1. Introduction
Carbon, the building block of life and the fourth most abundant element in the universe, is one of the most indispensable materials known to humankind. In addition to the two naturally occurring allotropes, graphite and diamond, carbon is also capable of taking several remarkable physical forms on the nanoscale. One such distinct allotrope in the family of carbon nanomaterials is the fullerene. Its discovery
[1] sparked a great deal of curiosity and interest amongst the scientific community due to its unique closed, cage-like structure, composed solely from sp
2 hybridized carbon
[2][3]. Within the first few years after the discovery of buckyball C
60, the term ‘fullerene’ achieved a uniform acceptance as the general name for these carbon cages. Fullerenes can have different numbers of carbon atoms, shapes, and symmetry; hence, the largely accepted notation system
[4] is to subscript the number of carbon atoms to state the class of the fullerene. The structure of C
60, the most abundant buckyball, has been verified to consist of 12 five-membered rings and 20 six-membered rings
[2]. Most of the fullerenes obey the isolated pentagon rule (IPR), where pentagons are exclusively surrounded by hexagons, which increases their stability. However, several fullerenes, especially a number of endohedral fullerenes (see next section), do not follow the IPR rule
[5]. Instead they follow the maximum pentagon separation rule, where they exhibit maximum pentagon separation for maximum stability
[6].
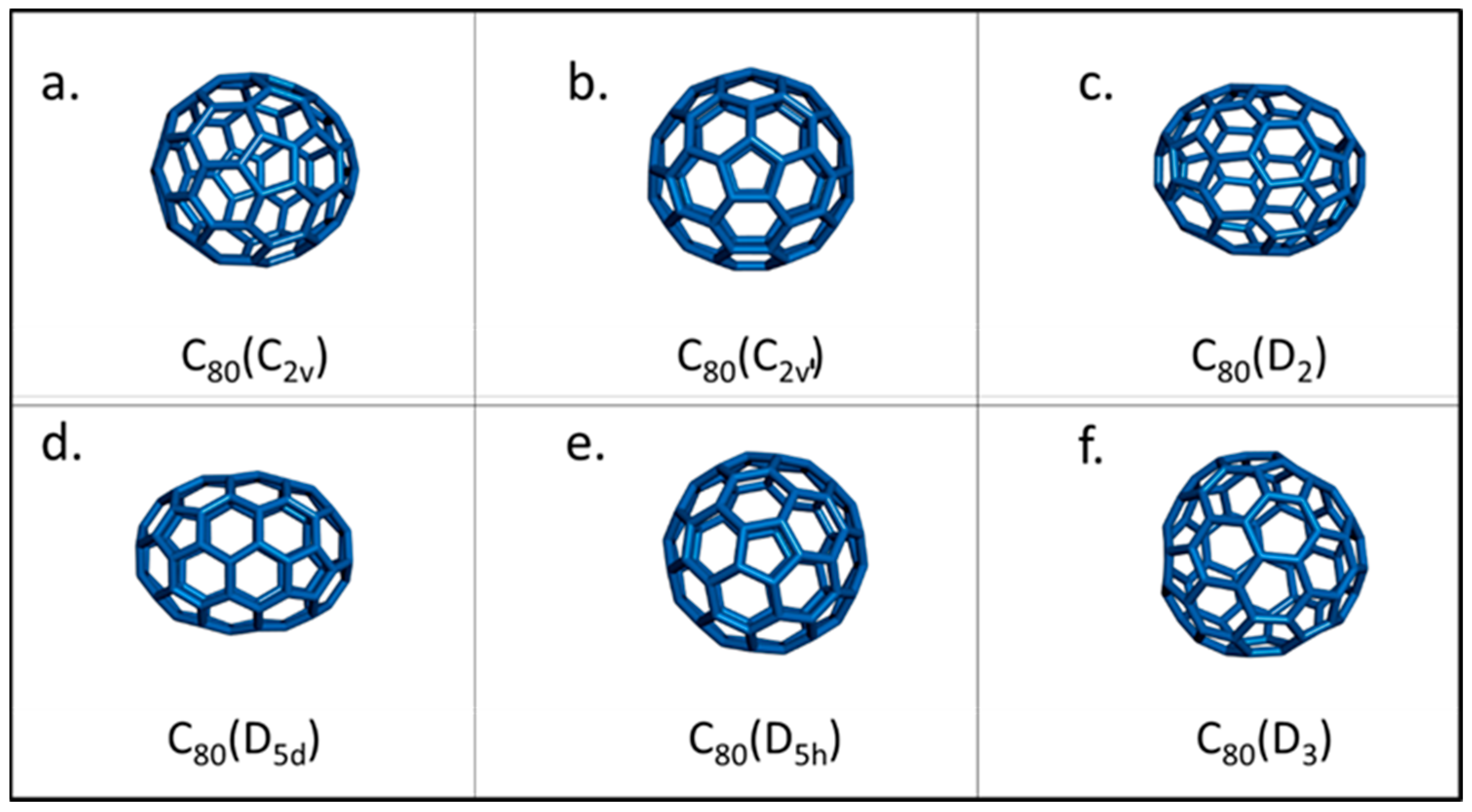
Fullerenes are known to have different isomers, with C
60 having 1812 theoretically different isomers
[7][8]. These isomers can have different symmetry, form, and energetic stability. An example of the different structures of the six most stable isomers of C
80 is shown in
Figure 1, with D
5d and D
2 being the most stable and abundantly found isomers
[9]. Fullerene isomers are also known to have different oxidation potential and chemical reactivity. For instance, the D
