, the oxygen consumption inhibitor atovaquone (ATO), and the photosensitizer hypericin (HY). In this system, oxygen production was effectively increased by MnO2 nanoparticles and the synergistic action of ATO with mitochondrial complex III resulted in the blockage of aerobic respiratory chain and the suppression of oxygen consumption. By suppressing hypoxia in TME of tumor-bearing mice, PDT application essentially promoted HY action to react with oxygen and promote increased antitumor effect. The antitumor activity of PDT originated from the elevated generation of ROS as an outcome of electron transfer mechanisms of the photosensitizer and the molecular oxygen intracellularly. A review of the mechanisms associated with PDT efficacy and the limitations induced by hypoxia has been authored by Zhou et al. [197]. In another study, Chang et al. [198] investigated the effect of lipid-PLGA particles co-delivering MnO2 nanoparticles and sorafenib as an effective approach for HCC. The nanoparticles promoted oxygen production in orthotopic HCC tumor mice xenografts, and this resulted in suppression of hypoxia and of MDR to sorafenib. As a result, inhibition of tumor cells proliferation and suppression of angiogenesis and metastasis were observed.
In addition to MnO2, iron oxide nanoparticles were also studied as Fenton catalysts triggering reduction of hydrogen peroxide to oxygen by ferrous ions. He et al. [199], described the in vitro and in vivo effectiveness of solid lipid calcium dioxide (CaO2) nanocarriers (SLNs) for co-delivery of doxorubicin and iron-oleate. At the tumor site, the SLNs dissociated by lipase overexpressed in cancer cells, enabling the release of iron-oleate and CaO2 particles. These, in response to acidic cellular environment, released DOX and produced hydrogen peroxide molecules. The Fe3+ ferrous ions released from iron-oleate reacted with H2O2 molecules to produce oxygen and the Fe2+ ions created hydroxyl radicals for antitumor chemodynamic therapy (CDT). The latter can induce oxidative damage to tumor tissues through Fenton or Fenton-like reactions of metal catalysts. In another interesting study by Ou et al. [200], the antitumor effect of CDT based on Fenton reaction was exploited. Βlack phosphorus nanosheets (BPNS) functionalized with active photosynthetic Chlorophyceae (Chl) cells and Fe3+ ions were synthesized. In this smart system, Chl cells exploited their inherent photosynthetic ability to produce O2 and metabolites that, in combination with the BPNS, improved 1O2 and O2 generation. The presence of Fe3+ ions resulted in the simultaneous consumption of glutathione and created hydroxyl radicals (·OH) through the reaction with hydrogen peroxide. The ultimate goal was the synergistic PDT/CDT and immune response, since Chl cells stimulate the proliferation and maturation of dendritic cells. Recently, Dong et al. [201] prepared Zn/Cu responsive nanoparticles with improved blood circulation time and increased tumor accumulation in animal models through the EPR effect. The pH-responsiveness of Cu/Zn nanoparticles originated from the ZnO particles which, at acidic pH, dissolve to Zn2+ ions. Enhanced CDT efficacy was observed at the acidic tumor pH, since Cu/Zn nanoparticles dissolved to Cu2+ ions that were further reduced by glutathione to Cu+. This effectively generates hydroxyl radical from hydrogen peroxide through a Fenton-like reaction. Moreover, CaO2 nanoparticles coated with a pH-sensitive methacrylate based copolymer were studied by Sheng et al. [202] to enable tumor oxygen generation and improved PDT therapy in MIA PaCa-2 tumor-bearing mice. The combination of CDT with immunotherapy was examined by Zhang et al. [203]. They applied liposome nanoparticles carrying copper-oleate and the HIF-1 inhibitor acriflavine (ACF), for combined antitumor immune responses. The liposomes dissociated in the acidic hypoxic TME and the released copper ions catalytically reduced hydrogen peroxide to highly active hydroxyl radicals. The activity of copper ions was supported by ACF that effectively inhibited the HIF-1/glutathione pathway. This suppressed the expression of programmed death ligand-1 (PD-L1), reduced the extracellular expression levels of lactate and adenosine, and promoted immunogenic cell death (ICD).
3.4.6. Synergistic Targeting with Antioxidants
The synergistic effect of hypoxia-targeting nanomedicines in combination with chemotherapeutic agents and antioxidants has been extensively investigated, due to their effects in reoxygenation and ROS expression levels [204–206]. The overproduction of ROS can promote oxidative stress (OS) and induce oxidative damage of biomolecules as DNA, lipids and proteins. This eventually promotes the death of normal cells while in tumor tissues, due to increased energy demand and metabolic modifications, the demand on ROS production is excessively increased. The reduction in antioxidant level and/or the disruption of redox equilibrium within TME can further promote tumor cell growth and progression. Among various antioxidants studied in tumor therapeutics, polyphenols are associated with cancer cell apoptosis, inhibition of proliferation, and downregulation of COX-2 and tumor gene expression. Vitamins and minerals elicit their antioxidant action by maintaining DNA methylation inhibiting cancer cell proliferation and progression [207]. Vitamins such as Vitamin C (ascorbic acid, AA) are capable of scavenging and neutralizing tumor-generated ROS, providing normalization of OS within local tumor sites. This interaction promotes the production of dehydroascorbic acid (DHAA) that is related with GLUT-1,3, and 4 transporters for effective and rapid cell influx. Intracellularly, DHAA is converted into AA depleting glutathione and ATP enzymes. This affects the expression levels of hypoxia signaling regulation factors [208]. However, the therapeutic exploitation of AA is limited by the ultra-high doses that are required for efficacy and by its chemical instability [209]. Thus, palmitoyl ascorbate (PA; an acylated derivative of AA) also features antitumor activities, and has been incorporated in ROS-scavenging nanomedicines. Sawant et al. [208] investigated the effect of palmitoyl ascorbate PEGylated liposomes (PEG-PAL) in vitro and in vivo in BALB/c mice bearing 4T1 tumors. PEG-PAL liposomes exhibited enhanced effectiveness in suppressing tumor growth compared to free ascorbic acid. The mechanism of action of PEG-PAL was similar to that of ascorbic acid, since liposomes acted as ROS scavengers inhibiting extracellular ROS. In another study, Yang et al. [209] examined the combinational delivery of PA and doxorubicin by liposomes for efficient synergistic effect in suppressing tumor growth. This is because PA has a similar mechanism of action with DOX associated with reduced cardiotoxicity without delaying DOX activity. The evaluation in BALB/c mice and SD rats showed the synergistic antitumor effect of the PA-DOX liposomes, which caused an increased expression of tumor apoptotic cells and a suppression of Ki-67 and CD31 protein expression levels.
