
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vincenzo Summa | -- | 3266 | 2024-01-24 18:04:06 | | | |
| 2 | Lindsay Dong | Meta information modification | 3266 | 2024-01-25 01:47:36 | | |
Video Upload Options
Unripe tomatoes represent an agri-food waste resulting from industrial by-processing products of tomatoes, yielding products with a high content of bioactive compounds with potential nutraceutical properties. The food-matrix biological properties are attributed to the high steroidal glycoalkaloid (SGA) content. Among them, α-tomatine is the main SGA reported in unripe green tomatoes.
1. Introduction
2. Unripe Green Tomatoes as a Source of Glycoalkaloids
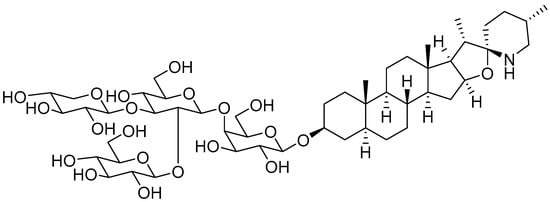
3. α-Tomatine Extraction from Green Tomatoes
4. α-Tomatine Analysis Methods
5. Nutraceutical Potential of Green Tomatoes
5.1. Antiviral, Antifungal, and Antibiotic Activity
5.2. Anti-Inflammatory Effects
5.3. Anti-Aging Effects
Green tomatoes and SGAs have shown promising anti-aging effects in many tissues, including the bones, brain, and muscles. A diet supplementation with a green tomato extract from “Korean chal tomato” (containing tomatidine in the amount of 1.06 ± 0.11 mg of tomatidine/100 g of dry weight) improved bone mineral density and overall bone quality in ovariectomizes rats, a model of postmenopausal osteoporosis [54]. The aglycone tomatidine inhibits osteoclastogenesis and reduces estrogenic deficiency-induced bone mass loss [75] through a mechanism that has not been fully elucidated, but that probably involves the modulation of the p53 and MAPK signaling pathways [76].
5.4. Anti-Tumoral Effects
5.5. Pharmacokinetics and Toxicological Aspects of Glycoalkaloids and Green Tomato Extracts
6. Conclusions
References
- Garcia-Marti, M.; Simal-Gandara, J. Chapter 5: Chemical and Biological Valorization of Tomato Waste. In Agri-Food Waste Valorisation; Royal Society of Chemistry: London, UK, 2023; Volume 78, p. 326.
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton, S.K.; Schwartz, S.J. Lycopene from Heat-Induced Cis-Isomer-Rich Tomato Sauce Is More Bioavailable than from All-Trans-Rich Tomato Sauce in Human Subjects. Br. J. Nutr. 2007, 98, 140–146.
- Carillo, P.; D’Amelia, L.; Dell’Aversana, E.; Faiella, D.; Cacace, D.; Giuliano, B.; Morrone, B. Eco-Friendly Use of Tomato Processing Residues for Lactic Acid Production in Campania. Chem. Eng. Trans. 2018, 64, 223–228.
- Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials 2021, 14, 4581.
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A.P.C. Characterization of Tomato Processing By-Product for Use as a Potential Functional Food Ingredient: Nutritional Composition, Antioxidant Activity and Bioactive Compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160.
- Maisto, M.; Annunziata, G.; Schiano, E.; Piccolo, V.; Iannuzzo, F.; Santangelo, R.; Ciampaglia, R.; Tenore, G.C.; Novellino, E.; Grieco, P. Potential Functional Snacks: Date Fruit Bars Supplemented by Different Species of Lactobacillus spp. Foods 2021, 10, 1760.
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010.
- Patel, A.H.; Sharma, H.P. Vaishali Physiological Functions, Pharmacological Aspects and Nutritional Importance of Green Tomato- a Future Food. Crit. Rev. Food Sci. Nutr. 2023, 1–23.
- El Mashad, H.M.; Zhao, L.; Zhang, R.; Pan, Z. Tomato. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–131. ISBN 9780128141397.
- Lu, S.; Chen, S.; Li, H.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; et al. Sustainable Valorization of Tomato Pomace (Lycopersicon esculentum) in Animal Nutrition: A Review. Animals 2022, 12, 3294.
- Faria-Silva, C.; de Sousa, M.; Carvalheiro, M.C.; Simões, P.; Simões, S. Alpha-Tomatine and the Two Sides of the Same Coin: An Anti-Nutritional Glycoalkaloid with Potential in Human Health. Food Chem. 2022, 391, 133261.
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 2367453.
