Fullerenes have a unique structure, capable of both encapsulating other molecules and reacting with those on the exterior surface. Fullerene derivatives have also been found to have enormous potential to address the challenges of the renewable energy sector and current environmental issues, such as in the production of n-type materials in bulk heterojunction solar cells, as antimicrobial agents, in photocatalytic water treatment processes, and in sensor technologies. Endohedral metallofullerenes, in particular, can possess unpaired electron spins, driven by the enclosed metal atom or cluster, which yield valuable magnetic properties. These properties have significant potential for applications in molecular magnets, spin probes, quantum computing, and devices such as quantum information processing,, atomic clocks, and molecular magnets.
- endohedral metallofullerenes
- EMFs
- arc discharge
- bottom-up synthesis
- in situ doping
1. Introduction
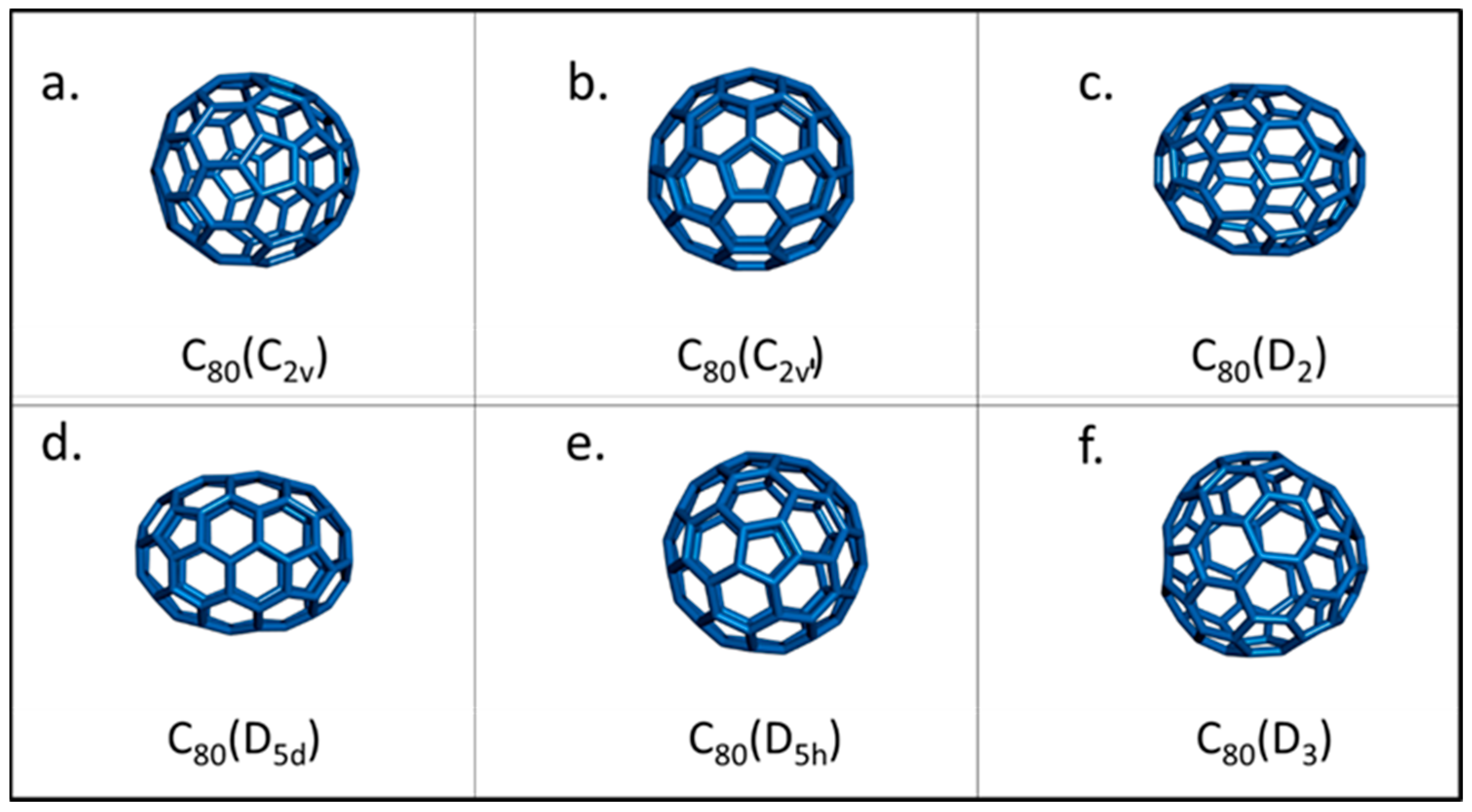
2. Structure
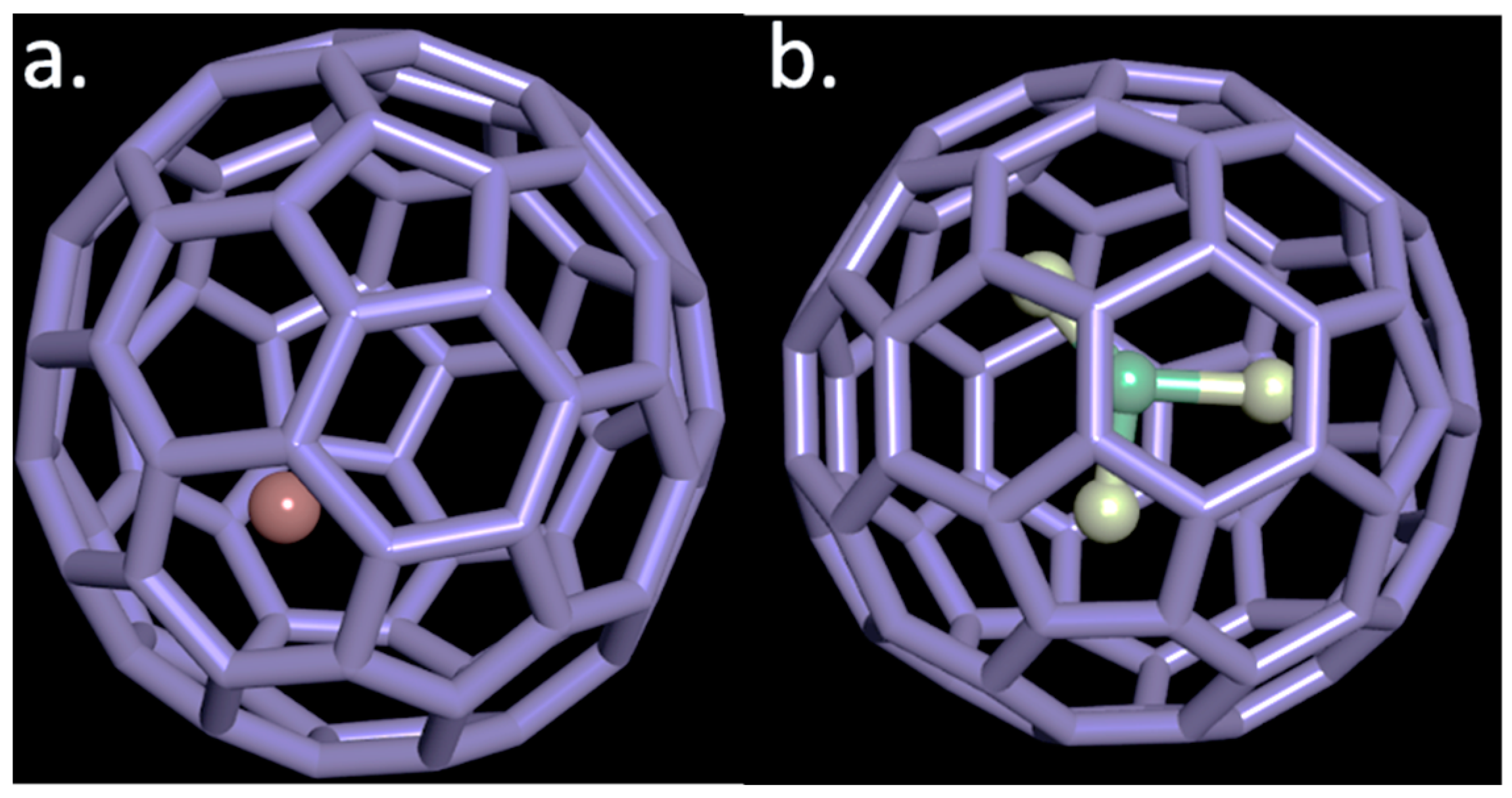
2.1. Endohedral Metallofullerenes: Endohedral Metallofullerenes
2.2. Environmental Application