Table 5. Nanomedicines based on biomaterials targeting tumor hypoxia.
|
Targeting Effects |
Carrier Type |
Therapeutic Agent |
Characteristics |
Ref. |
|
GLUT |
PLGA-chitosan particles |
GLUT-1 |
Glucose deprivation, increased apoptotic enzymes expression |
[161] |
|
Glucose-Methacrylate-OEGMA nanoparticles |
Interferon-α |
Tumor targeting and antitumor immunity |
[162] |
|
|
Cu particles/tumor cell membrane coating |
HIF-1α inhibitor/disulfiram |
Enhanced tumor sensitivity |
[167] |
|
|
Zn-imidazole–hyaluronic acid particles |
DNAzymes |
Antitumor effects inhibiting glucose energy |
[168] |
|
|
Nanopipette sensors |
Glucose Oxidase |
Identification of intracellular glucose level |
[169] |
|
|
Multidrug Resistance |
Se/chitosan nanoparticles |
Cisplatin |
Suppressed ROS formation, inhibited HIF-1α, MDR-2, P-gp |
[175] |
|
Organosilica particles |
Cisplatin/Acriflavine |
Inhibition of tumor growth and metastasis |
[176] |
|
|
Silk fibroin particles |
Doxorubicin/PX478 HIF inhibitor |
Downregulation of MDR1 and P-gp |
[177] |
|
|
PLA-diazobenzene-PEG polymersomes |
iRGD peptide/Doxorubicin |
Increased accumulation, inhibition of tumor growth |
[179] |
|
|
Chemo-Sensitivity |
Hyaluronic acid nanogels/DSPE-PEG nano-micelles |
Doxorubicin/TRPA-1 inhibitor |
Enhanced tumors sensitivity, antitumor and antimetastatic effects |
[183] |
|
Chitosan-FA particles |
Nitroreductase/Doxorubicin |
Hypoxia triggered effective antitumor action |
[184] |
|
|
CM-chitosan-maleimide particles |
Dihydroartemicin/PDT |
Suppression of HIF-1α and VEGF, inhibition of tumor metastasis |
[185] |
|
|
M1/M2 polarization |
Iron oxide-hyaluronic acid-chitosan nanoparticle |
HIF-1α siRNA/PGE2 receptor antagonist |
Suppression of proliferation, migration, angiogenesis, decreased protein levels |
[189] |
|
Combinational |
MnO2–hyaluronic acid nanoparticles |
Doxorubicin |
Inhibiting tumor growth and cell proliferation |
[193] |
|
Human serum albumin MnO2 nanoparticles |
Chlorin e6/PDT |
Tumor targeting ability, increased accumulation, elevated oxygen levels, tumor necrosis and apoptosis |
[194] |
|
|
MnO2–albumin nanoparticles |
Indocyanine green/PDT |
Enhanced oxygen production, antitumor effect |
[195] |
|
|
DSPE-PEG liposomes/MnO2-BSA nanoparticles |
Atovaquone/hypericin/PDT |
Suppressing hypoxia, increased antitumor effect |
[196] |
|
|
Lipid-PLGA-MnO2 particles |
Sorafenib |
Hypoxia suppression, inhibited tumor cells proliferation, suppressed angiogenesis and metastasis |
[198] |
|
|
Solid lipid calcium peroxide (CaO2) nanocarriers |
Doxorubicin/iron-oleate/Chemodynamic theapy |
Oxidative damage to tumor tissues |
[199] |
|
|
pH-sensitive methacrylate–CaO2 particles |
CaO2 particles/PDT |
Increased tumor oxygenation |
[200] |
|
|
Liposome nanoparticles |
Cu-oleate/Acriflavine |
Immunogenic cell death, combined antitumor immune responses |
[201] |
|
|
Antioxidants |
PEGylated liposomes |
Palmitoyl ascorbate |
Suppressed tumor growth |
[206] |
|
Liposomes |
Doxorubicin/Palmitoyl ascorbate |
Suppressed tumor growth |
[207] |
3.5. The Tumor Acidosis
Tumor and stromal cells use aerobic glycolysis for their amplified energy supply requirements, as a direct consequence of hypoxia and defective vasculature. Aerobic glycolysis is an oxygen-independent process known as the Warburg effect. However, even in normally oxygenated tumor regions, the main energy supplier remains aerobic glycolysis in about 80% of solid tumors [210]. In aerobic glycolysis, glucose constitutes the main macronutrient of tumor cells for their biosynthetic requirements and follows the lactate metabolic pathway, through GLUT transporters producing amplified levels of lactic acid (lactate). The main transcriptional factors of glycolytic activity regulating lactate production are the HIF-1α and c-Myc regulatory genes [211] that promote the overexpression of varied glycolytic enzymes such as lactate dehydrogenase A (LDHA), and monocarboxylate transporters (MCTs) such as MCT1 and MCT4 [212]. Mainly, the upregulation of LDHA gene favors the activity of LDH-5 and inhibits the activity of LDH-1, and promotes the conversion of pyruvate to lactate. Through this metabolic pathway, elevated amounts of lactate, protons (H+), and carbon dioxide (CO2) are secreted into TME. This leads to acidosis [213]. Acidosis regulates the metabolism of innate and adaptive immune cells by: (i) hindering the function of CD8+ T, natural killer (NK), natural killer T (NKT) and dendritic (DC) cells; (ii) supporting regulation of FOXP3+ T cells (Treg); and (iii) promoting M2 activated macrophage polarization. Overall, the acidic TME is an immunosuppressive incubator of pro-oncogenic and tumorigenic factors, and has been extensively studied for targeted nanomedicine applications [214–216]. The glycolytic metabolic pathway and acidic pH gradient are key participating factors in MDR due to their activation of enzymes and proteins responsible for resistance, efflux of drugs through P-gp, and stimulation of migration [217,218].