3 isomer of C
78 is easier to oxidize than the C
2v isomer of the same fullerene by about 0.25 V
[10]. Another very intriguing feature of the fullerenes is that they have a hollow space, which is large enough to encapsulate other atoms, ions, molecules, and various kinds of clusters, giving rise to a new class of hybrid materials called endohedral fullerenes.
Figure 1. (a–f) Schematic representation of the different isomers of the C80 cage.
2. Structure
The ability of different sizes of fullerene cages to incorporate different types of metals and clusters can lead to the formation of materials with novel solid-state properties. The robust nature of the carbon cage plays a very important role in isolating the atoms from the exterior environment, while the electronic properties of the cage can be tuned by the selection of the atoms inside. La@C
82 was among the first discovered endohedral fullerene, where the symbol @ denotes the inclusion of a La atom inside a C
82 cage. Since then, fullerenes encapsulating metallic
[11], carbide
[12][13], nitride
[14], and many methano-
[15] and oxide-
[16] clusters have been synthesized and isolated over the years. In fact, fullerenes containing single atoms of elements that otherwise are not stable, such as noble gases
[17][18][19] and non-metals
[20] such as nitrogen
[21], have also been synthesized. Transition metals and actinides were amongst the challenging elements to encapsulate within fullerenes, primarily due to their reactivity and the difficulties associated with their incorporation into the graphite rod for arc-discharge synthesis. However, over the past decade, significant progress has been made in also encapsulating various transition metals within fullerenes.
[22] In these cases, while describing the diversity of the fullerene cages, prefixes and cage symmetries are also used. For instance, Sc
4(μ
3-O)
3@
Ih(7)-C
80 represents the endohedral cluster group, cage structure, and point-group symmetry, following the nomenclature of Fowler and Manolopoulos
[4].