2.3. Synthesis
| EMF Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Arc discharge [72] |
|
|
| Laser ablation [72] |
|
|
| Molecular surgery [72][73] |
|
|
| Ion implantation [72] |
|
|
This entry is adapted from the peer-reviewed paper 10.3390/inorganics12020038
References
- Kroto, H.W.; Heath, J.R.; O Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163.
- Hawkins, J.M.; Meyer, A.; Lewis, T.A.; Loren, S.; Hollander, F.J. Crystal Structure of Osmylated C60: Confirmation of the Soccer Ball Framework. Science 1991, 252, 312–313.
- Wudl, F. The chemical properties of buckminsterfullerene (C60) and the birth and infancy of fulleroids. Accounts Chem. Res. 1992, 25, 157–161.
- Akasaka, T.; Lu, X. Structural and electronic properties of endohedral metallofullerenes. Chem. Rec. 2012, 12, 256–269.
- Kobayashi, K.; Nagase, S. A stable unconventional structure of Sc2@C66 found by density functional calculations. Chem. Phys. Lett. 2002, 362, 373–379.
- Rodríguez-Fortea, A.; Alegret, N.; Balch, A.L.; Poblet, J.M. The maximum pentagon separation rule provides a guideline for the structures of endohedral metallofullerenes. Nat. Chem. 2010, 2, 55–961.
