Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Spyridon Prountzos | -- | 2330 | 2024-01-31 13:12:36 | | | |
| 2 | Mona Zou | -3 word(s) | 2327 | 2024-02-01 08:36:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alexopoulou, E.; Prountzos, S.; Raissaki, M.; Mazioti, A.; Caro-Dominguez, P.; Hirsch, F.W.; Lovrenski, J.; Ciet, P. MRI in Community-Acquired Pneumonia in the Paediatric Population. Encyclopedia. Available online: https://encyclopedia.pub/entry/54591 (accessed on 07 February 2026).
Alexopoulou E, Prountzos S, Raissaki M, Mazioti A, Caro-Dominguez P, Hirsch FW, et al. MRI in Community-Acquired Pneumonia in the Paediatric Population. Encyclopedia. Available at: https://encyclopedia.pub/entry/54591. Accessed February 07, 2026.
Alexopoulou, Efthymia, Spyridon Prountzos, Maria Raissaki, Argyro Mazioti, Pablo Caro-Dominguez, Franz Wolfgang Hirsch, Jovan Lovrenski, Pierluigi Ciet. "MRI in Community-Acquired Pneumonia in the Paediatric Population" Encyclopedia, https://encyclopedia.pub/entry/54591 (accessed February 07, 2026).
Alexopoulou, E., Prountzos, S., Raissaki, M., Mazioti, A., Caro-Dominguez, P., Hirsch, F.W., Lovrenski, J., & Ciet, P. (2024, January 31). MRI in Community-Acquired Pneumonia in the Paediatric Population. In Encyclopedia. https://encyclopedia.pub/entry/54591
Alexopoulou, Efthymia, et al. "MRI in Community-Acquired Pneumonia in the Paediatric Population." Encyclopedia. Web. 31 January, 2024.
Copy Citation
The most common acute infection and leading cause of death in children worldwide is pneumonia. Clinical and laboratory tests essentially diagnose community-acquired pneumonia (CAP). CAP can be caused by bacteria, viruses, or atypical microorganisms. Imaging is usually reserved for children who do not respond to treatment, need hospitalisation, or have hospital-acquired pneumonia. Advances in MRI protocols make it a viable alternative for diagnosing CAP and its complications.
community-acquired pneumonia
complicated pneumonia
pediatrics
ultrasound
computed tomography
magnetic resonance imaging
1. Magnetic Resonance Imaging
Magnetic Resonance Imaging (MRI) is a radiation-free technique that provides excellent soft-tissue contrast and anatomical detail. Still, it comes at a higher cost per examination and is less available in low-income countries. MRI is usually a more time-consuming examination than CT, with an increased need for sedation in children younger than 5 years. However, the recently reported use of real-time MRI for imaging pneumonia is just as fast as a CT scan, and it no longer requires sedation. Movement and breathing by the child do not cause artefacts either [1].
2. Pleural Effusion—Empyema
A pleural effusion is formed when there is an imbalance between hydrostatic and oncotic pressure between the systemic and pulmonary circulations and the pleural space [2]. Additionally, the obstruction of lymphatic drainage by thick pleural fluid and debris further contributes to the accumulation of the effusion [3][4][5]. Parapneumonic effusion is a pleural effusion associated with an ipsilateral lung infection and can be simple or complicated (in the presence of infecting organisms). An empyema is a thick, purulent pleural effusion, which can be either free or loculated [6]. The progression of pleural effusion to empyema occurs in three stages: (a) the exudative stage, which is a simple parapneumonic effusion; (b) the fibrinopurulent stage, which is a complicated parapneumonic effusion; and (c) the organising phase, in which fibCRroblastic activity and peel formation occur.
In Chest radiography (CR), pleural effusion may have different appearances, such as blunting of the costophrenic angle or the meniscus sign when free fluid is present (Figure 1). A medial convex border to the lung parenchyma indicates a loculated effusion. Complete opacification of the ipsilateral hemithorax is present in large effusions where the underlying parenchyma is obscured. An ipsilateral concave scoliosis and contralateral mediastinal shift may be common associated findings, and further imaging is required to investigate the condition of the underlying lung and pleura (Figure 1).