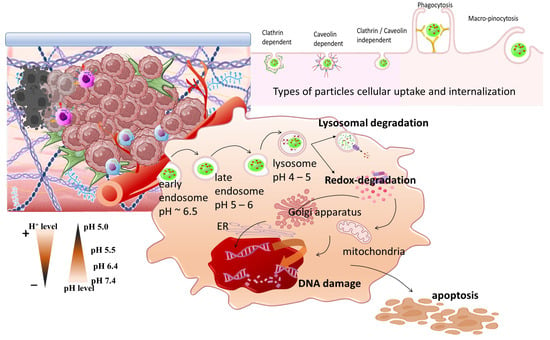
In tumors, a unique pH-gradient effect is established, with extracellular pH levels (pHe) being more acidic (6.4–7.0) and intracellular pH (pHi) being more alkaline (7.25–7.50) (Scheme 10). Distinct pH variations exist in tumor cell organelles, and they can be divided into acidic, such as nucleosomes and lysosomes with a pH of 5.5 and 5.0, respectively; or alkaline, such as mitochondria and cytoplasm, which have a corresponding pH of 8.0 and 7.2, respectively. The pH gradient is associated with the expression of membrane transporters such as MCT1, MCT4, carbonic anhydrases, and sodium-bicarbonate co-transporter (NBC). These participate in the translocation of lactic acid, CO2, and its bicarbonate ion byproducts. Other mechanisms influencing TME acidity are the efflux of endosomes acidic cargo and the release of the acidic intracellular comportments of necrotic cells. In stimuli responsive nanomedicine (Table 6), pH sensitivity has been highly exploited and reviewed [219–223]. Apart from drug delivery systems, tumor acidosis was targeted by pH-regulating molecular systems at various stages of clinical trials (this is described by Corbet et al.) [224], and by TME sensitive platforms for combined endogenous stimuli responsive effects (as reviewed by Wang et al. [225]).
3.5.1. pH-Sensitive Peptides in Acidic Tumor Targeting
A strategy for exploiting TME acidity is based on pH responsive peptides which under physiological conditions interact weakly with the cellular membrane but at TME create stable transmembrane complexes promoting nanomedicines internalization. Yadav et al. [226] examined chitosan nanoparticles modified with a pH-sensitive cRGD peptide (RGD-CHNP) for the delivery of raloxifene (Rlx) in NOD/SCID 4T1 tumor-bearing mice. The nanoparticles presented enhanced tumor accumulation by RGD peptide active targeting in αvβ3 integrin expressing breast cancer cells and expressed enhanced antitumor effect, inhibiting angiogenesis and migration by suppressing the regulation of osteopontin (OPN), thus inhibiting Akt and ERK signaling cascade. The combination of receptor-mediated specific binding and acidic pH was exploited by Han et al. [227]. They designed glycogen nanoparticles functionalized with doxorubicin via a pH responsive hydrazine-based bond and β-galactose, with selective binding affinity to the asialoglycoprotein transmembrane receptor (ASGPR) on hepatic cancer cells. Upon ASGPR binding, cellular internalization and degradation of the nanoparticles was triggered and pH-sensitive DOX release was promoted. The nanoparticles were evaluated in BALB/c nude, hepatic tumor-bearing mice. They exhibited enhanced accumulation at the tumor site and efficient antitumor activity of DOX inhibiting tumor growth. Palanikumar et al. [228] studied PLGA nanoparticles cross-linked with bovine serum albumin (BSA) and conjugated with pH-responsive membrane peptide (ATRAM) for the delivery of doxorubicin attached to triphenylphosphonium (TPP) in tumor-bearing C3H/HeJ mice. BSA provided long circulation time of the nanoparticles, and this resulted in effective intracellular localization in response to acidic pH, owing to the ATRAM peptide. The BSA coating was susceptible to GSH-mediated degradation that promoted the controlled release of DOX-TPP and resulted in enhanced mitochondria DOX accumulation. This effectively inhibited tumor volume and mass while exhibiting no apparent toxicity to healthy tissues.