- Schwerdtfeger, P.; Wirz, L.N.; Avery, J. The topology of fullerenes. WIREs Comput. Mol. Sci. 2015, 5, 96–145.
- Sure, R.; Hansen, A.; Schwerdtfeger, P.; Grimme, S. Comprehensive theoretical study of all 1812 C60 isomers. Phys. Chem. Chem. Phys. 2017, 19, 14296–14305.
- Rios, C.; Salcedo, R. Computational Termochemistry Study of the C80 Isomers and Their Endo Lanthanum Complexes by Applying Homodesmotic and Isodesmic Reactions. Molecules 2012, 17, 14588–14601.
- Yang, Y.; Arias, F.; Echegoyen, L.; Chibante, L.P.F.; Flanagan, S.; Robertson, A.; Wilson, L.J. Reversible Fullerene Electrochemistry: Correlation with the HOMO-LUMO Energy Difference for C60, C70, C76, C78, and C84. J. Am. Chem. Soc. 1995, 117, 7801–7804.
- Takata, M.; Nishibori, E.; Sakata, M.; Inakuma, M.; Yamamoto, E.; Shinohara, H. Triangle Scandium Cluster Imprisoned in a Fullerene Cage. Phys. Rev. Lett. 1999, 83, 2214–2217.
- Wang, C.R.; Kai, T.; Tomiyama, T.; Yoshida, T.; Kobayashi, Y.; Nishibori, E.; Shinohara, H. A Scandium Carbide Endohedral Metallofullerene: (Sc2C2)@C84. Angew. Chem. Int. Ed. 2001, 40, 397–399.