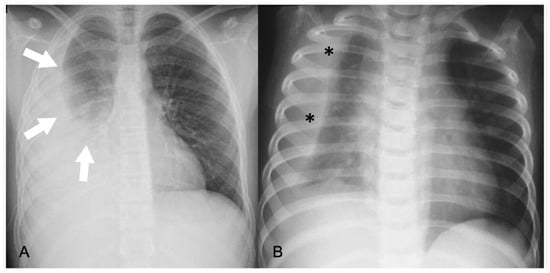
Figure 1. Pleural effusions on chest radiographs: (A) meniscus sign (arrows); (B) loculated effusion with convex borders (*) towards the spine.
Lung ultrasound (LUS) can provide information regarding the volume of the effusion (as small as 10 mL), the presence of fibrinous septations, and the echogenicity grade of the pleural fluid (Figure 2) [7]. LUS can be used to differentiate pleural effusions from consolidated lung and peripheral lung abscesses from empyema. In the appropriate clinical setting, the pulmonologist or thoracic surgeon may decide whether to perform thoracocentesis and put on a chest tube, fibrinolysis, or VATS based on the appearance and volume of the pleural effusion. The indication for performing thoracentesis arises when a pleural effusion, which may vary in volume, shows resistance to IV antibiotic treatment or when the patient continues to experience symptoms [8][9]. A few formulae can be used to calculate pleural effusion volume in adults; however, none of them are truly adapted to children. LUS also provides guidance for the insertion of draining catheters. Intravenous CEUS may be useful to better outline pleural effusions, whereas intracavitary contrast is helpful in determining catheter localization and patency, or the presence of loculation [10][11][12][13][14].
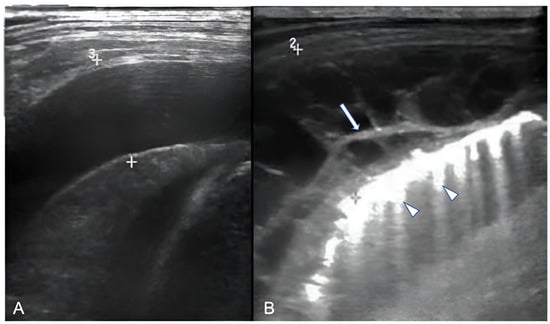
Figure 2. Ultrasonographic appearances of pleural effusions between cursors: (A) simple (anechoic), amenable for drainage under the appropriate clinical setting; (B) complicated, with internal septae (arrow). Also, note confluent B-lines (arrowheads).
Contrast-enhanced CT can demonstrate the location and volume of the effusion, although it might not show existing septations. The enhancement of thickened parietal pleura is usually depicted in this setting, although its absence does not exclude an infected effusion (Figure 3) [6].
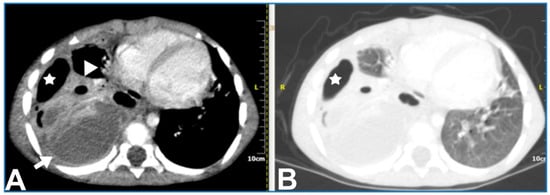
Figure 3. A 5.5-year-old boy with complicated pneumonia underwent a CT scan because of a differential diagnostic issue between empyema and lung abscesses on CR and LUS. Axial chest contrast-enhanced CT scan with soft-tissue window (A) and lung window (B) depicts a large multiloculated empyema (arrow), collapsed lung parenchyma with cavitary necrosis (arrowhead), and loculated pneumothorax (stars). Further, optimal treatment was drainage of the empyema based upon the chest CT findings.
Regarding MRI, simple effusions show crescentic distribution and low signal on T1-weighted sequences, high signal on T2-weighted sequences, and no contrast enhancement. Empyema shows a loculated effusion with thickened, enhancing pleura, septations, and heterogeneous contents on T1-weighted and T2-weighted images due to debris (Figure 4). The use of DWI can help in differentiating between simple effusion and empyema, as the latter shows restricted diffusivity [15][16].