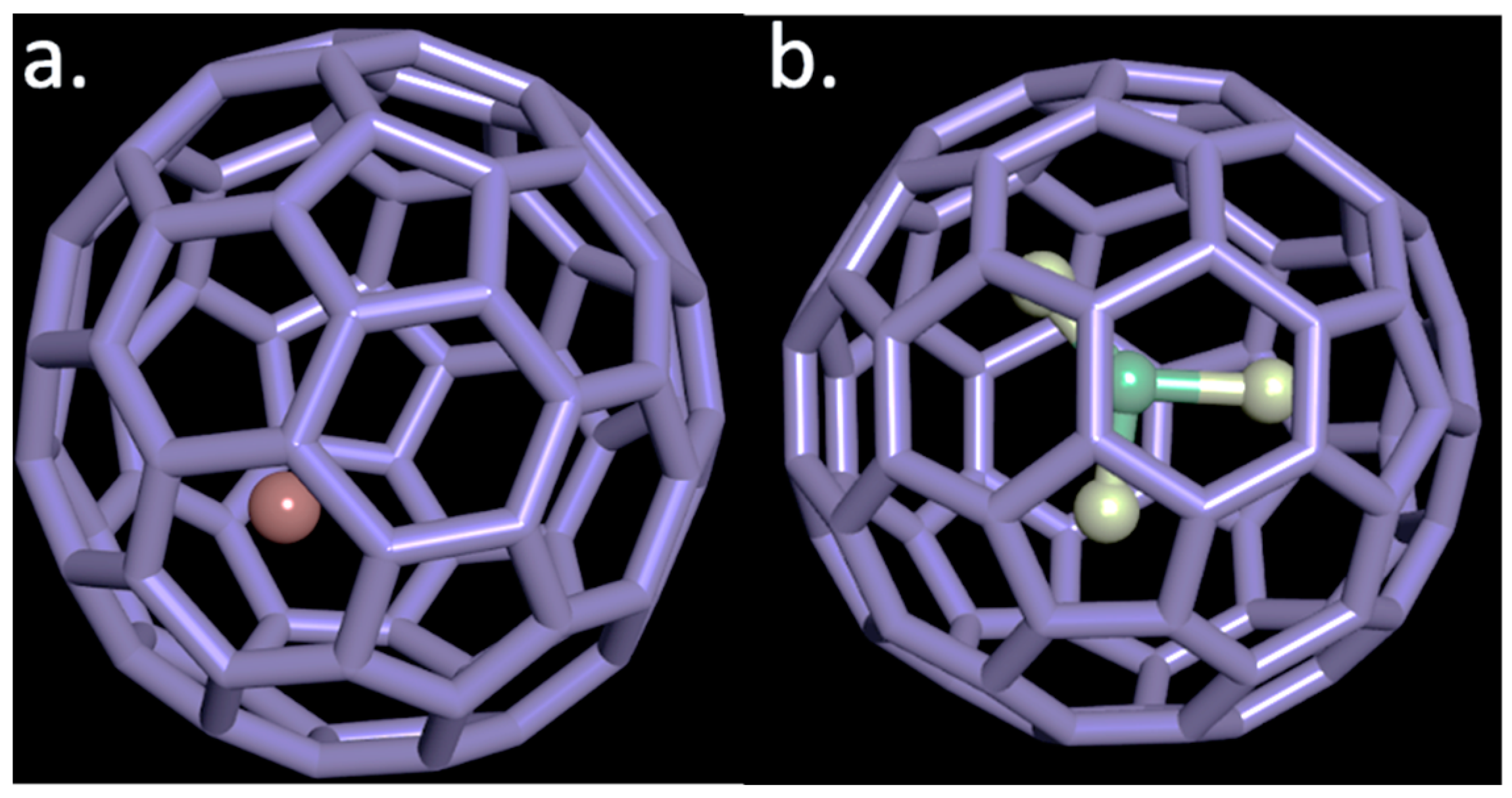
In 1991, researchers succeeded in encapsulating metal atoms, which opened a complete new set of possibilities in the field of material development, generally referred to as endohedral metallofullerenes
[23]. Among the endohedral fullerenes, the trimetallic nitride-containing cages of the type M
3N@C
2n (where M = Sc, Gd; 2n = 68–96), shown in
Figure 2, have been one of the most studied materials since their discovery in 1999
[14] due to their high yields during synthesis and their appropriate HOMO-LUMO levels, desired for applications in solar cells. However, despite the comparable high yields, empty fullerenes still constituted as major product during the synthesis process. The yields of M
3N@C
2n were further improved by Dunsch and Yang
[24], who introduced ammonia during fullerene synthesis and produced an endohedral fullerene, Sc
3N@
Ih-C
80, as the majority product for the first time. This family of fullerenes has an especially high yield because of the charge transfer from the encaged cluster, which results in a stable ion pair and stabilizes the higher fullerene cages of various isomeric structures. In addition, the large HOMO-LUMO gaps give further kinetic stability to the hybrid molecule.