- Wang, T.-S.; Chen, N.; Xiang, J.-F.; Li, B.; Wu, J.-Y.; Xu, W.; Jiang, L.; Tan, K.; Shu, C.-Y.; Lu, X.; et al. Russian-Doll-Type Metal Carbide Endofullerene: Synthesis, Isolation, and Characterization of Sc4C2@C80. J. Am. Chem. Soc. 2009, 131, 16646–16647.
- Stevenson, S.; Rice, G.; Glass, T.; Harich, K.; Cromer, F.; Jordan, M.R.; Craft, J.; Hadju, E.; Bible, R.; Olmstead, M.M.; et al. Small-bandgap endohedral metallofullerenes in high yield and purity. Nature 1999, 401, 55–57.
- Krause, M.; Ziegs, F.; Popov, A.A.; Dunsch, L. Entrapped Bonded Hydrogen in a Fullerene: The Five-Atom Cluster Sc 3 CH in C 80. ChemPhysChem 2007, 8, 537–540.
- Stevenson, S.; Mackey, M.A.; Stuart, M.A.; Phillips, J.P.; Easterling, M.L.; Chancellor, C.J.; Olmstead, M.M.; Balch, A.L. A Distorted Tetrahedral Metal Oxide Cluster inside an Icosahedral Carbon Cage. Synthesis, Isolation, and Structural Characterization of Sc4(μ3-O)2@Ih-C80. J. Am. Chem. Soc. 2008, 130, 11844–11845.
- Syamala, M.S.; Cross, R.J.; Saunders, M. 129Xe NMR Spectrum of Xenon Inside C60. J. Am. Chem. Soc. 2002, 124, 6216–6219.
- Saunders, M.; Jiménez-Vázquez, H.A.; Cross, R.J.; Poreda, R.J. Stable Compounds of Helium and Neon: He@C60 and Ne@C60. Science 1993, 259, 1428–1430.
- Saunders, M.; Jimenez-Vazquez, H.A.; Cross, R.J.; Mroczkowski, S.; Gross, M.L.; Giblin, D.E.; Poreda, R.J. Incorporation of helium, neon, argon, krypton, and xenon into fullerenes using high pressure. J. Am. Chem. Soc. 1994, 116, 2193–2194.
- Larsson, J.A.; Greer, J.C.; Harneit, W.; Weidinger, A. Phosphorous trapped within buckminsterfullerene. J. Chem. Phys. 2002, 116, 7849–7854.
- Mauser, H.; Hirsch, A.; van Eikema Hommes, N.J.; Clark, T.; Pietzak, B.; Weidinger, A.; Dunsch, L. Stabilization of Atomic Nitrogen Inside C60. Angew. Chem. Int. Ed. Engl. 1997, 36, 2835–2838.
- Cai, W.; Zhang, M.; Echegoyen, L.; Lu, X. Recent Advances in Endohedral Metallofullerenes. Fundam. Res. 2023; in press.
- Chai, Y.; Guo, T.; Jin, C.; Haufler, R.E.; Chibante, L.P.F.; Fure, J.; Wang, L.; Alford, J.M.; Smalley, R.E. Fullerenes with metals inside. J. Phys. Chem. 1991, 95, 7564–7568.
- Dunsch, L.; Yang, S. Metal Nitride Cluster Fullerenes: Their Current State and Future Prospects. Small 2007, 3, 1298–1320.
- Tsuchiya, T.; Wakahara, T.; Lian, Y.; Maeda, Y.; Akasaka, T.; Kato, T.; Mizorogi, N.; Nagase, S. Selective Extraction and Purification of Endohedral Metallofullerene from Carbon Soot. J. Phys. Chem. B 2006, 110, 22517–22520.
- Sato, S.; Maeda, Y.; Guo, J.D.; Yamada, M.; Mizorogi, N.; Nagase, S.; Akasaka, T. Mechanistic Study of the Diels–Alder Reaction of Paramagnetic Endohedral Metallofullerene: Reaction of La@C82 with 1,2,3,4,5-Pentamethylcyclopentadiene. J. Am. Chem. Soc. 2013, 135, 5582–5587.
