
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Parillo | -- | 3357 | 2024-01-31 09:43:38 | | | |
| 2 | Lindsay Dong | Meta information modification | 3357 | 2024-02-01 01:18:01 | | |
Video Upload Options
Brain spaces around (perivascular spaces) and alongside (paravascular or Virchow–Robin spaces) vessels have gained significant attention due to the advancements of in vivo imaging tools and to their crucial role in maintaining brain health, contributing to the anatomic foundation of the glymphatic system. In fact, it is widely accepted that peri- and para-vascular spaces function as waste clearance pathways for the brain for materials such as ß-amyloid by allowing exchange between cerebrospinal fluid and interstitial fluid. Visible brain spaces on magnetic resonance imaging are often a normal finding, but they have also been associated with a wide range of neurological and systemic conditions, suggesting their potential as early indicators of intracranial pressure and neurofluid imbalance.
1. Introduction
2. Anatomy
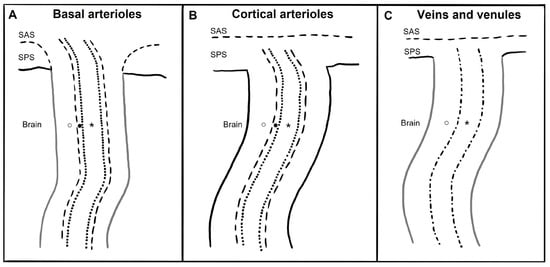
3. Imaging
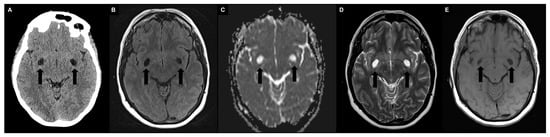
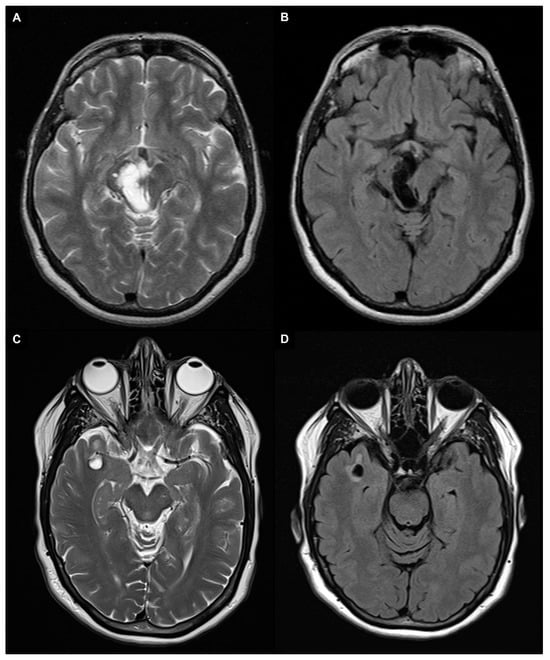
4. Quantification
4.1. Visual Scoring
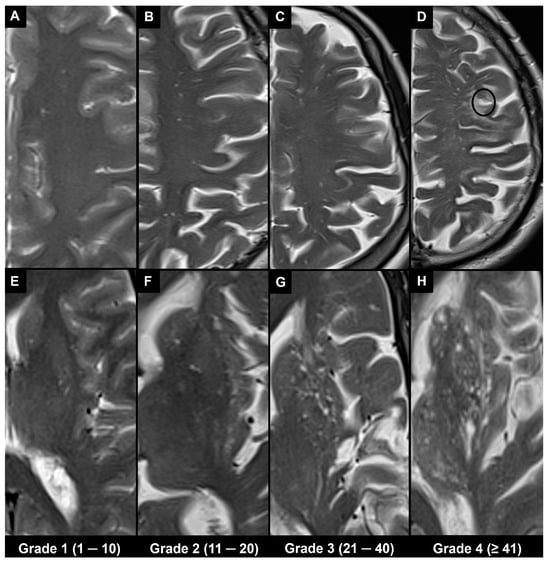
4.2. Automatic and Semi-Automatic Segmentation and Morphometry
5. Associations
5.1. Physiological
5.2. Pathological
6. Differential Diagnosis
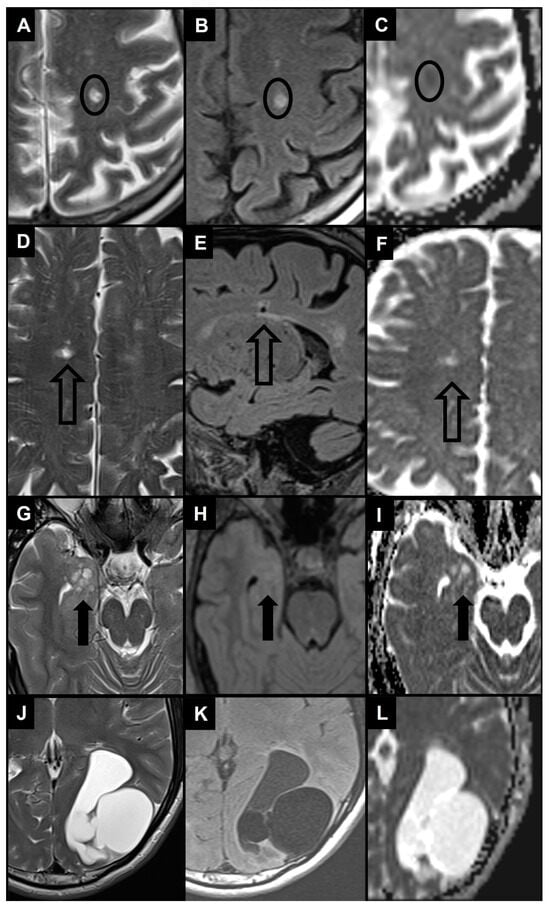
6.1. Recent Small Subcortical Infarct
6.2. Lacune of Presumed Vascular Origin
6.3. WMH
6.4. Benign Intracranial Cysts
6.5. Neoplastic Intracranial Cysts
6.6. Vascular and Inflammatory Cysts
6.7. Infectious Cysts
6.8. Inherited Cysts
7. Conclusions
References
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular Spaces in the Brain: Anatomy, Physiology and Pathology. Nat. Rev. Neurol. 2020, 16, 137–153.
- Robin, C. Recherches Sur Quelques Particularites de La Structure Des Capillaires de l’encephale. J. Physiol. Homme Anim. 1859, 2, 537–548.
