Solid tumors are composed of a highly complex and heterogenic microenvironment, with increasing metabolic status. This environment plays a crucial role in the clinical therapeutic outcome of conventional treatments and innovative antitumor nanomedicines. Scientists have devoted great efforts to conquering the challenges of the tumor microenvironment (TME), in respect of effective drug accumulation and activity at the tumor site. The main focus is to overcome the obstacles of abnormal vasculature, dense stroma, extracellular matrix, hypoxia, and pH gradient acidosis. In this endeavor, nanomedicines that are targeting distinct features of TME have flourished; these aim to increase site specificity and achieve deep tumor penetration. The development of such systems has significantly advanced the application of biomaterials in combinational therapies and in immunotherapies for improved anticancer effectiveness.
- biomaterials
- tumor microenvironment
- nanomedicine
- hypoxia
- acidosis
- resistance
- tumor vasculature
- targeting
- stimuli-responsiveness
1. Introduction
Cancer incidence and mortality has increased dramatically, with female breast cancer being the most commonly diagnosed, surpassing lung cancer [1][2][1,2]. It represents a major public health issue in emerging and developing countries, and poses great socioeconomic and psychological challenges. According to recent world statistics, cancer is the first or second leading cause of death; there are nearly 20 million new cases and almost 10 million deaths [1]. Global cancer statistics have estimated that near to 30 million new cases should be expected by 2040, and this is expected to affect more developed than emerging economies. Due to migration and demographic changes, the rate of cancer incidence is seriously affected by everyday risk factors, such as tobacco and alcohol use, unhealthy diet, and anxiety [1][2][1,2]. Despite the disappointing statistics, however, there was a decline in the overall cancer death rate of about 33% between 1991 and 2020. Furthermore, an estimated 4 million deaths were prevented. Moreover, a decline of 65% in cervical cancer incidence among women in their 20s during the period 2012 to 2019 was observed. This is due to the preventive effect of the human papillomavirus vaccine [1][2][1,2]. The decline in rates of cancer mortality can, undoubtedly, be related to increased research efforts in the field of cancer vaccines (RNA technologies) and personalized nanomedicines [3]. The greatest challenges for scientists are to ensure early diagnosis and prevention of cancer, which could effectively reduce cancer mortality.
Early diagnosis is crucial due to the high differentiation rate of tumor cells that promote the development of highly aggressive cancerous cells associated with multidrug resistance (MDR), stemness [4], and invasion [5]. A critical stimulus of MDR and invasion is tumor microenvironment (TME) (Scheme 1). This is a complicated interpenetrating network of varied cancerous and stromal cell types, extracellular matrix (ECM), and interstitial fluid (IF) [6][7][8][6–8]. TME is hostile to normal cells while being hospitable to stromal cells that are the nonmalignant components of solid tumors. These include endothelial cells (ECs), fibroblasts (FCs), immune cells (lymphocytes, macrophages, dendritic cells), and perivascular cells (PCs). These cells are interconnected in a protein-rich matrix that promotes angiogenesis and neovascularization [9][10][9,10]. Within the heterogenic TME, vasculature abnormalities are related to variations in oxygenation. Furthermore, the elevated presence of reactive oxygen species (ROS), glutathione (GSH), enzymes, and adenosine triphosphate (ATP) further promote a hypoxic status with acidic pH levels (pH 5.5–6.2). These features—in combination with secreted growth factors, cytokines, chemokines, and macromolecules such as proteases and proteins in the surrounding stroma—regulate the stimulation of cancer-associated fibroblasts (CAFs), and play key role in metastatic potency [11][12][13][11–13]. The stroma in combination with the highly dynamic ECM act as supportive reservoirs that directly or indirectly interconnect TME with capillary and vascular system cells, and immune system cells. This provide the essential nutrition components of oxygen, gas exchange, and metabolites withdrawal, to support tumorigenesis and continuous neovascularization [14][15][16][14–16].