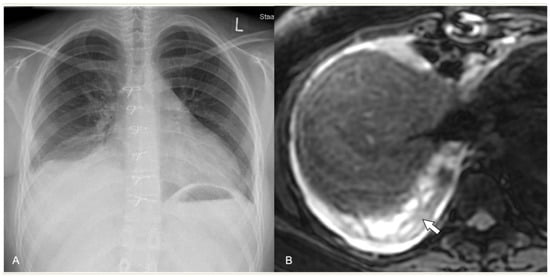
Figure 4. Complicated pneumonia following heart surgery. (A) Chest radiograph demonstrates the blunting of right pleurodiaphragmatic angle and a possible subpulmonary pleural effusion. (B) Axial MRI, T2-weighted sequence. Note the clear depiction of a pleural effusion with septations (arrow).
3. Acute Lung Parenchyma Complications
Three significant acute complications of severe pneumonia result from the extensive destruction and liquefaction of lung tissue caused by vascular thrombosis: necrotizing pneumonia, cavitary necrosis, and the formation of lung abscesses [3].
4. Necrotizing Pneumonia
Children with necrotizing pneumonia exhibit symptoms such as fever, cough, and tachypnoea that last for many days. Hypoxia, moderate anaemia, and hypoalbuminaemia are frequent symptoms, with pleural effusion commonly visible upon physical examination [3].
CR is not useful in the early stage of necrotizing pneumonia, as it is unable to depict liquefaction of the lung parenchyma. Cavitary necrosis typically exhibits the presence of air-filled cavities or air–fluid levels, which may require additional distinction from lung abscess or loculated empyema (Figure 5) [3][6].
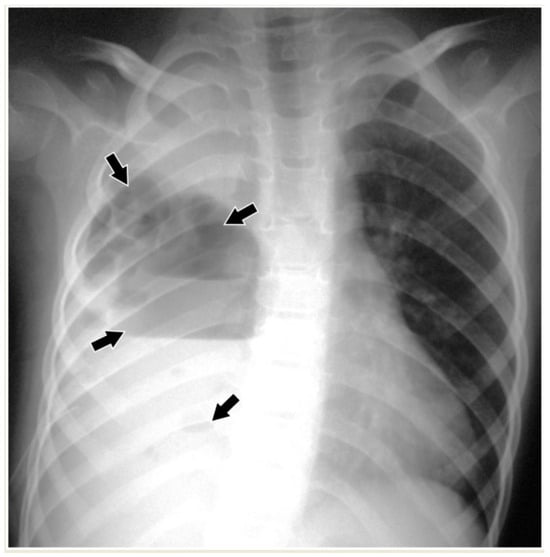
Figure 5. Necrotizing pneumonia in an 8-year-old boy. Chest radiograph shows opacification of the right hemithorax with ipsilateral concave scoliosis and multiple cavities (arrows) with air–fluid levels of variable size.
Necrosis is seen on LUS as ill-defined areas of low echogenicity and diminished vascularity (Figure 6). Attention needs to be paid to the differentiation between echogenic effusions and echogenic lung parenchyma due to pneumonia (Figure 7). When positioned in the peripheral lung, LUS demonstrates comparable accuracy to CT in identifying pneumonia sequelae, such as pleural effusion, necrosis, and abscesses of lung parenchyma. However, the LUS sensitivity is lower than that of CT for centrally located abnormalities. The presence of fluid and static air bronchograms within a consolidated region may indicate the early onset of parenchymal complications [17]. The role of intravenous CEUS in complicated pneumonia remains a subject of controversy. While some researchers have reported on its accurate identification of necrotizing pneumonia and the precise delineation of its extent, contrasting findings have emerged from other authors, who found that no significant additional information was garnered in comparison to the utilisation of Colour-Doppler [10][12][13][18][19].