- Periago, M.J.; Martínez-Valverde, I.; Chesson, A.; Provan, G. Phenolic Compounds, Lycopene and Antioxidant Activity in Commercial Varieties of Tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330.
- Kozukue, N.; Friedman, M. Tomatine, Chlorophyll, β-Carotene and Lycopene Content in Tomatoes during Growth and Maturation. J. Sci. Food Agric. 2003, 83, 195–200.
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of Phytochemicals Present in Tomato. J. Food Sci. Technol. 2018, 55, 2833–2849.
- Hernández-Ortega, M.; Ortiz-Moreno, A.; Hernández-Navarro, M.D.; Chamorro-Cevallos, G.; Dorantes-Alvarez, L.; Necoechea-Mondragón, H. Antioxidant, Antinociceptive, and Anti-Inflammatory Effects of Carotenoids Extracted from Dried Pepper (Capsicum annuum L.). J. Biomed Biotechnol. 2012, 2012, 524019.
- Subramoniam, A.; Asha, V.V.; Nair, S.A.; Sasidharan, S.P.; Sureshkumar, P.K.; Rajendran, K.N.; Karunagaran, D.; Ramalingam, K. Chlorophyll Revisited: Anti-Inflammatory Activities of Chlorophyll a and Inhibition of Expression of TNF-α Gene by the Same. Inflammation 2012, 35, 959–966.
- Maisto, M.; Iannuzzo, F.; Schiano, E.; Ciampaglia, R.; Labanca, A.; Montesano, D.; Piccolo, V.; Rossi, P.; Tenore, G.C. Effects of Fortified Laying Hen Diet with Moringa Oleifera Leaves and Goji Berries on Cholesterol and Carotenoid Egg Content. Foods 2022, 11, 3156.
- Zhang, Q.; Liu, D.; Cui, Y.; Xu, T.; Liu, X.; Liu, K.; Wang, Q.; Li, A.; Zhao, P.; Cheng, Z. Bioactivities and chemical profiling comparison and metabolomic variations of polyphenolics and steroidal glycoalkaloids in different parts of Solanum nigrum L. Phytochemical Analysis. 2023, 1–19.
- Fontaine, T.; Irving, G. Isolation and Partial Characterization of Crystalline Tomatine, an Antibiotic Agent from the Tomato Plant. Arch Biochem. 1948, 18, 467–475.
- Friedman, M.; Levin, C.E.; Mcdonald, G.M. α-Tomatine Determination in Tomatoes by HPLC Using Pulsed Amperometric Detection. Food Chem. 1994, 42, 1959–1964.
- Cárdenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. The Bitter Side of the Nightshades: Genomics Drives Discovery in Solanaceae Steroidal Alkaloid Metabolism. Phytochemistry 2015, 113, 24–32.
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419.
- Iijima, Y.; Watanabe, B.; Sasaki, R.; Takenaka, M.; Ono, H.; Sakurai, N.; Umemoto, N.; Suzuki, H.; Shibata, D.; Aoki, K. Steroidal Glycoalkaloid Profiling and Structures of Glycoalkaloids in Wild Tomato Fruit. Phytochemistry 2013, 95, 145–157.
- Friedman, M. Tomato Glycoalkaloids: Role in the Plant and in the Diet. J. Agric. Food Chem. 2002, 50, 5751–5780.
- Dzakovich, M.P.; Hartman, J.L.; Cooperstone, J.L. A High-Throughput Extraction and Analysis Method for Steroidal Glycoalkaloids in Tomato. Front. Plant Sci. 2020, 11, 767.
- Nepal, B.; Stine, K.J. Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems. Processes 2019, 7, 513.
- Monteiro, T.; Pinheiro, M.A. Lycopersicon Esculentum Miller: From by-Products towards Neuroprotection. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2013.
- Pardini, A.; Consumi, M.; Leone, G.; Bonechi, C.; Tamasi, G.; Sangiorgio, P.; Verardi, A.; Rossi, C.; Magnani, A. Effect of Different Post-Harvest Storage Conditions and Heat Treatment on Tomatine Content in Commercial Varieties of Green Tomatoes. J. Food Compos. Anal. 2021, 96, 103735.
- Koh, E.; Kaffka, S.; Mitchell, A.E. A Long-Term Comparison of the Influence of Organic and Conventional Crop Management Practices on the Content of the Glycoalkaloid α-Tomatine in Tomatoes. J. Sci. Food Agric. 2013, 93, 1537–1542.
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical Investigation and Antioxidant Properties of Unripe Tomato Cultivars (Solanum lycopersicum L.). Food Chem. 2023, 438, 137863.