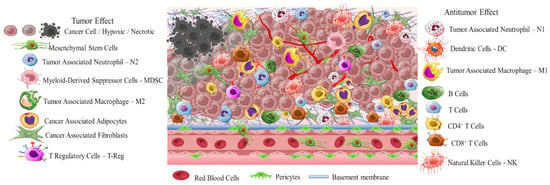
The greatest difficulty posed by the TME of solid tumors is MDR. This results in reduced therapeutic efficiency of traditional interventions such as chemo- and radiotherapy. The backbone of traditional therapeutic approaches is surgical ablation followed by chemo- and radiotherapy or a combination of both, depending on tumor severity. Chemo- and radiotherapy cause serious side effects for the patients within the therapeutic window of the administered doses [17]. Great progress has been achieved with advanced investigation of new therapeutic agents including peptides, antibodies, and prodrugs [18][19][20][21][22][18–22]. However, the success of these compounds is compromised by the limitations of abnormal vasculature, heterogenic basement membranes, and poor blood supply. These are all inherited by TME, and are the causes of therapeutic failure [23][24][25][23–25]. Nanomedicine represents an important strategy to improve the delivery of therapeutic agents such as drugs, peptides, antibodies, proteins, genes, and immunotherapeutic agents in a selective and controlled manner for efficient accumulation and stimuli responsiveness [26][27][28][29][30][31][32][33][34][26–34]. Although great progress has been achieved in this field, the clinical translation of nanomedicines is still limited. In this resviearch, the researchers aw, we aim to present a discussion on the field of responsive nanomedicines. This will emphasize the application of biomaterials including natural polymers such as polysaccharides, biodegradable polymers, and metal oxides, in targeting the TME of solid tumors. Biomaterials represent a field of distinct research interest, due to their unique inherent properties. Their structure allows for effective functionalization for the co-delivery of multiple compounds and for effective responsiveness to an internal (chemical and/or biological) or external physical stimulus (magnetic field, light, radiation, ultrasound). Biomaterials have proved to be great supporters of theranostic applications in cancer treatment [35][36][35,36]. Overall, in this resviearch the researchers w we will discuss the role of biomaterial-based nanomedicines in targeting the TME. This includes heterogenic vasculature, tumor stroma ECM, CAFs, tumor hypoxia, and acidosis. The researchers We will examine the most recent advances in therapeutic nanomedicine for solid tumors; these have the potential to improve clinical outcomes. Finally, the researchers we will summarize the challenges and future outlook for the application of nanomedicines in tumor immunotherapy and combinational therapy to overcome limitations and improve the therapeutic outcome.
2. Solid Tumor Nanomedicine: Distribution in Tumor Microenvironment
Intriguingly, solid tumors are pathological organ-like tissues with heterogenic TME and increased metabolic status, which promote and support processes mimicking normal tissues, as angiogenesis [37][38][39][37–39]. Due to the elevated dysregulation of angiogenetic factors, abnormal and destabilized blood and lymphatic vessels are developed. These have major variations in diameter, density, shape (spiral-like) and overall distribution within TME. Additionally, a simultaneous discontinuation of endothelium with leaking cell gaps, irregularly thick or thin basement membranes and disruption of blood flow cause excessive spatial stress and increased interstitial fluid pressure (IFP) [40][41][42][40–42]. These features promote the transport of nutrients, oxygen, and blood away from the central region of solid tumors. This stimulates ATP regulation, hypoxia, and acidosis. The same features prohibit the transfer of therapeutic drugs, and this results in an inferior targeting effect and heterogenic tumor distribution of drugs [43]. Nanomedicines improve targeting effectiveness and selectivity [44][45][46][44–46]. This is especially the case when they are supported by an enhanced permeability and retention (EPR) effect, which promotes extravasation and effectual intratumor localization [47] (Scheme 2). Despite progress being made, moderate clinical success has been achieved so far. This is because nanomedicine applications have encountered severe obstacles related to avascular tumor sites due to the TME’s characteristics restricting nanomedicines’ access only to highly vascular regions with increased perfusion [47][48][47,48]. These limitations in the therapeutic efficacy of nanomedicines have been tackled recently by exploiting the complex mechanisms and associated properties of TME. This improves intratumoral localization. Furthermore, specially designed nanomedicines exhibit stimuli-responsiveness in TME features, such as hypoxia and acidity, by combining ligand-mediated active targeting of selective receptors with growth factors, inhibitors, enzymes, and peptides. Combining the benefits of external stimuli-responsiveness has, beneficially, increased the targeting efficiency of nanomedicines and amplified the therapeutic activity [49][50][51][52][49–52].