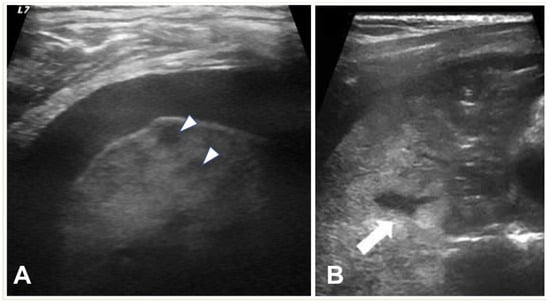
Figure 6. (A) LUS shows a consolidated lung with multiple hypoechoic areas (arrowheads) consistent with necrotising pneumonia in a 5.5-year-old boy. Pleural effusion is also present. (B) Necrotizing pneumonia in a 7-year-old girl; there is extensive consolidation containing hypoechoic areas with an ill-defined wall (arrow) consistent with necrosis.
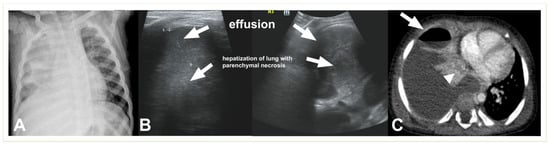
Figure 7. Complicated pneumonia in a 1-year-old boy. (A) Chest radiograph shows opacification of the right hemithorax with areas of consolidation and collapse. (B) Lung US shows an echogenic pleural effusion, making it difficult to distinguish from adjacent echogenic and necrotic lung parenchyma (C). Post-contrast CT scan images depict a large effusion, a loculated air–fluid level in the pleural space (thick arrow), and adjacent collapsed–consolidated lung parenchyma with necrotic areas (arrowhead).
Upon contrast-enhanced CT (CECT), necrotizing pneumonia is characterised by the presence of several patchy or widespread areas of diminished or absent enhancement, indicating liquefaction of the lung tissue (Figure 7). Cavitary necrosis is distinguished by the absence of a normal lung tissue structure, which is either diminished or lacking enhancement, and the appearance of many thin-walled cavities filled with either fluid or air. These cavities lack an enhancing border (Figure 8).
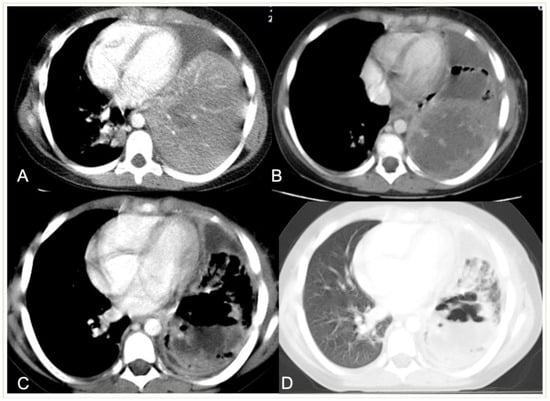
Figure 8. Development of necrotic and cavitary pneumonia in a 7-year-old girl. (A) There is a left basal consolidation with a central hypo-enhancing area, consistent with early necrosis. (B) Follow-up CT scan shows extensive areas of non-enhancement, consistent with extensive necrotic pneumonia. (C,D) Follow-up post-contrast scan, soft tissue, and lung window demonstrate cavitary necrosis containing multiple air–fluid levels.
Pulmonary necrosis on MRI is represented by areas of increased signal intensity on T2-weighed sequences, while cavitary necrosis is represented by fluid-filled and air-filled areas within a consolidation that do not enhance after the injection of contrast (Figure 9). Contrast-enhanced MRI shows a similar CECT pattern to identify necrotic lung parenchyma.