- Bickelhaupt, F.M.; Solà, M.; Fernández, I. Understanding the Reactivity of Endohedral Metallofullerenes: C78 versus Sc3N@C78. Chem. A Eur. J. 2015, 21, 5760–5768.
- Yutaka, M.; Akasaka, T.; Nagase, S. Advances in Carbon Nanomaterials; Jenny Stanford Publishing: Sigapore, 2012; Volume 269.
- Suzuki, T.; Maruyama, Y.; Kato, T.; Kikuchi, K.; Achiba, Y. Electrochemical properties of La@C82. J. Am. Chem. Soc. 1993, 115, 11006–11007.
- Kikuchi, K.; Suzuki, S.; Nakao, Y.; Nakahara, N.; Wakabayashi, T.; Shiromaru, H.; Saito, K.; Ikemoto, I.; Achiba, Y. Isolation and characterization of the metallofullerene LaC82. Chem. Phys. Lett. 1993, 216, 67–71.
- Takata, M.; Umeda, B.; Nishibori, E.; Sakata, M.; Saitot, Y.; Ohno, M.; Shinohara, H. Confirmation by X-ray diffraction of the endohedral nature of the metallofullerene Y@C82. Nature 1995, 377, 46–49.
- Yannoni, C.S.; Hoinkis, M.; de Vries, M.S.; Bethune, D.S.; Salem, J.R.; Crowder, M.S.; Johnson, R.D. Scandium Clusters in Fullerene Cages. Science 1992, 256, 1191–1192.
- Johnson, R.D.; de Vries, M.S.; Salem, J.; Bethune, D.S.; Yannoni, C.S. Electron paramagnetic resonance studies of lanthanum-containing C82. Nature 1992, 355, 239–240.
- Kirbach, U.; Dunsch, L. The Existence of Stable Tm@C82 Isomers. Angew. Chem. Int. Ed. Engl. 1996, 35, 2380–2383.
- Shinohara, H.; Sato, H.; Ohkohchi, M.; Ando, Y.; Kodama, T.; Shida, T.; Kato, T.; Saito, Y. Encapsulation of a scandium trimer in C82. Nature 1992, 357, 52–54.
- Campbell, E.E.; Fanti, M.; Hertel, I.V.; Mitzner, R.; Zerbetto, F. The hyperpolarisability of an endohedral fullerene: Li@C60. Chem. Phys. Lett. 1998, 288, 131–137.
- Deng, Q.; Junghans, K.; Popov, A.A. Carbide clusterfullerenes with odd number of carbon atoms: Molecular and electronic structures of Sc4C@C80, Sc4C@C82, and Sc4C3@C80. Theor. Chem. Accounts 2015, 134, 10.
- Dunk, P.W.; Mulet-Gas, M.; Nakanishi, Y.; Kaiser, N.K.; Rodríguez-Fortea, A.; Shinohara, H.; Poblet, J.M.; Marshall, A.G.; Kroto, H.W. Bottom-up formation of endohedral mono-metallofullerenes is directed by charge transfer. Nat. Commun. 2014, 5, 5844.
- Ito, Y.; Okazaki, T.; Okubo, S.; Akachi, M.; Ohno, Y.; Mizutani, T.; Shinohara, H. Enhanced 1520 nm Photoluminescence from Er3+ Ions in Di-erbium-carbide Metallofullerenes (Er2C2)@C82 (Isomers I, II, and III). ACS Nano 2007, 1, 456–462.
- Wang, J.; Zhao, Y.; Lee, P.-H.; Irle, S. Er3+ Photoluminescence in Er2@C82 and Er2C2@C82 Metallofullerenes Elucidated by Density Functional Theory. Inorg. Chem. 2017, 56, 6576–6583.