Nanomedicines that target solid tumors traverse a highly variable environment. This emphasizes the importance of adaptability and spatiotemporal pharmacological activity. The current treatment strategies that aim to overcome MDR and the multi-factorial TME rely on a combination of chemo-, radio-, and immunotherapies (Scheme 3). Approved nanomedicines are co-administered for improved synergistic therapeutic effect. In this way, biomaterials have greatly assisted in orchestrating the pharmacokinetics and targeting potential of nanomedicines. This is due to their inherent properties of biodegradability and ease of surface modification which offer effective sites for receptor-mediated ligand targeting (peptides, antibodies, nucleic acids, small organic molecules). Biomaterials also make possible theranostic applications of nanomedicines for real-time therapy and monitoring of tumor tissues. Moreover, biomaterials can be appropriately functionalized to obtain stimuli-responsiveness and manipulate TME hypoxia and spatial pH distribution (acidic extracellularly vs. basic intracellularly). In addition, the effectual combination of biomaterials with acquired responsiveness on varied external stimuli (hyperthermia, photodynamic, sonodynamic) has highly improved tumor-specific accumulation. This modulates cellular apoptotic cascades and promotes cellular death by associated gene regulation [53][54][55]. In this respect, biomaterials are important for TME-responsive applications, and they demonstrate promising results in combinational therapies and immunotherapies. Some of these are FDA-approved or under clinical trials for specific tumor types (Table 1) [56][57][58][59]. The clinical trials and applications of nanomedicines have mostly relied on improving drugs’ activity, as well as reducing side effects, and on enhancing biodistribution of drugs and agents within solid tumor tissues. For this, strategies have been developed to overcome physical, biological, and chemical obstacles in the TME, and achieve efficient specificity on molecular targets (such as cellular receptors, CAFs, CSC) and physiological factors (such as ECM, angiogenesis, IFP) [50][55][60]. The innate biological properties of biomaterials against opsonization have offered great support, because they provide longer and efficient systemic circulation time of the nanomedicines [61]. Natural polymers and synthetic biodegradable polymers which have been extensively reviewed and discussed for their application in the pharmaceutical and biomedical fields include: (i) polysaccharides such as alginic acid (alginate), dextran, agarose, hyaluronic acid, carrageenan, chitosan, and cyclodextrin: (ii) protein-based polymers such as gelatin, albumin, soy, and collagen; and (iii) synthetic polymers such as polyesters, polyamides, polyanhydrides, phosphorous based, and polyurethanes [62][63][64][65][66][67]. In solid tumor therapeutics, the benefits of novel theranostic and multifunctional nanomedicines are a niche research area that aims to overcome the limitations of TME and design novel therapies [67] (Scheme 1). In the following, the researchers discuss novel therapeutic and targeting concepts of biomaterial-based nanomedicines which exploit TME specificity and responsiveness.Nanomedicines that target solid tumors traverse a highly variable environment. This emphasizes the importance of adaptability and spatiotemporal pharmacological activity. The current treatment strategies that aim to overcome MDR and the multi-factorial TME rely on a combination of chemo-, radio-, and immunotherapies (Scheme 3). Approved nanomedicines are co-administered for improved synergistic therapeutic effect. In this way, biomaterials have greatly assisted in orchestrating the pharmacokinetics and targeting potential of nanomedicines. This is due to their inherent properties of biodegradability and ease of surface modification which offer effective sites for receptor-mediated ligand targeting (peptides, antibodies, nucleic acids, small organic molecules). Biomaterials also make possible