- Ngo, T.H.; Park, J.; Jo, Y.D.; Jin, C.H.; Jung, C.H.; Nam, B.; Han, A.R.; Nam, J.W. Content of Two Major Steroidal Glycoalkaloids in Tomato (Solanum lycopersicum Cv. Micro-Tom) Mutant Lines at Different Ripening Stages. Plants 2022, 11, 2895.
- Taveira, M.; Ferreres, F.; Gil-Izquierdo, A.; Oliveira, L.; Valentão, P.; Andrade, P.B. Fast Determination of Bioactive Compounds from Lycopersicon Esculentum Mill. Leaves. Food Chem. 2012, 135, 748–755.
- Friedman, M.; Levin, C.E. Tomatine Content in Tomato and Tomato Products Determined by HPLC with Pulsed Amperometric Detection. J. Agric. Food Chem. 1995, 43, 1507–1511.
- Friedman, M.; Kozukue, N.; Harden, L.A. Preparation and Characterization of Acid Hydrolysis Products of the Tomato Glycoalkaloid R-Tomatine. J. Agric. Food Chem. 1998, 46, 2096–2101.
- Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. Var. “Camone” Tomato in Spontaneously Hypertensive Rats. Molecules 2020, 25, 3758.
- Itkin, M.; Rogachev, I.; Alkan, N.; Rosenberg, T.; Malitsky, S.; Masini, L.; Meir, S.; Iijima, Y.; Aoki, K.; de Vos, R.; et al. GLYCOALKALOID METABOLISM1 Is Required for Steroidal Alkaloid Glycosylation and Prevention of Phytotoxicity in Tomato. Plant Cell 2011, 23, 4507–4525.
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of Matrix Effects and Extraction Efficiencies of LC-MS/MS Methods as the Essential Part for Proper Validation of Multiclass Contaminants in Complex Feed. J. Agric. Food Chem. 2020, 68, 3868–3880.
- Sonawane, P.D.; Gharat, S.A.; Jozwiak, A.; Barbole, R.; Heinicke, S.; Almekias-Siegl, E.; Meir, S.; Rogachev, I.; Connor, S.E.O.; Giri, A.P.; et al. A BAHD-Type Acyltransferase Concludes the Biosynthetic Pathway of Non-Bitter Glycoalkaloids in Ripe Tomato Fruit. Nat. Commun. 2023, 14, 4540.
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071.
- Kozukue, N.; Kozukue, E.; Yamashita, H.; Fujii, S. Alpha-Tomatine Purification and Quantification in Tomatoes by HPLC. J. Food Sci. 1994, 6, 1211–1212.
- Vaananen, T.; Kuronen, P.; Pehu, E. Comparison of Commercial Solid-Phase Extraction Sorbents for the Sample Preparation of Potato Glycoalkaloids. J. Chromatogr. A 2000, 869, 301–305.
- Choi, K.M.; Lee, Y.S.; Shin, D.M.; Lee, S.; Yoo, K.S.; Lee, M.K.; Lee, J.H.; Kim, S.Y.; Lee, Y.M.; Hong, J.T.; et al. Green Tomato Extract Attenuates High-Fat-Diet-Induced Obesity through Activation of the AMPK Pathway in C57BL/6 Mice. J. Nutr. Biochem. 2013, 24, 335–342.
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kim, H.J.; Lee, I.S.; Byun, J.O.; Kozukue, N. Tomatine-Containing Green Tomato Extracts Inhibit Growth of Human Breast, Colon, Liver, and Stomach Cancer Cells. J. Agric. Food Chem. 2009, 57, 5727–5733.
- Taveira, M.; Sousa, C.; Valentão, P.; Ferreres, F.; Teixeira, J.P.; Andrade, P.B. Neuroprotective Effect of Steroidal Alkaloids on Glutamate-Induced Toxicity by Preserving Mitochondrial Membrane Potential and Reducing Oxidative Stress. J. Steroid Biochem. Mol. Biol. 2014, 140, 106–115.
- Caprioli, G.; Cahill, M.G.; James, K.J. Mass Fragmentation Studies of α-Tomatine and Validation of a Liquid Chromatography LTQ Orbitrap Mass Spectrometry Method for Its Quantification in Tomatoes. Food Anal. Methods 2014, 7, 1565–1571.
- Leonardi, C.; Ambrosino, P.; Esposito, F.; Fogliano, V. Antioxidative Activity and Carotenoid and Tomatine Contents in Different Typologies of Fresh Consumption Tomatoes. J. Agric. Food Chem. 2000, 48, 4723–4727.