- Plant, S.R.; Dantelle, G.; Ito, Y.; Ng, T.C.; Ardavan, A.; Shinohara, H.; Porfyrakis, K. Acuminated fluorescence of Er3+ centres in endohedral fullerenes through the incarceration of a carbide cluster. Chem. Phys. Lett. 2009, 476, 41–45.
- Li, M.-Y.; Zhao, Y.-X.; Han, Y.-B.; Yuan, K.; Nagase, S.; Ehara, M.; Zhao, X. Theoretical Investigation of the Key Roles in Fullerene-Formation Mechanisms: Enantiomer and Enthalpy. ACS Appl. Nano Mater. 2020, 3, 547–554.
- Yang, S.; Zalibera, M.; Rapta, P.; Dunsch, L. Charge-Induced Reversible Rearrangement of Endohedral Fullerenes: Electrochemistry of Tridysprosium Nitride Clusterfullerenes Dy3N@C2n (2n =78, 80). Chem. A Eur. J. 2006, 12, 7848–7855.
- Popov, A.A.; Avdoshenko, S.M.; Cuniberti, G.; Dunsch, L. Dimerization of Radical-Anions: Nitride Clusterfullerenes versus Empty Fullerenes. J. Phys. Chem. Lett. 2011, 2, 1592–1600.
- Zhang, J.; Bowles, F.L.; Bearden, D.W.; Ray, W.K.; Fuhrer, T.; Ye, Y.; Dixon, C.; Harich, K.; Helm, R.F.; Olmstead, M.M.; et al. A missing link in the transformation from asymmetric to symmetric metallofullerene cages implies a top-down fullerene formation mechanism. Nat. Chem. 2013, 5, 880–885.
- Crichton, R.; Zhang, J. Handbook of Fullerene Science and Technology; Springer Nature: Singapore, 2022.
- Mulet-Gas, M.; Abella, L.; Dunk, P.W.; Rodríguez-Fortea, A.; Kroto, H.W.; Poblet, J.M. Small endohedral metallofullerenes: Exploration of the structure and growth mechanism in the Ti@C2n (2n = 26–50) family. Chem. Sci. 2014, 6, 675–686.
- Yao, Y.R.; Chen, Z.C.; Chen, L.; Zheng, S.Y.; Yang, S.; Deng, S.L.; Zheng, L.S. Two Metastable Endohedral Metallofullerenes Sc2C2@C1(39656)-C82 and Sc2C2@C1(51383)-C84:Direct-C2-Insertion Products from Their Most Stable Precursors. J. Am. Chem. Soc. 2023, 145, 16778–16786.
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral Metal Atoms in Pristine and Functionalized Fullerene Cages. Acc. Chem. Res. 2009, 43, 92–102.
- Takata, M. The MEM/Rietveld method with nano-applications—Accurate charge-density studies of nano-structured materials by synchrotron-radiation powder diffraction. Acta Crystallogr. A 2008, 64, 232–245.
- Shinohara, H.; Inakuma, M.; Hayashi, N.; Sato, H.; Saito, Y.; Kato, T.; Bandow, S. Spectroscopic Properties of Isolated Sc3@C82 Metallofullerene. J. Phys. Chem. 1994, 98, 8597–8599.
- Senapati, L.; Schrier, J.; Whaley, K.B. Reply to Comment on “Electronic Transport, Structure, and Energetics of Endohedral Gd@C82 Metallofullerenes”. Nano Lett. 2005, 5, 2341.
- Kobayashi, K.; Nagase, S. Theoretical study of structures and dynamic properties of Sc3@C82. Chem. Phys. Lett. 1999, 313, 45–51.
- Yamada, M.; Wakahara, T.; Lian, Y.; Tsuchiya, T.; Akasaka, T.; Waelchli, M.; Mizorogi, N.; Nagase, S.; Kadish, K.M. Analysis of Lanthanide-Induced NMR Shifts of the Ce@C82 Anion. J. Am. Chem. Soc. 2006, 128, 1400–1401.