theranostic applications of nanomedicines for real-time therapy and monitoring of tumor tissues. Moreover, biomaterials can be appropriately functionalized to obtain stimuli-responsiveness and manipulate TME hypoxia and spatial pH distribution (acidic extracellularly vs. basic intracellularly). In addition, the effectual combination of biomaterials with acquired responsiveness on varied external stimuli (hyperthermia, photodynamic, sonodynamic) has highly improved tumor-specific accumulation. This modulates cellular apoptotic cascades and promotes cellular death by associated gene regulation [53–55]. In this respect, biomaterials are important for TME-responsive applications, and they demonstrate promising results in combinational therapies and immunotherapies. Some of these are FDA-approved or under clinical trials for specific tumor types (Table 1) [56–59]. The clinical trials and applications of nanomedicines have mostly relied on improving drugs’ activity, as well as reducing side effects, and on enhancing biodistribution of drugs and agents within solid tumor tissues. For this, strategies have been developed to overcome physical, biological, and chemical obstacles in the TME, and achieve efficient specificity on molecular targets (such as cellular receptors, CAFs, CSC) and physiological factors (such as ECM, angiogenesis, IFP) [50,55,60]. The innate biological properties of biomaterials against opsonization have offered great support, because they provide longer and efficient systemic circulation time of the nanomedicines [61]. Natural polymers and synthetic biodegradable polymers which have been extensively reviewed and discussed for their application in the pharmaceutical and biomedical fields include: (i) polysaccharides such as alginic acid (alginate), dextran, agarose, hyaluronic acid, carrageenan, chitosan, and cyclodextrin: (ii) protein-based polymers such as gelatin, albumin, soy, and collagen; and (iii) synthetic polymers such as polyesters, polyamides, polyanhydrides, phosphorous based, and polyurethanes [62–67]. In solid tumor therapeutics, the benefits of novel theranostic and multifunctional nanomedicines are a niche research area that aims to overcome the limitations of TME and design novel therapies [67] (Scheme 1). In the following, we discuss novel therapeutic and targeting concepts of biomaterial-based nanomedicines which exploit TME specificity and responsiveness.
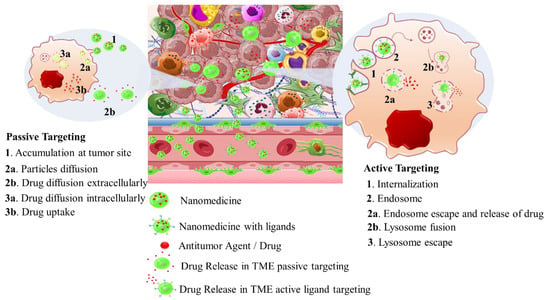
Table 1.
Therapeutic nanomedicines approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
|
Carrier Type |
Product Name |
Therapeutic Agent |
Cancer Type |
Stage |
Ref. |
|---|---|---|---|---|---|
|
Liposomes |
Zolsketil® |
Doxorubicin |
Metastatic breast cancer, advanced ovarian cancer, multiple myeloma, AIDS-related Kaposi’s sarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/zolsketil-pegylated-liposomal (accessed on 15 January 2024) |
Approved (EMA, 2022) |
|
|
Vyxeos® |
Cytarabine: daunorubicin |
Newly diagnosed therapy-related acute myeloid leukemia, acute myeloid leukemia with myelodysplasia related changes (https://www.ema.europa.eu/en/medicines/human/EPAR/vyxeos-liposomal-previously-known-vyxeos, accessed on 15 January 2024) |
Approved (EMA, 2018) (FDA, 2017) |
||
|
Onivyde®/CPX-351 |
Irinotecan |
Pancreatic cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/onivyde-pegylated-liposomal-previously-known-onivyde, accessed on 15 January 2024) |
Approved (EMA, 2016) (FDA, 2015) |
[56][57]
[56,57] |
|
|
Mepact® |
Mifamurtide |
Osteosarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/mepact, accessed on 15 January 2024) |
Approved (EMA, 2009) |
||
|
Ameluz® |
5-aminolevulinic acid |
Superficial and/or nodular basal cell carcinoma (https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz, accessed on 15 January 2024) |
Approved (EMA, 2011) |
||
|
DaunoXome® |
Daunorubicin |
Kaposi’s sarcoma |
Approved (FDA 1996) Discontinued (FDA, 2021) |
||
|
Iron Oxide nanoparticles |
NanoTherm® |
Fe2O3 |
Glioblastoma, prostate, and pancreatic cancer (https://www.eib.org/en/stories/new-cancer-treatments, accessed on 15 January 2024) |