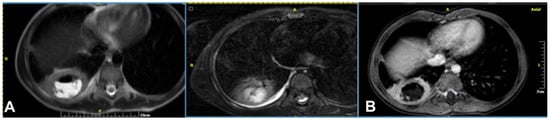
Figure 9. A 6-year-old boy, referred to MRI for exclusion of a lung abscess. (A) T2-weighted sequence shows a right lower lobe consolidation with a single air–fluid cavity (area with high and very low signal intensity), without a clearly depicted wall (area of isointensity surrounding the cavity) and an adjacent small pleural effusion (crescent-shaped line of high signal intensity). (B) Post-contrast T1-weighted sequence shows contrast enhancement by the adjacent consolidated lung parenchyma, consistent with the presence of cavitary necrosis.
5. Abscess
Children with lung abscesses often have an extended cough and mild fever, with less common symptoms including chest discomfort, dyspnoea, sputum production, and hemoptysis. A chest exam may show normal or symptoms of consolidation [3].
A lung abscess is a single cavity with a thick wall and purulent content resulting from suppuration and necrosis of the parenchyma. After the initial stage, the abscess can be partially or even completely air-filled. This is an uncommon complication in immunocompetent children with CAP but, when present, it may be associated with a pre-existing pulmonary cavity, such as cystic pulmonary adenomatoid malformations (CPAM). Severe forms of necrotizing pneumonia have also been associated with Staphylococcus aureus strains expressing the Panton–Valentine leukocidin cytotoxic agent [3][20]. A lung abscess on CR appears as a round or oval-shaped area that is denser than the surrounding lung parenchyma. The edges of the abscess might be irregular or ill-defined, and there may be air–fluid levels inside, indicating the presence of gas and fluid. On LUS, a lung abscess typically appears as a hypoechoic area within the lung tissue with irregular borders. Surrounding the hypoechoic area, there might be a hyperechoic rim, which corresponds to the inflamed tissue. On CECT, this appears as a hypodense cavity surrounded by a well-defined enhancing wall without central enhancement and a variable proportion of air and/or fluid content (Figure 10). On MRI, a rounded, T2-hyperintense area surrounded by a hypointense wall can be seen, which is usually thick and irregular and may contain an air–fluid level or be completely air-filled (Figure 11 and Figure 12). A characteristic, restricted diffusion can be seen in DWI sequences, with peripheral wall enhancement after the injection of intravenous contrast [15][16][21][22][23][24].
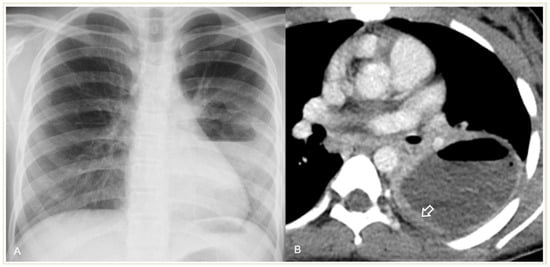
Figure 10. Lung abscess complicated CAP in a 12-year-old girl: (A) chest radiograph shows a cavity with an air–fluid level; (B) contrast-enhanced CT scan demonstrates a cavity with a thick enhancing wall (arrow) containing an air–fluid level, favoring the diagnosis of an abscess.
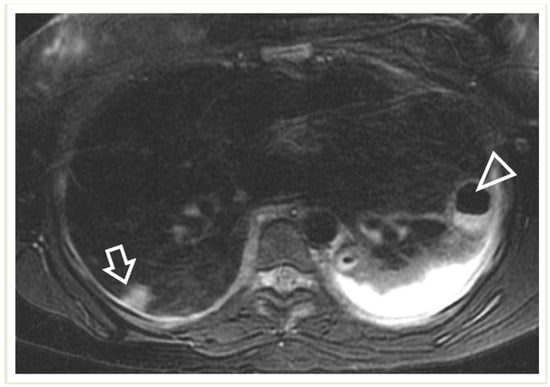
Figure 11. A 17-year-old girl with a pulmonary abscess following septic embolism. Lung MRI, in an axial T2-weighted image, shows areas of high signal intensity consistent with subpleural septic emboli (arrow) and pleural effusion (very high-signal-intensity area dorsally, mostly on the left side). Note the abscess at the left lung base, exhibiting a low signal and relatively thick wall, and containing an air–fluid level (arrowhead).