Among pH-sensitive nanomedicines for tumor therapy, nanogels have been extensively investigated owing to their unique characteristics. These include self-assembly ability, stability upon systemic circulation, improved drug delivery compared to polymeric nanoparticles, high specificity and tissue penetration through EPR due to their small size, and bioconjugation activity for microenvironment responsive therapeutics [229]. Biomaterials such as hyaluronic acid, chitosan, DNA, and alginate were evaluated for tumor targeting nanogels with pH sensitivity that was due to pH-responsive peptides or pH-sensitive degradation of the cross-linked drugs and molecules. Ding et al. [230] studied hyaluronic acid nanogels cross-linked with pH-sensitive E3 (GY(EIAALEK)3GC) and K3 (GY-(KIAALKE)3GC) peptides (HA-cNCs) for targeted delivery of cytochrome C (CC) and saporin proteins to CD44 overexpressing MCF-7 breast cancer cells. The intracellular localization of the nanogels was promoted by CD44 receptor-mediated endocytosis due to HA, which triggered the endosomal degradation of the E3/K3 pH-sensitive cross-linked peptides and the release of the loaded proteins. The triggered release of CC and saporin from the nanogels resulted in a combined antitumor effect against breast cancer cells. CC is a hemeprotein weakly connected in the inner mitochondrial membrane, that participates in ATP synthesis. During the early apoptotic phase, detachment of CC is stimulated by ROS production. This leads to CC efflux into the cytosol, and acts as a regulator of apoptotic stimuli in cancer cells. Moreover, saporin is a ribosome-inactivating protein involved in the inhibition of protein synthesis in the cytosol; this results in cell death.
3.5.2. Metals and Metal Oxides in Acidic Tumor Targeting
Metal oxide nanoparticles have attracted research interest in emerging tumor therapeutic and diagnostic applications. Investigation of these nanoparticles has expanded on varied strategies including conjugation, combination with radiotherapy or chemotherapy, and activity based on external or internal stimuli. Several research approaches that combine the effects of metal oxides (MO) with targeting the acidic TME were developed in order to obtain enhanced antitumor efficacy. The interest on MO nanoparticles is due to their pro-apoptotic activity; inhibition of tumor cell growth and metastasis, and ROS production [231,232]. A characteristic example of an MO is cerium oxide nanoparticles (nanoceria). These are inorganic antioxidants that at physiological pH express catalytic mimicking activity to quench ROS effect. At acidic pH, however, they function as oxidases and increase oxidative stress and apoptosis. Gao et al. [233] studied multi-responsive nanoceria particles for the delivery of doxorubicin. The particles were coated with glycol chitosan and were bestowed with tumor-targeting ability by CXCR4 antagonist (AMD11070). An important axis connecting tumor cells and TME is the CXCR4/CXCL12 signaling, based on the CXC G protein-coupled chemokine receptor 4 (CXCR4 or CD184) that is overexpressed in various human tumors including human retinoblastoma. CXC chemokine ligand 12 (CXCL12, or stromal-derived-factor-1, SDF-1) is a ligand that acts through binding to the CXCR4 and promotes cancer stem cell phenotype, tumor progression, invasion, and metastasis. The nanoceria particles were evaluated for their antitumor activity on retinoblastoma cells. They expressed elevated internalization that significantly increased ROS production at acidic pH. This resulted in the inhibition of tumor growth, and there was substantial tumor size suppression and reduction in blood vessel leakages, in orthotopic models of genetic p107s mice.
Manganese dioxide nanoparticles (MnO2) represent promising theranostic candidates, combining TME oxygenation triggered by MnO2 reduction effect on ROS, with photodynamic therapy and pH-responsiveness. Yang et al. [234] studied hollow MnO2 nanoparticles functionalized with PEG for the combined delivery of doxorubicin and the photodynamic agent Ce6. At the acidic tumor pH, the degradation of MnO2 nanoparticles was promoted by reaction with protons and GSH. This generated Mn2+ ions and led to the oxygenation of the tumors and the combined release of DOX and Ce6. This further promoted the inhibition of tumor growth. Antitumor immune responses were also observed. For example, there was significantly decreased population of M2 macrophages and suppressed expression levels of IL-10. Tumor acidosis was exploited as an endogenous stimulus by Chen et al. [235] for the targeting effect of FA-conjugated MnO2-coated mesoporous silicon nanoparticles. The nanoparticles were loaded with metformin (Me), an oral drug for type 2 diabetes, and fluvastatin sodium (Flu), an inhibitor of monocarboxylate transporter 4 (MCT4 protein) that is responsible for mediating the intracellular lactate/H+ efflux. The nanoparticles expressed effective targeting affinity to folate receptor for enhanced internalization and intracellular degradation of the MnO2 particles by GSH, through oxidation reduction; this resulted in the release of Me and Flu. The synergistic effect of the drugs successfully regulated the pyruvate metabolic pathway, and promoted the production of elevated lactate levels and suppression of the lactate efflux. This further induced intracellular acidosis that promoted tumor cell death, suppressed tumor growth, and inhibited metastasis in MCF-7 tumor-bearing nude mice.