- Virchow, R. Ueber die Erweiterung kleinerer Gefäfse. Arch. Für Pathol. Anat. Physiol. Für Klin. Med. 1851, 3, 427–462.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111.
- Gouveia-Freitas, K.; Bastos-Leite, A.J. Perivascular Spaces and Brain Waste Clearance Systems: Relevance for Neurodegenerative and Cerebrovascular Pathology. Neuroradiology 2021, 63, 1581–1597.
- Weller, R.O.; Hawkes, C.A.; Kalaria, R.N.; Werring, D.J.; Carare, R.O. White Matter Changes in Dementia: Role of Impaired Drainage of Interstitial Fluid. Brain Pathol. Zur. Switz. 2015, 25, 63–78.
- Absinta, M.; Ha, S.-K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and Nonhuman Primate Meninges Harbor Lymphatic Vessels That Can Be Visualized Noninvasively by MRI. eLife 2017, 6, e29738.
- Bouvy, W.H.; Biessels, G.J.; Kuijf, H.J.; Kappelle, L.J.; Luijten, P.R.; Zwanenburg, J.J.M. Visualization of Perivascular Spaces and Perforating Arteries with 7 T Magnetic Resonance Imaging. Investig. Radiol. 2014, 49, 307–313.
- Weller, R.O.; Djuanda, E.; Yow, H.-Y.; Carare, R.O. Lymphatic Drainage of the Brain and the Pathophysiology of Neurological Disease. Acta Neuropathol. 2009, 117, 1–14.
- Ringstad, G.; Valnes, L.M.; Dale, A.M.; Pripp, A.H.; Vatnehol, S.-A.S.; Emblem, K.E.; Mardal, K.-A.; Eide, P.K. Brain-Wide Glymphatic Enhancement and Clearance in Humans Assessed with MRI. JCI Insight 2018, 3, e121537.
- Eide, P.K.; Vatnehol, S.A.S.; Emblem, K.E.; Ringstad, G. Magnetic Resonance Imaging Provides Evidence of Glymphatic Drainage from Human Brain to Cervical Lymph Nodes. Sci. Rep. 2018, 8, 7194.
- Roher, A.E.; Kuo, Y.-M.; Esh, C.; Knebel, C.; Weiss, N.; Kalback, W.; Luehrs, D.C.; Childress, J.L.; Beach, T.G.; Weller, R.O.; et al. Cortical and Leptomeningeal Cerebrovascular Amyloid and White Matter Pathology in Alzheimer’s Disease. Mol. Med. Camb. Mass. 2003, 9, 112–122.