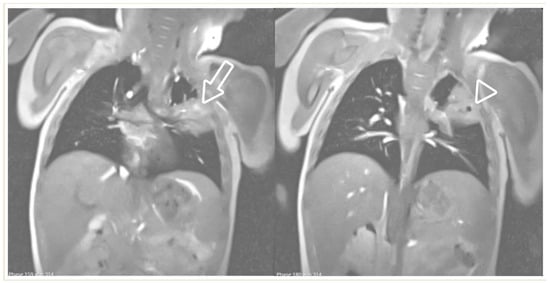
Figure 12. Real-time MRI sequence in the coronal plane in a 1-year-old girl with left upper-lobe pneumonia. Selected images from the free-breathing scan, which took 16 s to acquire and reconstruct. Within the isointense pulmonary infiltrate, there are areas of bright signal intensity (arrow) and a round area of very low (almost black) signal intensity, suggestive of air inclusions (arrowhead), with an appearance consistent with cavitary necrosis.
6. Pneumatocele
A pneumatocele is a thin-walled intraparenchymal cystic structure that is filled with air (or little-to-no fluid content) and is formed by a valve-type mechanism that allows for the one-way passage of air into the interstitial space. Pneumatocele are often the results of severe infections, trauma, and mechanical ventilation. Pneumatocele appear as bubble-like structures on imaging studies like X-rays or CT scans [25]. Pneumatocele are difficult to identify on LUS because they are usually filled with air and therefore have an anechoic appearance.
7. Pleural Fistulas
A pleural fistula can form during severe pneumonia as a result of the erosion or perforation of lung tissue that connects the bronchi to the pleural space. Pleural fistula is a serious and potentially life-threatening complication in severe pneumonia, leading to respiratory distress, sepsis, and pneumothorax. The diagnosis of bronchopleural fistula is usually definite on CT when a direct communication between the lung parenchyma and the pleural space is identified. It is also suspected when there is a persistent air leak in the pleural space for more than 24 h (Figure 13) or when pneumothorax recurs following the removal of the drains [26]. CECT is the optimal technique for diagnosing a pleural fistula due to its high resolution, which enables the identification of direct connections between small peripheral bronchi and the pleural space [27].
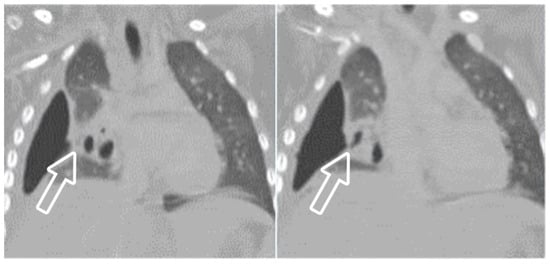
Figure 13. A 4-year-old girl with complicated pneumonia and a surgically confirmed broncho-pleural fistula. CT scan and coronal reconstruction show right loculated pneumothorax and collapsed lung parenchyma, and suggest the fistula to be an indentation (arrow) at the visceral pleura adjacent to a pneumatocele.
8. Pediatric Acute Respiratory Distress Syndrome (PARDS)
PARDS is defined as the occurrence of signs and symptoms of pulmonary oedema that are not fully explained by cardiac failure or fluid overload within 7 days of a clinical insult [28]. Various epidemiological factors and etologies play a significant role in the development of PARDS. The most common PARDS risk factors include pneumonia or lower respiratory tract infections [29]. One of the clinical and laboratory criteria used to diagnose PARDS is the detection of bilateral lung infiltrates on chest radiography (Figure 14) [28].
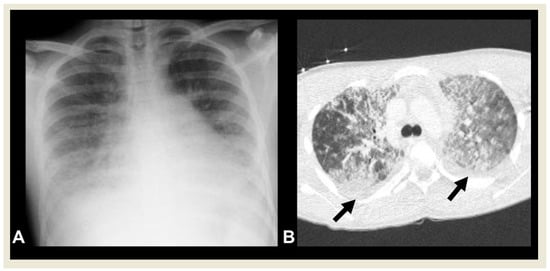
Figure 14. A 15-year-old boy with PARDS. (A) Chest radiography shows bilateral, diffuse lower lung opacities, and pleural effusions. (B) CECT shows bilateral ground-glass and alveolar opacities, and interlobular septal thickening with concomitant pleural effusions (arrows).