Gold nanostructures are highly applied in tumor targeting, since upon internalization by tumor cells they act as sensitizers to radiation therapy. The advantages of gold nanoparticles include efficient transportation through the leaky tumor vasculature, surface modification by thiol linkages, and use in clinical applications. Rauta et al. [236] studied the conjugation of gold nanorods with charge-reversal poly(Glu-co-Lys) polypeptides with pH responsiveness. They effectively switched charge at the acidic extracellular TME, and this enabled their internalization in tumor cells. The evaluation of the Au nanorods in orthotopic pancreatic tumors resulted in enhanced accumulation at the tumors’ periphery and the hypoxic core of large tumors. No abnormalities were observed in normal organs and there were no hematological deviations; this indicates the safety of the gold nanorods. Another example of charge-reversal responsive polymers induced by pH acidity was studied by Xue et al. [237] in doxorubicin-loaded superparamagnetic iron oxide nanoparticles (SPIONs) modified with citraconic anhydride-dextran (Dex-COOH) and cystamine-dextran (Dex-SS-NH2). The nanoparticles showed a pH-responsive negative charge decline due to the acid-sensitive dextran coating; this enabled the internalization of the nanoparticles and the lysosomal escape by switching the charge from negative to positive. Subsequently, the nanoparticles—due to the presence of the disulfide bond—decomposed under the effect of GSH, and this triggered DOX release that promoted antitumor activity. This is shown by the significant inhibition of tumor volume in CT26 tumor-bearing mice. Effective accumulation of the nanoparticles in tumor tissue was observed with low non-specific tissue toxicity. In a study by Angelopoulou et al. [238], SPIONs functionalized with PMAA-g-PEGMA polymers and conjugated with canagliflozin via pH-sensitive bond were evaluated in PDV C57 tumor-bearing mice for their antitumor effect. Canagliflozin is a type 2 diabetes drug that acts through inhibition of sodium-glucose transporter protein (SGLT2), and takes advantage of the TME hypoxia. The nanoparticles expressed enhanced tumor accumulation by the application of a static magnetic field gradient and the pH-sensitive canagliflozin release was triggered. This provided efficient antitumor activity that, in combination with radiotherapy, inhibited tumor growth at a significantly higher degree compared to either monotherapy (drug or radiotherapy).
3.5.3. Biomaterial Based Polymeric Nanomedicines in Acidic Tumor Targeting
Another highly investigated and widely reviewed type of nanomedicine is polymeric systems combined with biomaterials for pH-responsive TME targeting. These include hydrogels [239], polymer nanoparticles [240,241], and micelles [242]. Despite the effort, the complex biological characteristics and aggressiveness of the acidic microenvironment of solid tumors remains a challenge for effective delivery. Since TME acidosis is not considered a limiting barrier, but signifies a micromilieu for smart targeted drug delivery, promising polymeric nanomedicine strategies were studied. Among them, hydrogels are injectable systems for in situ administration of drugs that enable the localized application at tumor site and can be designed in order to acquire pH-stimuli responsiveness and self-healing properties. N-carboxyethyl chitosan (CEC) hydrogels cross-linked with dibenzaldehyde-terminated poly(ethylene glycol) (PEGDA) and conjugated with doxorubicin were injected upon subcutaneous injection in hepatocellular liver carcinoma-bearing rats, to be evaluated for their antitumor activity. The hydrogels effectively accumulated at the tumor site and pH-responsive DOX release was triggered. Moreover, the hydrogel promoted self-healing activities due to the Schiff-base linkage between CEC and PEGDA [243].
In another study, Megahed et al. [244] evaluated pH-sensitive PEGylated chitosan niosomes for the delivery of Tamoxifen (Tam); this is a hormone antagonist used in breast cancer therapy. Chitosan was used as a pH-sensitive polymer and PEG provided the necessary long-circulation properties. Tam is a selective estrogen receptor modulator (SERM) with the activity of binding to estrogen receptors and promoting agonist or antagonist effects, depending on the targeted tissue. Tam represents a promising treatment for estrogen receptor-positive (ER+) breast cancer and for stromal targeting of pancreatic ductal adenocarcinoma (PDAC). The evaluation of cell cycle analysis revealed that the presence of chitosan and PEG in niosomes had a great influence on the induced apoptosis. Chitosan promoted apoptosis over necrosis of tumor cells, while PEG presence increased apoptotic and necrotic populations. The evaluation of the niosomes in breast tumor-bearing rats showed elevated antitumor efficacy and increased Tam accumulation at the tumor site. Chitosan is preferentially applied in tumor acidosis, because its abundant amino groups on the polysaccharide chain obtain a positive charge under acidic pH. Thus, an innate pH-responsiveness is generated by chitosan, and this enables its application in screening even for deep analysis of invasive cancer cells. This was reported by Ivanova et al. [245]. Chitosan micro-sized particles were evaluated for screening of tumor progression, in response to acquired resistance of the acidic TME. Toxicity was hypothesized to be associated with biological and chemical metabolic changes of acidic microenvironment and pH gradient effect. The highly invasive metastatic tumor cells have a strong negative charge, and thus they electrostatically attach to the chitosan micro-formulation, and this enables the screening of tumor metastasis.