- Bakker, E.N.T.P.; Bacskai, B.J.; Arbel-Ornath, M.; Aldea, R.; Bedussi, B.; Morris, A.W.J.; Weller, R.O.; Carare, R.O. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2016, 36, 181–194.
- Bacyinski, A.; Xu, M.; Wang, W.; Hu, J. The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy. Front. Neuroanat. 2017, 11, 101.
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance Systems in the Brain-Implications for Alzheimer Disease. Nat. Rev. Neurol. 2015, 11, 457–470.
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.R.; Perry, V.H.; Weller, R.O. Solutes, but Not Cells, Drain from the Brain Parenchyma along Basement Membranes of Capillaries and Arteries: Significance for Cerebral Amyloid Angiopathy and Neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144.
- George, I.C.; Arrighi-Allisan, A.; Delman, B.N.; Balchandani, P.; Horng, S.; Feldman, R. A Novel Method to Measure Venular Perivascular Spaces in Patients with MS on 7T MRI. AJNR Am. J. Neuroradiol. 2021, 42, 1069–1072.
- Jochems, A.C.C.; Blair, G.W.; Stringer, M.S.; Thrippleton, M.J.; Clancy, U.; Chappell, F.M.; Brown, R.; Jaime Garcia, D.; Hamilton, O.K.L.; Morgan, A.G.; et al. Relationship Between Venules and Perivascular Spaces in Sporadic Small Vessel Diseases. Stroke 2020, 51, 1503–1506.
- Brown, W.R.; Moody, D.M.; Challa, V.R.; Thore, C.R.; Anstrom, J.A. Venous Collagenosis and Arteriolar Tortuosity in Leukoaraiosis. J. Neurol. Sci. 2002, 203–204, 159–163.
- Barisano, G.; Lynch, K.M.; Sibilia, F.; Lan, H.; Shih, N.-C.; Sepehrband, F.; Choupan, J. Imaging Perivascular Space Structure and Function Using Brain MRI. NeuroImage 2022, 257, 119329.
- Montagne, A.; Nikolakopoulou, A.M.; Zhao, Z.; Sagare, A.P.; Si, G.; Lazic, D.; Barnes, S.R.; Daianu, M.; Ramanathan, A.; Go, A.; et al. Pericyte Degeneration Causes White Matter Dysfunction in the Mouse Central Nervous System. Nat. Med. 2018, 24, 326–337.
- Perosa, V.; Oltmer, J.; Munting, L.P.; Freeze, W.M.; Auger, C.A.; Scherlek, A.A.; van der Kouwe, A.J.; Iglesias, J.E.; Atzeni, A.; Bacskai, B.J.; et al. Perivascular Space Dilation Is Associated with Vascular Amyloid-β Accumulation in the Overlying Cortex. Acta Neuropathol. 2022, 143, 331–348.
- Oztürk, M.H.; Aydingöz, U. Comparison of MR Signal Intensities of Cerebral Perivascular (Virchow-Robin) and Subarachnoid Spaces. J. Comput. Assist. Tomogr. 2002, 26, 902–904.
- Naganawa, S.; Nakane, T.; Kawai, H.; Taoka, T. Differences in Signal Intensity and Enhancement on MR Images of the Perivascular Spaces in the Basal Ganglia versus Those in White Matter. Magn. Reson. Med. Sci. MRMS Off. J. Jpn. Soc. Magn. Reson. Med. 2018, 17, 301–307.
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging Standards for Research into Small Vessel Disease and Its Contribution to Ageing and Neurodegeneration. Lancet Neurol. 2013, 12, 822–838.
- Yu, L.; Hu, X.; Li, H.; Zhao, Y. Perivascular Spaces, Glymphatic System and MR. Front. Neurol. 2022, 13, 844938.
- Mallio, C.A.; Quattrocchi, C.C.; Rovira, À.; Parizel, P.M. Gadolinium Deposition Safety: Seeking the Patient’s Perspective. AJNR Am. J. Neuroradiol. 2020, 41, 944–946.
- Parillo, M.; Mallio, C.A.; Van der Molen, A.J.; Rovira, À.; Ramalho, J.; Ramalho, M.; Gianolio, E.; Karst, U.; Radbruch, A.; Stroomberg, G.; et al. Skin Toxicity after Exposure to Gadolinium-Based Contrast Agents in Normal Renal Function, Using Clinical Approved Doses: Current Status of Preclinical and Clinical Studies. Investig. Radiol. 2023, 58, 530–538.