- Guo, Y.-J.; Zheng, H.; Yang, T.; Nagase, S.; Zhao, X. Theoretical Insight into the Ambiguous Endohedral Metallofullerene Er3C74: Covalent Interactions among Three Lanthanide Atoms. Inorg. Chem. 2015, 54, 8066–8076.
- Lu, X.; Akasaka, T.; Nagase, S. Carbide Cluster Metallofullerenes: Structure, Properties, and Possible Origin. Accounts Chem. Res. 2013, 46, 1627–1635.
- Popov, A.A.; Zhang, L.; Dunsch, L. A Pseudoatom in a Cage: Trimetallofullerene Y3@C80 Mimics Y3N@C80 with Nitrogen Substituted by a Pseudoatom. ACS Nano 2010, 4, 795–802.
- Zhao, P.; Zhao, X.; Ehara, M. Theoretical Insight into Sc2C76: Carbide Clusterfullerene Sc2C2@C74 versus Dimetallofullerene Sc2@C76. Inorg. Chem. 2017, 56, 10195–10203.
- Jin, P.; Hao, C.; Li, S.; Mi, W.; Sun, Z.; Zhang, J.; Hou, Q. Theoretical Study on the Motion of a La Atom Inside a C82 Cage. J. Phys. Chem. A 2006, 111, 167–169.
- Liu, F.; Velkos, G.; Krylov, D.S.; Spree, L.; Zalibera, M.; Ray, R.; Samoylova, N.A.; Chen, C.-H.; Rosenkranz, M.; Schiemenz, S.; et al. Air-stable redox-active nanomagnets with lanthanide spins radical-bridged by a metal–metal bond. Nat. Commun. 2019, 10, 571.
- Hao, Y.; Wang, Y.; Dubrovin, V.; Avdoshenko, S.M.; Popov, A.A.; Liu, F. Caught in Phase Transition: Snapshot of the Metallofullerene Sc3N@C70 Rotation in the Crystal. J. Am. Chem. Soc. 2021, 143, 612–616.
- Speller, E.M. The significance of fullerene electron acceptors in organic solar cell photo-oxidation. Mater. Sci. Technol. 2017, 33, 924–933.
- Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Review: Fullerene based acceptors for efficient bulk heterojunction organic solar cell applications. Sol. Energy Mater. Sol. Cells 2017, 161, 102–148.
- Ross, R.B.; Cardona, C.M.; Guldi, D.M.; Sankaranarayanan, S.G.; Reese, M.O.; Kopidakis, N.; Peet, J.; Walker, B.; Bazan, G.C.; Van Keuren, E.; et al. Endohedral fullerenes for organic photovoltaic devices. Nat. Mater. 2009, 8, 208–212.
- Li, T.; Xiao, L.; Yang, J.; Ding, M.; Zhou, Z.; LaConte, L.; Jin, L.; Dorn, H.C.; Li, X. Trimetallic Nitride Endohedral Fullerenes Carboxyl-Gd3N@C80: A New Theranostic Agent for Combating Oxidative Stress and Resolving Inflammation. ACS Appl. Mater. Interfaces 2017, 9, 17681–17687.
- Lu, J.; Sabirianov, R.F.; Mei, W.N.; Gao, Y.; Duan, C.G.; Zeng, X. Structural and Magnetic Properties of Gd3N@C80. J. Phys. Chem. B 2006, 110, 23637–23640.
- Zhang, J.; Ye, Y.; Chen, Y.; Pregot, C.; Li, T.; Balasubramaniam, S.; Dorn, H.C. Gd3N@C84(OH)x: A New Egg-Shaped Metallofullerene Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc. 2014, 136, 2630–2636.
- Weaver, J.; Chai, Y.; Kroll, G.; Jin, C.; Ohno, T.; Haufler, R.; Guo, T.; Alford, J.; Conceicao, J.; Chibante, L.; et al. XPS probes of carbon-caged metals. Chem. Phys. Lett. 1992, 190, 460–464.