Stimuli pH-responsive polymeric nanoparticles are the focus of interest in a wide range of cancer-targeting applications. Relative research includes engineered nanoparticles that are able to respond to TME endogenous stimuli [246]; pH-responsive activity is based on charge-shifting polymer structures, acid labile linkages, and pH-responsive cross-linkers [240]. Zhao et al. [247] studied cross-linked polymeric nanoparticles with folic acid (FA) and galactose (GAL) targeting activity and dual pH/redox-sensitivity due to the PDPA and PDEMA cross-linked block copolymers, respectively. The amphiphilic cross-linked polymers formed self-assembled nanoparticles loaded with doxorubicin and were evaluated in HepG2 hepatocellular carcinoma cells. GAL was responsible for selectively binding to asialoglycoprotein (ASGPR) receptors of HepG2 cells and FA to folate receptors, and promoting dual active targeting for efficient internalization. Due to the protonation of the tertiary amine at acidic pH and the reduction of the disulfide bond by GSH, increased DOX release was promoted intracellularly; this resulted in increased cytotoxicity and apoptosis. Another example of charge-shifting polymers was reported by Yuan et al. [248], who studied zwitterionic polymers based on block copolymers of PCL-b-PAEP. The copolymers were composed of equal anion and cation groups on their backbone chain, giving them high hydrophilicity that promotes resistance to protein adsorption, avoidance of rapid recognition by immune system, and delayed blood clearance; these, therefore, represent dynamic alternatives to PEG. The PCL-b-PAEP block copolymers were further grafted with thiol derivatives of cysteamine hydrochloride and TMA, resulting in positively charged polymers that were further reacted with 2,3-dimethylmaleic anhydride to acquire pH-sensitivity. The polymers were self-assembled in micelles encapsulating doxorubicin with surface charge-switching ability in response to the acidic TME. The evaluation of the micelles in MDA-MB-231 tumor-bearing mice, provided evidence for enhanced tumor cell internalization and inhibition of tumor growth. Wang et al. [249] investigated charge-shifting PDPA polymers in micelle-type nanoparticles which were loaded with iron oxide nanoparticles (IONPs) and β-lapachone (La). The pH-responsive PDPA-modified IONPs were further incorporated in H2O2-responsive polymeric prodrugs of PEG-polycamptothecin. Thus, dually responsive nanoparticles were obtained, and they expressed pH and H2O2 sensitivity. This resulted in acidic-mediated degradation in the endosome/lysosome environments due to the shifting pH-responsiveness of PDPA. Thus, La was released and catalyzed by nicotinamide adenine dinucleotide (phosphate): quinone oxidoreductase 1 (NAD(P)H: NQO1), and this produced elevated levels of hydrogen peroxide. Then the newly produced H2O2 reacted with iron ions to further promote the generation of toxic ROS levels with elevated expression of hydrogen peroxide species promoting the degradation of the peroxalate ester linkages. Thus, the release of camptothecin was triggered. The synergistic effect of the nanoparticles resulted in effective antitumor activity in A549 tumor-bearing mice, and this significantly inhibited tumor volume and tumor growth (IRG) and caused low systemic toxicity.
PLGA nanoparticles have been highly evaluated in nanomedicine, including pH-responsive applications, owing to excellent biocompatibility, biodegradability, and ease of functionalization properties. Liang et al. [250] studied PLGA nanoparticles coated with BSA and encapsulating doxorubicin and graphene quantum dots (GQDs). GQDs have fluorescence properties for cellular imaging. The pH-responsive DOX release was accomplished by the biodegradation of the PLGA structure and the protonation of daunosamine group in the acidic environment. In another study by Meng et al. [251], PLGA nanoparticles were evaluated for the combined delivery of doxorubicin, sodium carbonate (Na2CO3) and liquid perfluorocarbon (PFC) for ultrasound-responsive antitumor treatment. The liquid PFC nanodroplets were evaporated by ultrasound to stimulate rapid Na2CO3 release. Na2CO3 acting as a neutralizing agent regulated the cellular proton pumps, and this resulted in inhibition of lactate acidosis and enhanced DOX release; thus increasing tumor growth inhibition.
As outlined by Shi et al. [252], the pH-sensitivity of drug delivery by nanomedicines can be contributed to by various mechanisms including protonation of biomolecules (drugs, peptides, and polymers) and degradation of pH-sensitive bonds. The study of nanoparticles composed of pH-sensitive copolymers selectively dissociating was investigated by Wang et al. [253]. They studied polymeric micelles composed of two types of polymers. One type was a Ce6-modified acid-responsive poly(ethylene glycol)-b-poly(2-(hexamethyleneimino) ethyl methacrylate) (PEG-b-PHMA) copolymers. The Chlorin e6 (Ce6) photosensitizer was grafted onto the acid-sensitive PHMA diblock. The second type of polymer was a iRGD peptide-modified polymeric prodrug of doxorubicin (iPDOX). The acid-responsive and peptide polymers were combined into one single nanoplatform (termed iPAPD). At physiological pH, the PEG-b-PHMA matrix was rigid, and this protected DOX prodrug from degradation and non-specific activity; while at the acidic tumor pH the PEG-b-PHMA chain was susceptible to degradation, releasing the DOX prodrug and restoring the Ce6 activity for real-time fluorescence imaging. The nanoparticles were evaluated in tumor spheroids and tumor-bearing animal models, and this showed effective tumor accumulation and increased tumor penetration due to the iRGD peptide. The P85 pluronic blocked the P-gp pumps and prevented DOX efflux; this further caused an elevated antitumor effect. The combination with PDT resulted in activation of Ce6 which induced ROS production, promoted DOX diffusion inside the tumor mass, and inhibited acquired drug resistance by altering the gene expression profile of the tumor cells. Another example of acid-responsive polymers was described by Liu et al. [254], who developed pH-sensitive amphiphilic block PCL-b-PEG copolymers for the co-delivery of paclitaxel (PTX) and acetazolamide (ACE). ACE is an inhibitor of carbonic anhydrase IX (CA IX) which is related to acidic tumor pH and MDR. The pH-responsiveness was attributed to the pH-cleavable hydrazine bond, which promoted the degradation of the polymeric shell and the release of ACE and PTX. In vivo administration of these dual-drug loaded nanoparticles resulted in successful tumor accumulation and tumor growth inhibition; this increased the survival rate of tumor-bearing mice. The ability of these nanoparticles to restore tumor acidity resulted in the enhanced effectiveness of paclitaxel.