Approved (EMA, 2013) |
[57] |
|
Albumin nanoparticles |
Abraxane® |
Paclitaxel |
Metastatic breast cancer, locally advanced or metastatic non-small cell lung cancer, metastatic adenocarcinoma of the pancreas (https://www.ema.europa.eu/en/medicines/human/EPAR/abraxane, accessed on 15 January 2024) |
Approved (EMA 2008) (FDA 2005) |
|
|
Pazenir® |
Paclitaxel |
Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, non-small cell lung cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/pazenir, accessed on 15 January 2024) |
Approved (EMA 2019) |
[58] |
|
|
Vaccines |
Adstiladrin® |
Adenoviral vector-based gene therapy |
Bacillus Calmette–Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-adstiladrin-nadofaragene-firadenovec-vncg-patients-high-risk, accessed on 15 January 2024) |
Approved (FDA 2022) |
[59] |
|
Provenge® |
Autologous peripheral-blood mononuclear cells |
Metastatic castration-resistant prostate cancer (mCRPC) (https://www.drugs.com/history/provenge.html, accessed on 15 January 2024) |
Approved (EMA 2013) (FDA 2010) Discontinued (EMA, 2015) |
[59] |
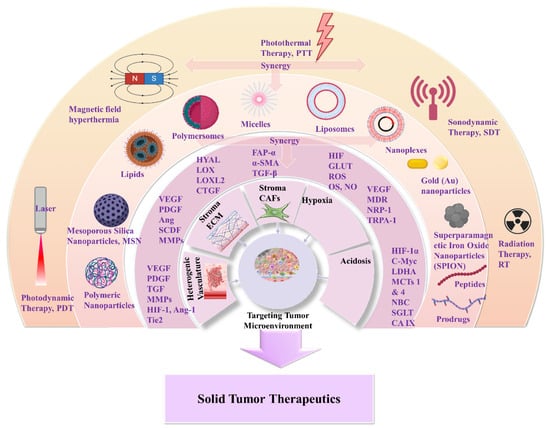
3. Nanomedicines for Targeting TME: Application of Natural and Synthetic Biomaterials
3.1. The Heterogenic Vasculature
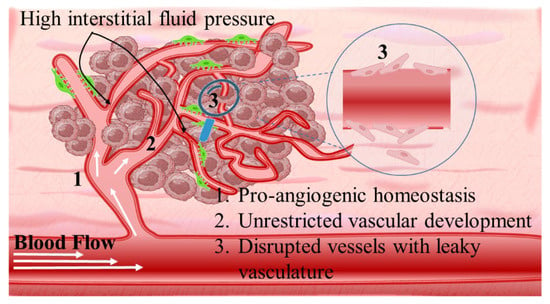
Angiogenetic mechanism is divided into two phases: the avascular, wherein tumor progression is suppressed due to controlled homeostasis of pro- and anti-angiogenetic factors; and the vascular, wherein tumor development is promoted by a switched homeostasis favoring a pro-angiogenetic environment. For solid tumors, in order to progress and develop, a de facto ultimate need is presented for blood, oxygen, and nutrient supply. This is supported by the continuously evolving tumor vasculature (Scheme 4) [68][69][70]. Thus, regulating angiogenesis is a key step in tackling TME abnormal vasculature that is being targeted by nanomedicines through angiogenetic inhibitors. The intention is to promote tumor suppression mechanisms by limiting the unrestricted vascular development [71]. The main target of angiogenetic therapeutics is the inhibition of growth factors of the pro-angiogenetic domain that present elevated affinity with surface receptors of ECs. These include: (i) soluble factors such as vascular endothelial growth factor (VEGF family factors comprising A to F members), platelet-derived growth factor (PDGF), beta and alpha transforming growth factors (TGF-α, -β), angiopoietins (Ang); and (ii) insoluble membrane-bound proteins such as ephrins, integrins, cadherins, matrix metalloproteinases (MMPs), and hypoxia-inducible factor-1 (HIF-1) (Scheme 5) [72].Angiogenetic mechanism is divided into two phases: the avascular, wherein tumor progression is suppressed due to controlled homeostasis of pro- and anti-angiogenetic factors; and the vascular, wherein tumor development is promoted by a switched homeostasis favoring a pro-angiogenetic environment. For solid tumors, in order to progress and develop, a de facto ultimate need is presented for blood, oxygen, and nutrient supply. This is supported by the continuously evolving tumor vasculature (Scheme 4) [68–70]. Thus, regulating angiogenesis is a key step in tackling TME abnormal vasculature that is being targeted by nanomedicines through angiogenetic inhibitors. The intention is to promote tumor suppression mechanisms by limiting the unrestricted vascular development [71]. The main target of angiogenetic therapeutics (Table 2) is the inhibition of growth factors of the pro-angiogenetic domain that present elevated affinity with surface receptors of ECs. These include: (i) soluble factors such as vascular endothelial growth factor (VEGF family factors comprising A to F members), platelet-derived growth factor (PDGF), beta and alpha transforming growth factors (TGF-α, -β), angiopoietins (Ang); and (ii) insoluble membrane-bound proteins such as ephrins, integrins, cadherins, matrix metalloproteinases (MMPs), and hypoxia-inducible factor-1 (HIF-1) (Scheme 5) [72].