Figure 2. A representative figure of two endohedral metallofullerenes, (a) monometallic in a C82 cage and (b) trimetallic nitride encapsulated in a C80 fullerene cage. The red and the yellow balls correspond to the metal ion encapsulated in the carbon cage and the green to nitrogen.
2.1. Endohedral Metallofullerenes: Endohedral Metallofullerenes
In the years following the discovery of C
60, the study of empty cage fullerenes heavily dominated the field of nanomaterials. The focus on empty cage buckyballs often resulted in the blind assumption that endohedral metallofullerenes (denoted as EMFs) possessed the same structural or chemical properties as the corresponding empty cages. However, over the years, after more studies were conducted on individual EMFs, it became established that they exhibit novel structural, chemical, and electronic properties, which were completely different to those of their parent empty cages. For instance, carbon cages of EMFs have different isomeric structures as compared to their parent fullerene cage, and they also show different solubility in individual solvents
[25]. As for their electronic and chemical behaviour, their chemical reactions with radicals
[26] and many other cyclo-addition
[27] reactions show significantly different patterns and redox potentials
[28]. For instance, the reduction potentials of La@C
82 and La
2@C
80 are 0.70 V and 0.81 V lower than that of C
60 [29]. The reason for such a huge difference is largely attributed to the covalent interaction between the enclosed species and the carbon cage as well as the charge transfer phenomenon from the metallic species to the fullerene cage.
The most studied metallofullerenes involve rare earth metals, such as La, Sc, Dy, and Tm
[23][30][31][32][33][34][35], and main group metals such as Li and Ca
[36]. There is an extensive array of EMFs that have one, two, three, or even four metal atoms trapped inside them
[37]. Alternatively, they can also contain M
3N
[14] or M
2C
2 units
[12], where M refers to metals. The key to understanding the electronic properties of these hybrid materials is through studying the degree of charge transfer from the metal species to the exterior cage, which alters its electronic state. While the number of electrons transferred from a majority of metal atoms to the cage is known and verified using X-ray diffraction, absorption, and other spectroscopic methods
[31][32][33][34][35], researchers observed that contradictory results still exist in the literature regarding the effect of the various parameters on the arc synthesis and the formation mechanism of these fullerenes. For instance, Dunk et al. claimed that the formation mechanism is governed by a charge transfer between the metal and the cage
[38]. Through detailed mechanistic studies, they revealed that the C
2 insertion during the bottom-up synthesis becomes more difficult as the oxidation state of the metal increases. This charge transfer and the nature of the cluster inside the cage also dictates a series of properties, such as electrochemical behaviour and light emission. In fact, Ito et al. studied Er
2@C
82 and Er
2C
2@C
82, which showed nearly identical cage properties with a 6- negatively charged C
826−, using fluorescence spectroscopy
[39]. Most notably, this cage charge contradicted an earlier publication by Wang et al. claiming that the Er
2@C
82 and Er
2C
2@C
82 compounds have a 4- negatively charged cage, i.e., C
824− [40]. Furthermore, Ito et al. showed that the characteristic emission of the erbium ions at 1520 nm was influenced by the symmetry of the cage, an observation later echoed by Plant et al.