An alternative to polymers was provided by biomimetic nanoparticles composed of membranes originating from natural cells. Liu et al. [255] studied hybrid DSPE-PEOz pH-sensitive liposomes loaded with doxorubicin and incorporated into platelet membrane-coated nanoparticles (platesomes). Platesomes have a notable active tumor targeting behavior, since their membrane expresses several surface proteins including integrin α6, CD41, and CD62p; these specifically bind to the CD44 receptor of tumor cells. The hybrid nanoparticles expressed increased plasma half-life and elevated tumor accumulation, and this enables the selective release of DOX from the pH-sensitive liposomes in response to the acidity of lysosomes. Platesomes exhibited a significantly enhanced antitumor effect in 4T1 tumor-bearing BALB/c mice. Platelet membrane nanoparticles were also studied by Luo et al. [256] for their synergistic effect against tumor acidosis and hypoxia. Zeolitic imidazolate framework-8 nanoparticles (ZIF8) delivering doxorubicin, hemoglobin (Hb), and lactate oxidase (LOX) were further coated with platelet membrane to enhance passive targeting, increase circulation time, and lower toxicity in the biological environment. The nanoparticles synergistically combined the anticancer activity of DOX with the anti-hypoxia activity of oxygen-carrying hemoglobin and with the LOX catalytic activity in converting lactic acid to pyruvate and hydrogen peroxide. The evaluation in BALB/c tumor-bearing mice revealed an elevated tumor targeting effect of the nanoparticles; this degraded intracellularly to release Hb and LOX, thus inhibiting tumor hypoxia and acidity through oxygenation and lactate decomposition, respectively. The produced hydrogen peroxide resulted in oxidative stress of the tumor cells that, in combination with DOX, enhanced cellular apoptosis. The synergistic effects of the platelet nanoparticles resulted in suppressed tumor hypoxia, remodeling of tumor acidity, and inhibition of tumor growth.
Table 6. pH-sensitive nanomedicines based on biomaterials targeting tumor acidosis.|
Targeting Effects |
Carrier Type |
Therapeutic Agent |
Characteristics |
Ref. |
|
pH-sensitive peptides |
Chitosan nanoparticles/cRGD peptide |
Raloxifene |
Increased accumulation, enhanced antitumor effect inhibiting angiogenesis and migration |
[226] |
|
Glycogen nanoparticles/hydrazine-based bond |
Doxorubicin/β-galactose |
Enhanced accumulation, inhibiting tumor growth |
[227] |
|
|
PLGA–BSA particles ATRAM peptide |
Doxorubicin/TPP |
Enhanced mitochondria targeting, inhibited tumor volume and mass |
[228] |
|
|
Hyaluronic acid nanogels E3/K3 peptides |
Cytochrome C (CC)/saporin proteins |
Inhibition of protein synthesis in the cytosol, efficient antitumor effect |
[230] |
|
|
Metals/Metal Oxides Chemo-Sensitivity |
Cerium oxide–glycol chitosan nanoparticles |
CXCR4 antagonist/Doxorubicin |
Elevated internalization, increased ROS production at acidic pH, tumor size suppression, and reduced blood vessel leakage |
[233] |
|
PEG–MnO2 nanoparticles |
Doxorubicin/Ce6 PDT |
Tumor oxygenation, inhibition of tumor growth, elevated antitumor immune responses |
[234] |
|
|
MnO2-coated mesoporous silicon nanoparticles |
Metformin/fluvastatin sodium |
Induced intracellular acidosis promoting tumor cell death, suppressed tumor growth and metastasis |
[235] |
|
|
Au nanorods/P(Glu-co-Lys) polypetides |
Au nanorods |
Enhanced accumulation in tumors periphery and hypoxic core |
[236] |
|
|
Iron oxide SPIONs/cystamine-dextran |
Doxorubicin |
Increased pH-triggered internalization, inhibition of tumor volume |
[237] |
|
|
Iron oxide SPIONs/PMAA-g-PEGMA |
Canagliflozin/Radiotherapy |
Accumulation in tumor tissue, inhibition of tumor growth |
[238] |
|
|
pH-sensitive Polymeric particles |
Carboxyethyl chitosan–PEGDA hydrogels |
Doxorubicin |
Self-healing properties, antitumor effect |
[243] |
|
Chitosan-PEG niosomes |
Tamoxifen |
Increased drug accumulation and antitumor efficacy |
[244] |
|
|
Chitosan microparticles |
- |
Screening of tumor progression |
[245] |
|
|
FA-PMgDP-PDPA-PDEMA particles |
Doxorubicin/Galactose |
Efficient internalization, increased toxicity, and apoptosis |
[247] |
|
|
PCL-b-PAEP-TMA-Cya/DMA micelles |
Doxorubicin |
Enhanced internalization, inhibition of tumor growth |
[248] |
|
|
Iron oxide-PDPA particles |
PEG-polycamptothecin prodrug |
Effective antitumor activities, effective antitumor activities |
[249] |
|
|
Graphene quantum dots-PLGA-BSA particles |
Doxorubicin |
Sufficient internalization and in vitro toxicity |
[250] |
|
|
PLGA particles |
Doxorubicin/sodium carbonate/liquid perfluorocarbon |
Tumor accumulating ability, and inhibited tumor growth |
[251] |
|
|
PEG-b-PHMA particles |
Doxorubicin-P85 prodrug/iRGD peptide/Ce6 PDT |
Elevated antitumor effect and complete suppression of tumor growth |
[253] |
|
|
PCL-PEG particles |
Paclitaxel/Acetazolamide |
Inhibitory effect on tumor growth, increasing the survival rate |
[254] |
|
|
DSPE-PEOz liposomes in platelet membrane particles |