- Barisano, G.; Sheikh-Bahaei, N.; Law, M.; Toga, A.W.; Sepehrband, F. Body Mass Index, Time of Day and Genetics Affect Perivascular Spaces in the White Matter. J. Cereb. Blood Flow. Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2021, 41, 1563–1578.
- Rudie, J.D.; Rauschecker, A.M.; Nabavizadeh, S.A.; Mohan, S. Neuroimaging of Dilated Perivascular Spaces: From Benign and Pathologic Causes to Mimics. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2018, 28, 139–149.
- Papayannis, C.E.; Saidon, P.; Rugilo, C.A.; Hess, D.; Rodriguez, G.; Sica, R.E.P.; Rey, R.C. Expanding Virchow Robin Spaces in the Midbrain Causing Hydrocephalus. AJNR Am. J. Neuroradiol. 2003, 24, 1399–1403.
- Loh, D.D.L.; Swaminathan, S.K. Tumefactive Perivascular Spaces Causing Obstructive Hydrocephalus. Radiology 2023, 307, e221724.
- Kwee, R.M.; Kwee, T.C. Tumefactive Virchow-Robin Spaces. Eur. J. Radiol. 2019, 111, 21–33.
- Cheraya, G.; Bajaj, S.; Sharma, S.; Gandhi, D.; Shah, J.; Parikh, N.; Gupta, N. Giant Perivascular Spaces in Brain: Case Report with a Comprehensive Literature Review. Precis. Cancer Med. 2022, 5, 29.
- Lakhani, D.A.; Joseph, J. Giant Tumefactive Perivascular Spaces. Radiology 2023, 307, 222559.
- Rawal, S.; Croul, S.E.; Willinsky, R.A.; Tymianski, M.; Krings, T. Subcortical Cystic Lesions within the Anterior Superior Temporal Gyrus: A Newly Recognized Characteristic Location for Dilated Perivascular Spaces. AJNR Am. J. Neuroradiol. 2014, 35, 317–322.
- Lim, A.T.; Chandra, R.V.; Trost, N.M.; McKelvie, P.A.; Stuckey, S.L. Large Anterior Temporal Virchow-Robin Spaces: Unique MR Imaging Features. Neuroradiology 2015, 57, 491–499.
- Potter, G.M.; Chappell, F.M.; Morris, Z.; Wardlaw, J.M. Cerebral Perivascular Spaces Visible on Magnetic Resonance Imaging: Development of a Qualitative Rating Scale and Its Observer Reliability. Cerebrovasc. Dis. Basel Switz. 2015, 39, 224–231.
- Moses, J.; Sinclair, B.; Law, M.; O’Brien, T.J.; Vivash, L. Automated Methods for Detecting and Quantitation of Enlarged Perivascular Spaces on MRI. J. Magn. Reson. Imaging JMRI 2023, 57, 11–24.
- Zong, X.; Park, S.H.; Shen, D.; Lin, W. Visualization of Perivascular Spaces in the Human Brain at 7T: Sequence Optimization and Morphology Characterization. NeuroImage 2016, 125, 895–902.
- Boespflug, E.L.; Schwartz, D.L.; Lahna, D.; Pollock, J.; Iliff, J.J.; Kaye, J.A.; Rooney, W.; Silbert, L.C. MR Imaging-Based Multimodal Autoidentification of Perivascular Spaces (mMAPS): Automated Morphologic Segmentation of Enlarged Perivascular Spaces at Clinical Field Strength. Radiology 2018, 286, 632–642.
- Francis, F.; Ballerini, L.; Wardlaw, J.M. Perivascular Spaces and Their Associations with Risk Factors, Clinical Disorders and Neuroimaging Features: A Systematic Review and Meta-Analysis. Int. J. Stroke Off. J. Int. Stroke Soc. 2019, 14, 359–371.
- Kim, H.G.; Shin, N.-Y.; Nam, Y.; Yun, E.; Yoon, U.; Lee, H.S.; Ahn, K.J. MRI-Visible Dilated Perivascular Space in the Brain by Age: The Human Connectome Project. Radiology 2023, 306, e213254.
- Piantino, J.; Boespflug, E.L.; Schwartz, D.L.; Luther, M.; Morales, A.M.; Lin, A.; Fossen, R.V.; Silbert, L.; Nagel, B.J. Characterization of MR Imaging-Visible Perivascular Spaces in the White Matter of Healthy Adolescents at 3T. AJNR Am. J. Neuroradiol. 2020, 41, 2139–2145.