References
- Hirsch, F.W.; Sorge, I.; Vogel-Claussen, J.; Roth, C.; Gräfe, D.; Päts, A.; Voskrebenzev, A.; Anders, R.M. The current status and further prospects for lung magnetic resonance imaging in pediatric radiology. Pediatr. Radiol. 2020, 50, 734–749.
- Calder, A.; Owens, C.M. Imaging of parapneumonic pleural effusions and empyema in children. Pediatr. Radiol. 2009, 39, 527–537.
- De Benedictis, F.M.; Kerem, E.; Chang, A.B.; Colin, A.; Zar, H.J.; Bush, A. Complicated pneumonia in children. Lancet 2020, 396, 786–798.
- Feller-Kopman, D.; Light, R. Pleural Disease. N. Engl. J. Med. 2018, 378, 740–751.
- Lai-Fook, S.J. Pleural Mechanics and Fluid Exchange. Physiol. Rev. 2004, 84, 385–410.
- Eslamy, H.K.; Newman, B. Pneumonia in Normal and Immunocompromised Children: An Overview and Update. Radiol. Clin. N. Am. 2011, 49, 895–920.
- Laya, B.F.; Concepcion, N.D.P.; Garcia-Peña, P.; Naidoo, J.; Kritsaneepaiboon, S.; Lee, E.Y. Pediatric Lower Respiratory Tract Infections: Imaging Guidelines and Recommendations. Radiol. Clin. N. Am. 2022, 60, 15–40.
- Tracy, M.C.; Mathew, R. Complicated pneumonia: Current concepts and state of the art. Curr. Opin. Pediatr. 2018, 30, 384–392.
- Fischer, G.B.; Mocelin, H.T.; Andrade, C.F.; Sarria, E.E. When should parapneumonic pleural effusions be drained in children? Paediatr. Respir. Rev. 2018, 26, 27–30.
- Lovrenski, J. Pediatric lung ultrasound—Pros and potentials. Pediatr. Radiol. 2020, 50, 306–313.
- James, C.A.; Lewis, P.S.; Moore, M.B.; Wong, K.; Rader, E.K.; Roberson, P.K.; Ghaleb, N.A.; Jensen, H.K.; Pezeshkmehr, A.H.; Stroud, M.H.; et al. Efficacy of standardizing fibrinolytic therapy for parapneumonic effusion. Pediatr. Radiol. 2022, 52, 2413–2420.
- Deganello, A.; Rafailidis, V.; Sellars, M.E.; Ntoulia, A.; Kalogerakou, K.; Ruiz, G.; Cosgrove, D.O.; Sidhu, P.S. Intravenous and Intracavitary Use of Contrast-Enhanced Ultrasound in the Evaluation and Management of Complicated Pediatric Pneumonia. J. Ultrasound Med. 2017, 36, 1943–1954.
- Tomà, P. Lung ultrasound in pediatric radiology—Cons. Pediatr. Radiol. 2020, 50, 314–320.
- Chen, H.-J.; Yu, Y.-H.; Tu, C.-Y.; Chen, C.-H.; Hsia, T.-C.; Tsai, K.-D.; Shih, C.-M.; Hsu, W.-H. Ultrasound in Peripheral Pulmonary Air-Fluid Lesions. Chest 2009, 135, 1426–1432.
- Liszewski, M.C.; Görkem, S.; Sodhi, K.S.; Lee, E.Y. Lung magnetic resonance imaging for pneumonia in children. Pediatr. Radiol. 2017, 47, 1420–1430.
- Kapur, S.; Bhalla, A.S.; Jana, M. Pediatric Chest MRI: A Review. Indian J. Pediatr. 2019, 86, 842–853.