Doxorubicin |
Enhanced antitumor effect |
[255] |
|
|
Zeolitic imidazolate framework-8 nanoparticles |
Doxorubicin/hemoglobin/LOX |
Tumor targeting effect, suppressed tumor hypoxia, remodeled tumor acidity and inhibited tumor growth |
[256] |
3.6. Tumor Immunotherapies
Another important area of nanomedicine research is tumor immunotherapy. Tumor immunotherapies may represent a vital solution in TME limitation and can be divided into immune checkpoint inhibitors (ICI), immune tumor vaccines, and chimeric antigen receptor-modified CAR T cells. The development of nanomedicines for cancer immunotherapy is largely based on the application of biomaterials such as polysaccharides (e.g., hyaluronic acid, alginic acid, chitosan, and dextran) and synthetic polymers including PEG, PLA, PLGA, PCL, and polypeptides [257]. The development of immunotherapeutic nanomedicines based on biomaterials has emerged as a strategy to improve therapeutic outcome by inducing tissue-specific immunomodulation through cell, antibody, and gene immune responses [258]. Opportunities to apply biomaterials in tumor immunotherapy and their combination with conventional therapies have been interestingly reviewed [259–261]. Among immunotherapies, immune checkpoint inhibitors present promising therapies that mainly target cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1), and programmed cell death receptor-1 ligand (PD-L1). Immune checkpoints are proteins on the surface of immune T cells that recognize and bind to partner proteins on tumor cells, and promote intracellular inhibitory signals and immunosuppressive enzymes; this suppresses host immune T cell attack against tumor cells. Hu et al. [262] developed amphiphilic nanoparticles with a core from hydrophobic ROS-sensitive poly(thioketal phosphoester) and a shell from hydrophilic lecithin/DSPE-PEG, encapsulating doxorubicin, Ce6 photosensitizer, and anti-PD-L1 antibody for ICI. The nanoparticles were evaluated in 4T1 tumor-bearing mice. Rapid degradation of the ROS-responsive core triggered DOX release and, in combination with laser irradiation, promoted effective PDT activity due to the Ce6 component of the nanoparticles. The combinational effect with the anti-PD-L1 antibody ICI promoted the maturation of DCs, and this significantly suppressed the growth of primary tumors and effectively inhibited distant tumor growth. Another type of immunotherapy is the delivery of immunostimulatory agents that directly activate immune T cells by binding of agonistic antibodies on surface receptors such as CD40, OX40 (CD134), and CD137; this promotes downstream signaling pathways for T cell-mediated antitumor activity [263–266].
The application of biomaterials in cancer vaccines has made significant progress due to the advantageous effect on enhancing the safety profile and stimulating antigen-specific T cell response. Immunotherapy vaccines aim to activate the immune system attack against tumor cells by downregulating the immune tolerance to tumor antigens, through the combined delivery of immunostimulatory agents to activate host immune cells and immunogenic epitopes of specific tumor antigens [263,264]. Τhe effective delivery of the immunotherapy vaccines to dendritic cells is crucial, because DCs are the main antigen presenting immune cells. Rosalia et al. [264], reported that PLGA nanoparticles coated with agonistic aCD40-mAb and encapsulating tumor associated Ag protein successfully primed CD8+ T cells; thus, the survival of tumor-bearing mice was prolonged. In another study, Wang et al. [265] investigated amphiphilic pH-sensitive galactosyl dextran-retinal (GDR) nanogels with a pH-sensitive hydrazone bond for dextran conjugation with all-trans retinal (a metabolite of vitamin A). The nanogels were galactosylated to acquire DC-targeting ability and were effective vaccine delivery systems due to their ability to successfully amplify major histocompatibility class I, MHC I, antigen expression in DCs, and induced effective antitumor immune responses.
The most recent immunotherapies are CAR T cell and CAR T cell receptor therapies. CAR T cells are engineered immune cells originating from the peripheral blood mononuclear cells of the patient’s blood that are harvested and stimulated to become T cells with specific DNA encoding to recognize certain tumor antigens. There are two FDA-approved CAR T cell therapies for blood cancer; however, their application in solid tumors remains challenging. Nevertheless, CAR T cell therapies against solid tumors are being studied ongoing clinical trials [263,264]. Biomaterials are promising candidates for CAR T cell modification improving the therapeutic efficacy and immune-editing processes [266]. Moreover, nano-biomaterials can improve the effect of immunotherapies, and trigger enhanced therapeutic outcomes and regulation of immune cells by overcoming the barriers of the cold tumor immune microenvironment [267].
4. Discussion
5. Conclusions
6. Future Directions
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics16020179