- Murphy, T.A.; Pawlik, T.; Weidinger, A.; Höhne, M.; Alcala, R.; Spaeth, J.M. Observation of Atomlike Nitrogen in Nitrogen-Implanted Solid C60. Phys. Rev. Lett. 1996, 77, 1075–1078.
- Funasaka, H.; Yamamoto, K.; Sakurai, K.; Ishiguro, T.; Sugiyama, K.; Takahashi, T.; Kishimoto, Y. Preparation of Fullerene Derivatives by Resistive Heating with Graphite Crucible. Full- Sci. Technol. 1993, 1, 437–448.
- Sinitsa, A.S.; Chamberlain, T.W.; Zoberbier, T.; Lebedeva, I.V.; Popov, A.M.; Knizhnik, A.A.; McSweeney, R.L.; Biskupek, J.; Kaiser, U.; Khlobystov, A.N. Formation of Nickel Clusters Wrapped in Carbon Cages: Toward New Endohedral Metallofullerene Synthesis. Nano Lett. 2017, 17, 1082–1089.
- Popov, A.A. Synthesis and Molecular Structures of Endohedral Fullerenes. In Nanostructure Science and Technology; Lockwood, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–34.
- Bloodworth, S.; Whitby, R.J. Synthesis of endohedral fullerenes by molecular surgery. Commun. Chem. 2022, 5, 121.
- Churilov, G.; Popov, A.; Vnukova, N.; Dudnik, A.; Samoylova, N.; Glushenko, G. Controlled synthesis of fullerenes and endohedral metallofullerenes in high frequency arc discharge. Full-Nanotub. Carbon Nanostructures 2016, 24, 675–678.
- Shinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 63, 843–892.
- Chen, N.; Ortiz, A.L.; Echegoyen, L. Fullerenes: Principles and Applications, 2nd ed; The Royal Society of Chemistry: London, UK, 2011; Chapter 2; pp. 12–65.
- Yang, S. Synthesis, Separation, and Molecular Structures of Endohedral Fullerenes. Curr. Org. Chem. 2012, 16, 1079–1094.
- Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012, 41, 7723–7760.
- Lu, X.; Bao, L.; Akasaka, T.; Nagase, S. Recent progress in the chemistry of endohedral metallofullerenes. Chem. Commun. 2014, 50, 14701–14715.
- Jin, P.; Tang, C.; Chen, Z. Carbon atoms trapped in cages: Metal carbide clusterfullerenes. Coord. Chem. Rev. 2014, 270–271, 89–111.
- Shinohara, H.; Yamaguchi, H.; Hayashi, N.; Sato, H.; Ohkohchi, M.; Ando, Y.; Saito, Y. Isolation and spectroscopic properties of scandium fullerenes (Sc2@C74, Sc2@C82, and Sc2@C84). J. Phys. Chem. 1993, 97, 4259–4261.
- Liu, B.-B.; Xu, W.-G.; Liu, Z.-Y.; Yang, H.-B.; Li, M.-H.; Liu, S.-Y.; Zou, G.-T. High yield synthesis and extraction of La@C2n. Solid State Commun. 1996, 97, 407–410.
- Bandow, S.; Shinohara, H.; Saito, Y.; Ohkohchi, M.; Ando, Y. High yield synthesis of lanthanofullerenes via lanthanum carbide. J. Phys. Chem. 1993, 97, 6101–6103.
- Lu, W.; Baek, J.B.; Dai, L. (Eds.) Carbon Nanomaterials for Advanced Energy Systems; Wiley: Hoboken, NJ, USA, 2015.
- Kyesmen, P.I.; Onoja, A.; Amah, A.N. Fullerenes synthesis by combined resistive heating and arc discharge techniques. SpringerPlus 2016, 5, 1323.