- Kikuta, J.; Kamagata, K.; Takabayashi, K.; Taoka, T.; Yokota, H.; Andica, C.; Wada, A.; Someya, Y.; Tamura, Y.; Kawamori, R.; et al. An Investigation of Water Diffusivity Changes along the Perivascular Space in Elderly Subjects with Hypertension. AJNR Am. J. Neuroradiol. 2022, 43, 48–55.
- Mahammedi, A.; Wang, L.L.; Williamson, B.J.; Khatri, P.; Kissela, B.; Sawyer, R.P.; Shatz, R.; Khandwala, V.; Vagal, A. Small Vessel Disease, a Marker of Brain Health: What the Radiologist Needs to Know. AJNR Am. J. Neuroradiol. 2022, 43, 650–660.
- Cervantes-Arslanian, A.; Garcia, H.H.; Rapalino, O. Imaging of Infectious and Inflammatory Cystic Lesions of the Brain, a Narrative Review. Expert. Rev. Neurother. 2023, 23, 237–247.
- Sharma, V.; Prabhash, K.; Noronha, V.; Tandon, N.; Joshi, A. A Systematic Approach to Diagnosis of Cystic Brain Lesions. South Asian J. Cancer 2013, 2, 98–101.
- Erkinjuntti, T.; Inzitari, D.; Pantoni, L.; Wallin, A.; Scheltens, P.; Rockwood, K.; Roman, G.C.; Chui, H.; Desmond, D.W. Research Criteria for Subcortical Vascular Dementia in Clinical Trials. J. Neural Transm. Suppl. 2000, 59, 23–30.
- Robles, L.A.; Paez, J.M.; Ayala, D.; Boleaga-Duran, B. Intracranial Glioependymal (Neuroglial) Cysts: A Systematic Review. Acta Neurochir. 2018, 160, 1439–1449.
- Altafulla, J.J.; Suh, S.; Bordes, S.; Prickett, J.; Iwanaga, J.; Loukas, M.; Dumont, A.S.; Tubbs, R.S. Choroidal Fissure and Choroidal Fissure Cysts: A Comprehensive Review. Anat. Cell Biol. 2020, 53, 121–125.
- Yamashita, K.; Zong, X.; Hung, S.-C.; Lin, W.; Castillo, M. Hippocampal Sulcus Remnant: Common Finding in Nonelderly Adults on Ultra-High-Resolution 7T Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2020, 44, 43–46.
- Buffa, G.B.; Chaves, H.; Serra, M.M.; Stefanoff, N.I.; Gagliardo, A.S.; Yañez, P. Multinodular and Vacuolating Neuronal Tumor of the Cerebrum (MVNT): A Case Series and Review of the Literature. J. Neuroradiol. J. Neuroradiol. 2020, 47, 216–220.
- Luzzi, S.; Elia, A.; Del Maestro, M.; Elbabaa, S.K.; Carnevale, S.; Guerrini, F.; Caulo, M.; Morbini, P.; Galzio, R. Dysembryoplastic Neuroepithelial Tumors: What You Need to Know. World Neurosurg. 2019, 127, 255–265.
- Abergel, A.; Lacalm, A.; Massoud, M.; Massardier, J.; des Portes, V.; Guibaud, L. Expanding Porencephalic Cysts: Prenatal Imaging and Differential Diagnosis. Fetal Diagn. Ther. 2017, 41, 226–233.
- Kwee, R.M.; Kwee, T.C. Virchow-Robin Spaces at MR Imaging. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2007, 27, 1071–1086.
- Hinojosa-Rodríguez, M.; Harmony, T.; Carrillo-Prado, C.; Van Horn, J.D.; Irimia, A.; Torgerson, C.; Jacokes, Z. Clinical Neuroimaging in the Preterm Infant: Diagnosis and Prognosis. NeuroImage Clin. 2017, 16, 355–368.
- Zafeiriou, D.I.; Batzios, S.P. Brain and Spinal MR Imaging Findings in Mucopolysaccharidoses: A Review. AJNR Am. J. Neuroradiol. 2013, 34, 5–13.
- Matheus, M.G.; Castillo, M.; Smith, J.K.; Armao, D.; Towle, D.; Muenzer, J. Brain MRI Findings in Patients with Mucopolysaccharidosis Types I and II and Mild Clinical Presentation. Neuroradiology 2004, 46, 666–672.