3.2. The Tumor Stroma Extracellular Matrix
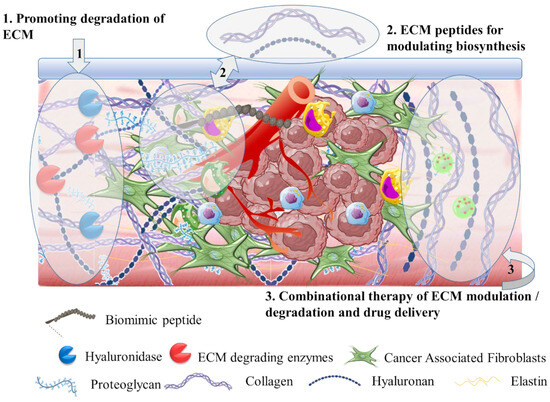
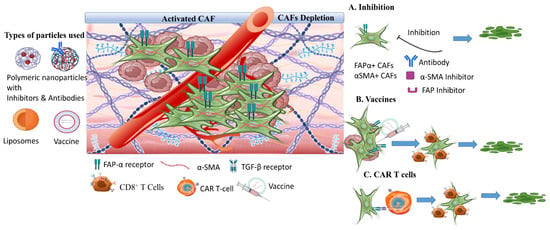
3.4. The Tumor Hypoxia
3.4. The Tumor Hypoxia
Another therapeutic target of responsive nanomedicine is the TME hypoxia (Scheme 9). This is a direct consequence of heterogenic vasculature and fluctuating blood flow, and results in insufficient oxygen diffusion and perfusion within the tumor environment. The rapid proliferation rate of tumor and stromal cells creates excessive consumption of supplied oxygen, nutrients, and energy [87][155]. Imbalance in the diffusion mechanisms of oxygen supply is observed at depths after 70–150 μm from peripheral tumor blood vessels. This results in gas oxygen (gas-O2) levels falling below 1–2% in hypoxic solid tumors. There are two types of hypoxia: the chronic, wherein oxygen’s concentration is characterized by a longitudinal gradient drop for a prolonged time period of several hours; and the acute, in which tumor ECs and stromal cells are attached to vasculature with deprived oxygen perfusion [88][156]. Extensive research has resulted in the understanding of hypoxia mechanisms and their effects on tumor biology by participating in the regulation of angiogenesis, metastasis, and multidrug resistance [89][90] (Table 5) [157,158]. Hypoxia-regulated genes are expressed among various tumor types with high hypoxic gene expressions such as the squamous cell carcinoma (SCC) of the head and neck, lung, and cervix tumors; these have also been investigated [91][159].