[41] Until as recently as 2020, previously unknown factors, such as enantiomers and enthalpy, were discovered to play vital and evolving roles in the synthesis mechanism for fullerenes C
2n (50 ≤ 2n ≤ 70)
[42]. After a critical analysis of research articles
[43][44], it can be concluded that the complete mechanism of their formation, either through a bottom-up or top-down approach, and the charge transfer itself remains elusive and highly controversial
[45]. In fact, the insights derived from experimental results have also been contradictory to theoretical results in the field. For instance, it has been suggested that factors such as the C
2 concentration and temperature may dictate the more favourable direction of the reversible C
2 injection/ejection reaction during the synthetic process
[46]. However, theoretical studies have highlighted that the C
2 injection is more energetically favourable than its C
2 ejection counterpart, contradicting the experimental data on the importance of temperature and concentration
[47][48]. The missing key links and low synthesis yield of fullerenes make it difficult to investigate the fullerene formation mechanism. Utilizing both experimental and theoretical frameworks is therefore necessary to answer some of the fundamental questions of this field.
As briefly mentioned in the previous section, the isomeric structures of the carbon cages encapsulating metals differ from their empty counterparts
[34]. Further study of the positions and movements of the encapsulated atoms is also important in determining the chemical and physical properties of EMFs, including their magnetic behaviour, electron paramagnetic resonance (EPR), and electrochemical properties
[49]. The structure of the EMFs themselves had long been speculated, but the first crucial evidence came through in 1995 when Shinohara et al. experimentally confirmed that the metal atom is indeed encapsulated within the fullerene cage
[31]. The synchrotron X-ray powder diffraction patterns of Y@C
82 and the maximum entropy method (MEM) revealed that the yttrium atom was displaced from the centre and instead tightly bound to the cage
[50]. The study also confirmed strong dipole–dipole and charge transfer interaction between the different molecules
[49]. MEM/Rietveld analysis has long been used to determine the structures of metallofullerenes
[31][50]. However, the results (structure and position of metals inside the carbon cage) obtained by this method have been in question in recent years. This started when Wang et al.
[12] discovered that the scandium carbide endohedral fullerene, Sc
3C
2@C
80, was, in fact, what had long been considered to be a tri-metallofullerene Sc
3@C
82 [51]. These incoherences emphasize the importance of
13C NMR and synchrotron X-ray structural analysis in determining the structures and encapsulated cluster inside the fullerene cage and suggest the unreliability of the decades of results obtained exclusively using MEM/Rietveld analysis when determining the structures of metallofullerenes
[52][53][54]. Consequently, the structures and positions of metals inside a fullerene have been re-examined in the past few years
[55][56][57][58]. Furthermore, it was also observed that these metal clusters had motions and trajectories inside the cage
[59], which also changed positions depending on the temperature
[60][61].
2.2. Environmental Application
A massive number of publications in fullerene-related research show the possibility of using fullerenes for a variety of environmental and energy applications. The characteristics of fullerenes that give them an advantage over other materials include their robust carbon structure, absorption of light in the visible range, electron-accepting capabilities, and more recently, superconductivity
[62]. All these properties make fullerenes especially sought-after materials for energy applications, especially organic solar cells
[63]. They have become, and remain, an important part of high-performance organic solar cells.
2.3. Synthesis
Over the past few decades, EMFs have been proposed for a number of applications, including energy harvesting in organic solar cells
[64] and MRI contrast agents
[65][66][67]. EMFs have been synthesized through a range of different methodologies, such as laser ablation of graphite
[23][68], arc discharge
[30][33], ion bombardment
[36][69], resistive heating
[70], and electron initiation inside carbon nanotubes (CNTs) within aberration-corrected high-resolution transmission electron spectroscopy (AC-HRTEM) (CNT acts as a nanoreactor container to facilitate the formation of endohedral fullerenes from metals and the amorphous carbon atoms trapped inside. The process is energetically enabled under the presence of an electron beam inside an AC-HRTEM.)