- Musolino, A.M.; Tomà, P.; Supino, M.C.; Scialanga, B.; Mesturino, A.; Scateni, S.; Battaglia, M.; Pirozzi, N.; Bock, C.; Buonsenso, D. Lung ultrasound features of children with complicated and noncomplicated community acquired pneumonia: A prospective study. Pediatr. Pulmonol. 2019, 54, 1479–1486.
- Dietrich, C.F.; Buda, N.; Ciuca, I.M.; Dong, Y.; Fang, C.; Feldkamp, A.; Jüngert, J.; Kosiak, W.; Mentzel, H.J.; Pienar, C.; et al. Lung ultrasound in children, WFUMB review paper (part 2). Med. Ultrason. 2021, 23, 443–452.
- Rafailidis, V.; Andronikou, S.; Mentzel, H.-J.; Piskunowicz, M.; Squires, J.H.; Barnewolt, C.E. Contrast-enhanced ultrasound of pediatric lungs. Pediatr. Radiol. 2021, 51, 2340–2350.
- Eber Fabio, E.M. (Ed.) ERS Handbook of Paediatric Respiratory Medicine; The European Respiratory Society: Lausanne, Switzerland, 2021.
- Konietzke, P.; Mueller, J.; Wuennemann, F.; Wagner, W.L.; Schenk, J.-P.; Alrajab, A.; Kauczor, H.-U.; Stahl, M.; Mall, M.A.; Wielpütz, M.O.; et al. The value of chest magnetic resonance imaging compared to chest radiographs with and without additional lung ultrasound in children with complicated pneumonia. PLoS ONE 2020, 15, e0230252.
- Gorkem, S.B.; Coskun, A.; Yikilmaz, A.; Zurakowski, D.; Mulkern, R.V.; Lee, E.Y. Evaluation of Pediatric Thoracic Disorders: Comparison of Unenhanced Fast-Imaging-Sequence 1.5-T MRI and Contrast-Enhanced MDCT. Am. J. Roentgenol. 2013, 200, 1352–1357.
- Baez, J.C.; Ciet, P.; Mulkern, R.; Seethamraju, R.T.; Lee, E.Y. Pediatric Chest MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 337–349.
- Ciet, P.; Tiddens, H.A.W.M.; Wielopolski, P.A.; Wild, J.M.; Lee, E.Y.; Morana, G.; Lequin, M.H. Magnetic resonance imaging in children: Common problems and possible solutions for lung and airways imaging. Pediatr. Radiol. 2015, 45, 1901–1915.
- Al-Saleh, S.; Grasemann, H.; Cox, P. Necrotizing Pneumonia Complicated by Early and Late Pneumatoceles. Can. Respir. J. 2008, 15, 129–132.
- Andronikou, S.; Goussard, P.; Sorantin, E. Computed tomography in children with community-acquired pneumonia. Pediatr. Radiol. 2017, 47, 1431–1440.
- Kapoor, H.; Gulati, V.; Gulati, A.; Donuru, A.; Parekh, M. Comprehensive Imaging Review of Pleural Fistulas from Diagnosis to Management. RadioGraphics 2022, 42, 1940–1955.
- Shein, S.L.; Maddux, A.B.; Klein, M.J.; Bhalla, A.; Briassoulis, G.; Dahmer, M.K.; Emeriaud, G.; Flori, H.R.; Gedeit, R.; Ilia, S.; et al. Epidemiology and Outcomes of Critically Ill Children at Risk for Pediatric Acute Respiratory Distress Syndrome: A Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology Study. Crit. Care Med. 2022, 50, 363–374.
- Kohne, J.G.; Flori, H.R. Risk Factors and Etiologies of Pediatric Acute Respiratory Distress Syndrome. In Pediatric Acute Respiratory Distress Syndrome; Springer: Cham, Switzerland, 2019; pp. 33–46.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
677
Revisions:
2 times
(View History)
Update Date:
01 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