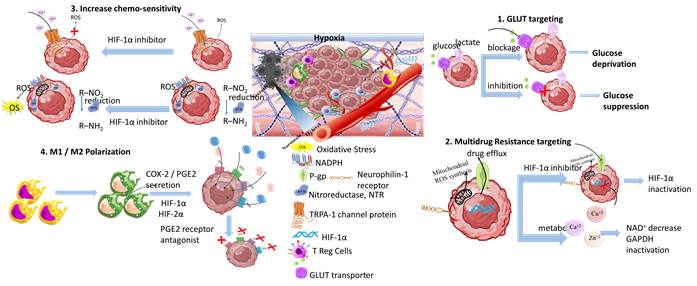
Scheme 7. Tumor hypoxia represents a significant target for responsive nanomedicines. Important axes of nanomedicine research are GLUT targeting, multidrug resistance targeting, increase of chemo-sensitivity, M1/M2 macrophage polarization, increase of tumor oxygenation, and antioxidants. Biomaterials used for hypoxia targeting nanomedicines include polysaccharides, polymers, hybrid nanoparticles, metal oxides and hybrid metal–polymer nanoparticles, polymersomes, nanogels, and liposomes in combination with chemotherapeutic drug targeting (CDT), magnetic targeting, and PDT/PTT therapy. (Created with the assistance of BioRender.com https://app.biorender.com/user/signin?illustrationId=6156d45891063d00af8af51d (accessed on 1 September 2023 up to 31 November 2023), and Microsoft ppt).
3.5. The Tumor Acidosis
3.5. The Tumor Acidosis
Tumor and stromal cells use aerobic glycolysis for their amplified energy supply requirements, as a direct consequence of hypoxia and defective vasculature. Aerobic glycolysis is an oxygen-independent process known as the Warburg effect. However, even in normally oxygenated tumor regions, the main energy supplier remains aerobic glycolysis in about 80% of solid tumors [210]. In aerobic glycolysis, glucose constitutes the main macronutrient of tumor cells for their biosynthetic requirements and follows the lactate metabolic pathway, through GLUT transporters producing amplified levels of lactic acid (lactate). The main transcriptional factors of glycolytic activity regulating lactate production are the HIF-1α and c-Myc regulatory genes [211] that promote the overexpression of varied glycolytic enzymes such as lactate dehydrogenase A (LDHA), and monocarboxylate transporters (MCTs) such as MCT1 and MCT4 [212]. Mainly, the upregulation of LDHA gene favors the activity of LDH-5 and inhibits the activity of LDH-1, and promotes the conversion of pyruvate to lactate. Through this metabolic pathway, elevated amounts of lactate, protons (H+), and carbon dioxide (CO2) are secreted into TME. This leads to acidosis [213]. Acidosis regulates the metabolism of innate and adaptive immune cells by: (i) hindering the function of CD8+ T, natural killer (NK), natural killer T (NKT) and dendritic (DC) cells; (ii) supporting regulation of FOXP3+ T cells (Treg); and (iii) promoting M2 activated macrophage polarization. Overall, the acidic TME is an immunosuppressive incubator of pro-oncogenic and tumorigenic factors, and has been extensively studied for targeted nanomedicine applications [214–216]. The glycolytic metabolic pathway and acidic pH gradient are key participating factors in MDR due to their activation of enzymes and proteins responsible for resistance, efflux of drugs through P-gp, and stimulation of migration [217,218].
In tumors, a unique pH-gradient effect is established, with extracellular pH levels (pHe) being more acidic (6.4–7.0) and intracellular pH (pHi) being more alkaline (7.25–7.50) (Scheme 10). Distinct pH variations exist in tumor cell organelles, and they can be divided into acidic, such as nucleosomes and lysosomes with a pH of 5.5 and 5.0, respectively; or alkaline, such as mitochondria and cytoplasm, which have a corresponding pH of 8.0 and 7.2, respectively. The pH gradient is associated with the expression of membrane transporters such as MCT1, MCT4, carbonic anhydrases, and sodium-bicarbonate co-transporter (NBC). These participate in the translocation of lactic acid, CO2, and its bicarbonate ion byproducts. Other mechanisms influencing TME acidity are the efflux of endosomes acidic cargo and the release of the acidic intracellular comportments of necrotic cells. In stimuli responsive nanomedicine (Table 6), pH sensitivity has been highly exploited and reviewed [219–223]. Apart from drug delivery systems, tumor acidosis was targeted by pH-regulating molecular systems at various stages of clinical trials (this is described by Corbet et al.) [224], and by TME sensitive platforms for combined endogenous stimuli responsive effects (as reviewed by Wang et al. [225]).