- Abbasi-Parizad, P.; Salvino, R.A.; Passera, A.; Follador, A.R.V.; Cosentino, C.; Jucker, C.; Savoldelli, S.; Bacenetti, J.; Casati, P.; Scaglia, B. Development of a Cascade Production System Finalized to the Extraction of All-Tomatine-Rich Fraction Using the Tomato Cannery Waste as Feedstock. J. Clean Prod. 2023, 401, 136743.
- Roddick, J.G.; Melchers, G. Steroidal glycoalkaloid content of potato, tomato and their somatic hybrids. Theor. Appl. Genet. 1985, 70, 655–660.
- Drummer, O.H. Chromatographic screening techniques in systematic toxicological analysis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 1–2, 27–45.
- Klein- Júnior, L.C.; Heyden, Y.V.; Henriques, A.T. Enlarging the bottleneck in the analysis of alkaloids: A review on sample preparation in herbal matrices. Trends Anal. Chem. 2016, 80, 66–82.
- Bushway, R.J.; Perkins, B.; Paradis, L.R.; Vanderpan, S. High-Performance Liquid Chromatographic Determination of the Tomato Glycoalkaloid, Tomatine, in Green and Red Tomatoes. J. Agric. Food Chem. 1994, 42, 2824–2829.
- Figueiredo-González, M.; Valentão, P.; Andrade, P.B. Tomato Plant Leaves: From by-Products to the Management of Enzymes in Chronic Diseases. Ind. Crop. Prod. 2016, 94, 621–629.
- Nirmala, F.S.; Lee, H.; Kim, J.S.; Ha, T.; Jung, C.H.; Ahn, J. Green Tomato Extract Prevents Bone Loss in Ovariectomized Rats, a Model of Osteoporosis. Nutrients 2020, 12, 3210.
- Wolfender, J.L. HPLC in Natural Product Analysis: The Detection Issue. Planta Med. 2009, 75, 719–734.
- Moco, S.; Bino, R.J.; Vorst, O.; Verhoeven, H.A.; De Groot, J.; Van Beek, T.A.; Vervoort, J.; Ric De Vos, C.H. A Liquid Chromatography-Mass Spectrometry-Based Metabolome Database for Tomato. Plant. Physiol. 2006, 141, 1205–1218.
- Kozukue, N.; Kim, D.S.; Choi, S.H.; Mizuno, M.; Friedman, M. Isomers of the Tomato Glycoalkaloids α-Tomatine and Dehydrotomatine: Relationship to Health Benefits. Molecules 2023, 28, 3621.
- Cichon, M.J.; Riedl, K.M.; Wan, L.; Thomas-Ahner, J.M.; Francis, D.M.; Clinton, S.K.; Schwartz, S.J. Plasma Metabolomics Reveals Steroidal Alkaloids as Novel Biomarkers of Tomato Intake in Mice. Mol. Nutr. Food Res. 2017, 61, 1700241.
- Cooperstone, J.L.; Tober, K.L.; Riedl, K.M.; Teegarden, M.D.; Cichon, M.J.; Francis, D.M.; Schwartz, S.J.; Oberyszyn, T.M. Tomatoes Protect against Development of UV-Induced Keratinocyte Carcinoma via Metabolomic Alterations. Sci. Rep. 2017, 7, 5106.
- Dzakovich, M.P.; Goggans, M.L.; Thomas-Ahner, J.M.; Moran, N.E.; Clinton, S.K.; Francis, D.M.; Cooperstone, J.L.; Cooperstone, J. Transcriptomics and Metabolomics Reveal Tomato Consumption Alters Hepatic Xenobiotic Metabolism and Induces Steroidal Alkaloid Metabolite Accumulation in Mice. Mol. Nutr. Food Res. 2023, 16, 2300239.
- Yamanaka, T.; Vincken, J.P.; De Waard, P.; Sanders, M.; Takada, N.; Gruppen, H. Isolation, Characterization, and Surfactant Properties of the Major Triterpenoid Glycosides from Unripe Tomato Fruits. J. Agric. Food Chem. 2008, 56, 11432–11440.
- Rial, C.; Gómez, E.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Ecological Relevance of the Major Allelochemicals in Lycopersicon Esculentum Roots and Exudates. J. Agric. Food Chem. 2018, 66, 4638–4644.