[71]. Some of these popular methods are summarized in
Table 1 below.
The Kratschmer–Huffman arc method has been widely used and accepted as the most efficient and cost-effective technique to produce EMFs.
While the Kratschmer–Huffman method immediately became the most popular choice of EMF synthesis, it was not exempt from certain limitations. Most notably, only 60–70% of the graphite electrode was transferred to the carbon condensate (CC) containing the fullerenes and EMF, while the remaining 30–40% was lost as graphite build-up on the cathode. This disadvantage has now been eliminated by utilizing a high frequency arc discharge to supply the arc. The symmetrical setup of the electrodes allowed reaching up to a 100% conversion of the electrode material into the fullerenes and EMF containing CC
[74]. Additionally, high-frequency arc discharge can also be used for further mechanistic investigation of fullerene synthesis.
A metal oxide/graphite composite is used as the anode, whereas graphite is used as the cathode, and both of them are arced in the D.C. mode in the presence of an inert gas (usually helium). Despite three decades of extensive research and various proposed pathways, a well-established formation mechanism for the synthesis of fullerenes and EMFs is still missing. Their ambiguous reaction pathways, alongside the violent conditions involving the plasma formation, arc, and chemical ionization that are required for the synthesis, are a major drawback in studying the synthesis mechanisms of these unique carbon nanostructures. Furthermore, a few other drawbacks hinder the scaling-up of their synthesis and consequently the applications of endohedral metallofullerenes. The overall yield of the arc-discharge synthesis of EMFs is low, with the ratio of EMF-to-empty-cage typically at around 1%
[49]. As a result, a mixture of empty cage and endohedral fullerenes is typically produced, thus limiting the applications of the existing chromatographic methodologies for their fast and efficient separation. This results in the use of excessive amounts of solvents to efficiently separate them, which is neither ecological nor environmentally friendly. Therefore, it becomes important to review the synthesis of fullerenes. Shinohara published the first detailed scientific review of EMF research in 2000
[75], followed by an extensive update on endohedral fullerenes by Popov et al. in 2012
[76]. Many other groups have addressed the synthesis and properties of endohedral fullerenes
[76][77][78][79], including nitride fullerenes
[24], carbide cluster fullerenes
[80], and oxide cluster fullerenes. Herein, researchers focus primarily on critically discussing the key findings in the field for optimizing of synthesis parameters of endohedral metallofullerenes and the new chemically activated method, with a particular emphasis on the post-2000 science along with comparisons to the early methodologies. The yield of the EMF has been observed to be highly dependent on the pressure of He gas, the arc current, the arc gap, and the composition of anodes, among other factors
[51][81]. Liu
[82] and Bandow
[83] have argued that other parameters, such as the presence of catalysts (Cu, Fe, etc.), the back-burning of anodes, and in situ activation during arc discharge, also have an effect on the EMF yield. However, the latter two parameters would not increase the yield by a huge fraction because they do not modify the arc conditions drastically or change/influence the synthesis chemistry, e.g., lower the activation energy of the formation of the endohedral metallofullerenes. However, in order to maximize the efficiency of the method, a balance between the time spent during the synthesis and increase in yield needs to be established. Predominant aspects of overcoming the above challenge are anode composition, ionization potential, catalysts, and helium pressure in synthesis
[74].
In recent years, the Kratschmer–Huffman arc design has also been modified mechanically in several ways to make it more environmentally friendly. For instance, the use of demineralized coal electrodes, minimizing the use of current and using direct current instead of high alternating current are a few ways in which the process has been made more efficient and less energy intensive
[84]. Recently, Kyesmen et al. showed scaled-up production yield of fullerenes by employing such methods and including efficient energy usage by using resistive heating of one of the electrodes along its length
[85]. Such green methodologies should be further explored for future research purposes and industrial-scale production of fullerenes to ensure an environment friendly approach towards using nanomaterials.