3.6. Tumor Immunotherapies
Another important area of nanomedicine research is tumor immunotherapy. Tumor immunotherapies may represent a vital solution in TME limitation and can be divided into immune checkpoint inhibitors (ICI), immune tumor vaccines, and chimeric antigen receptor-modified CAR T cells. The development of nanomedicines for cancer immunotherapy is largely based on the application of biomaterials such as polysaccharides (e.g., hyaluronic acid, alginic acid, chitosan, and dextran) and synthetic polymers including PEG, PLA, PLGA, PCL, and polypeptides [257]. The development of immunotherapeutic nanomedicines based on biomaterials has emerged as a strategy to improve therapeutic outcome by inducing tissue-specific immunomodulation through cell, antibody, and gene immune responses [258]. Opportunities to apply biomaterials in tumor immunotherapy and their combination with conventional therapies have been interestingly reviewed [259–261]. Among immunotherapies, immune checkpoint inhibitors present promising therapies that mainly target cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1), and programmed cell death receptor-1 ligand (PD-L1). Immune checkpoints are proteins on the surface of immune T cells that recognize and bind to partner proteins on tumor cells, and promote intracellular inhibitory signals and immunosuppressive enzymes; this suppresses host immune T cell attack against tumor cells. Hu et al. [262] developed amphiphilic nanoparticles with a core from hydrophobic ROS-sensitive poly(thioketal phosphoester) and a shell from hydrophilic lecithin/DSPE-PEG, encapsulating doxorubicin, Ce6 photosensitizer, and anti-PD-L1 antibody for ICI. The nanoparticles were evaluated in 4T1 tumor-bearing mice. Rapid degradation of the ROS-responsive core triggered DOX release and, in combination with laser irradiation, promoted effective PDT activity due to the Ce6 component of the nanoparticles. The combinational effect with the anti-PD-L1 antibody ICI promoted the maturation of DCs, and this significantly suppressed the growth of primary tumors and effectively inhibited distant tumor growth. Another type of immunotherapy is the delivery of immunostimulatory agents that directly activate immune T cells by binding of agonistic antibodies on surface receptors such as CD40, OX40 (CD134), and CD137; this promotes downstream signaling pathways for T cell-mediated antitumor activity [263–266].
The application of biomaterials in cancer vaccines has made significant progress due to the advantageous effect on enhancing the safety profile and stimulating antigen-specific T cell response. Immunotherapy vaccines aim to activate the immune system attack against tumor cells by downregulating the immune tolerance to tumor antigens, through the combined delivery of immunostimulatory agents to activate host immune cells and immunogenic epitopes of specific tumor antigens [263,264]. Τhe effective delivery of the immunotherapy vaccines to dendritic cells is crucial, because DCs are the main antigen presenting immune cells. Rosalia et al. [264], reported that PLGA nanoparticles coated with agonistic aCD40-mAb and encapsulating tumor associated Ag protein successfully primed CD8+ T cells; thus, the survival of tumor-bearing mice was prolonged. In another study, Wang et al. [265] investigated amphiphilic pH-sensitive galactosyl dextran-retinal (GDR) nanogels with a pH-sensitive hydrazone bond for dextran conjugation with all-trans retinal (a metabolite of vitamin A). The nanogels were galactosylated to acquire DC-targeting ability and were effective vaccine delivery systems due to their ability to successfully amplify major histocompatibility class I, MHC I, antigen expression in DCs, and induced effective antitumor immune responses.
The most recent immunotherapies are CAR T cell and CAR T cell receptor therapies. CAR T cells are engineered immune cells originating from the peripheral blood mononuclear cells of the patient’s blood that are harvested and stimulated to become T cells with specific DNA encoding to recognize certain tumor antigens. There are two FDA-approved CAR T cell therapies for blood cancer; however, their application in solid tumors remains challenging. Nevertheless, CAR T cell therapies against solid tumors are being studied ongoing clinical trials [263,264]. Biomaterials are promising candidates for CAR T cell modification improving the therapeutic efficacy and immune-editing processes [266]. Moreover, nano-biomaterials can improve the effect of immunotherapies, and trigger enhanced therapeutic outcomes and regulation of immune cells by overcoming the barriers of the cold tumor immune microenvironment [267].