- Bachmann, H.; Autzen, S.; Frey, U.; Wehr, U.; Rambeck, W.; McCormack, H.; Whitehead, C.C. The Efficacy of a Standardised Product from Dried Leaves of Solanum Glaucophyllum as Source of 1,25-Dihydroxycholecalciferol for Poultry. Br. Poult. Sci. 2013, 54, 642–652.
- Altria, K.D.; Marsh, A.; Sänger-van de Griend, C. Capillary Electrophoresis for the Analysis of Small-Molecule Pharmaceuticals. Electrophoresis 2006, 27, 2263–2282.
- Bailly, C. The steroidal alkaloids α-tomatine and tomatidine: Panorama of their mode of action and pharmacological properties. Steroids 2021, 176, 108933.
- Tam, C.C.; Nguyen, K.; Nguyen, D.; Hamada, S.; Kwon, O.; Kuang, I.; Gong, S.; Escobar, S.; Liu, M.; Kim, J.; et al. Antimicrobial Properties of Tomato Leaves, Stems, and Fruit and Their Relationship to Chemical Composition. BMC Complement. Med. Ther. 2021, 21, 229.
- Sandrock, R.W.; Vanetten, H.D.; Sandrock, W. Biochemistry and Cell Biology Fungal Sensitivity to and Enzymatic Degradation of the Phytoanticipin α-Tomatine. Phytopathology 1998, 88, 137–143.
- Blankemeyer, J.T.; White, J.B.; Stringer, B.K.; Friedman, M. Effect of c,Tomatine and Tomatidine on Membrane Potential of Frog Embryos and Active Transport of Ions in Frog Skin. Food Chem. Toxicol. 1997, 35, 639–646.
- Poprawski, T.J.; Greenberg, S.M.; Ciomperlik, M.A. Effect of Host Plant on Beauveria Bassiana-and Paecilomyces Fumosoroseus-Induced Mortality of Trialeurodes Vaporariorum (Homoptera: Aleyrodidae). Environ. Entomol. 2000, 29, 1048–1053.
- McKee, R.K. Affecting the Toxicity of Solanine and Related Alkaloids to Fusarium Caeruleum. Microbiology 1959, 20, 686–696.
- Ito, S.-I.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaal, F.F.; El-Sayed, M.A. α-Tomatine, the Major Saponin in Tomato, Induces Programmed Cell Death Mediated by Reactive Oxygen Species in the Fungal Pathogen Fusarium Oxysporum. FEBS Lett. 2007, 581, 3217–3222.
- Elwan, H.A.M.; Elnesr, S.S.; Mohany, M.; Al-Rejaie, S.S. The Effects of Dietary Tomato Powder (Solanum lycopersicum L.) Supplementation on the Haematological, Immunological, Serum Biochemical and Antioxidant Parameters of Growing Rabbits. J. Anim. Physiol. Anim. Nutr. 2019, 103, 534–546.
- Zhao, B.; Zhou, B.; Bao, L.; Yang, Y.; Guo, K. Alpha-Tomatine Exhibits Anti-Inflammatory Activity in Lipopolysaccharide-Activated Macrophages. Inflammation 2015, 38, 1769–1776.
- Kovacs, B.A.; Wakkary, J.A.; Goodfriend, L.; Rose, B. Isolation of an Antihistaminic Principle Resembling Tomatine from Crown Gall Tumors. Science 1964, 144, 295–296.
- Hu, B.; Sun, X.; Yang, Y.; Ying, Z.; Meng, J.; Zhou, C.; Jiang, G.; Li, S.; Wu, F.; Zhao, X.; et al. Tomatidine Suppresses Osteoclastogenesis and Mitigates Estrogen Deficiency-Induced Bone Mass Loss by Modulating TRAF6-Mediated Signaling. FASEB J. 2019, 33, 2574–2586.
- Yu, T.; Wu, Q.; You, X.; Zhou, H.; Xu, S.; He, W.; Li, Z.; Li, B.; Xia, J.; Zhu, H.; et al. Tomatidine Alleviates Osteoporosis by Downregulation of P53. Med. Sci. Monit. 2020, 26, e923996-1–e923996-9.
- Huang, H.; Chen, S.; Van Doren, J.; Li, D.; Farichon, C.; He, Y.; Zhang, Q.; Zhang, K.; Conney, A.H.; Goodin, S.; et al. α-Tomatine Inhibits Growth and Induces Apoptosis in HL-60 Human Myeloid Leukemia Cells. Mol. Med. Rep. 2015, 11, 4573–4578.
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk Assessment of Glycoalkaloids in Feed and Food, in Particular in Potatoes and Potato-Derived Products. EFSA J. 2020, 18, e06222.




