Unripe tomatoes represent an agri-food waste resulting from industrial by-processing products of tomatoes, yielding products with a high content of bioactive compounds with potential nutraceutical properties. The food-matrix biological properties are attributed to the high steroidal glycoalkaloid (SGA) content. Among them, α-tomatine is the main SGA reported in unripe green tomatoes.
- green tomatoes
- glycoalkaloids
- α-tomatine
1. Introduction
2. Unripe Green Tomatoes as a Source of Glycoalkaloids
SGAs are a class of secondary metabolites produced by some plants in the Solanaceae family, such as tomatoes, potatoes, and eggplants. They are distributed in different botanical parts, which include the leaves, flowers, stems, roots, and unripe fruits [20][19]. Tomatine was firstly isolated by Fontaine et al., in tomato leaves and represents the main SGA isolated from the whole tomato plant [21][20]. Tomatine was originally isolated as a mixture of two SGAs, α-tomatine and dehydrotomatine, which differ based on a double bond in the aglycon moiety across positions 5 and 6. However, the chemical composition of tomatine commercial standards were characterized as a mixture of 90% α-tomatine and 10% dehydrotomatine in 1994 [22][21]. Therefore, all the studies conducted up to that year on tomatine actually referred to a mixture of the two compounds, presumably with the ratio 9/1. α-tomatine (C50H83NO21; molecular weight: 1034.2) is characterized by a nitrogen-containing spirostanic aglycone, called tomatidine (C27H45NO2; molecular weight: 415.7), attached to a tetrasaccharide unit in position 3β (S configuration). It is referred to as lycotetraose, which consists of two D-glucose and single D-galactose and D-xylose units [23][22]. The chemical structure is reported in Figure 1. α-tomatine belongs to the class of phytoanticipins, which are natural antimicrobial compounds that protect the plant against potential pathogens as fungi [24][23]. Although α-tomatine represents the most abundant tomato SGA, several secondary metabolites have been identified, both as biosynthetic precursors and as products of α-tomatine metabolism in the plant. The chemical diversity of α-tomatine derivatives is derived from some chemical modifications, such as isomerization, hydroxylation, acetylation, and glycosylation [25][24]. α-tomatine is present in all tomato parts, and the fruit concentration depends on the stage of maturity. Indeed, this compound is accumulated in unripe and green tomatoes, whereas during fruit maturation, it drastically decreases [26][25]. During the transition from green to red fruit, it is metabolized to esculeoside A, which represents the main mature tomato SGA [25][24].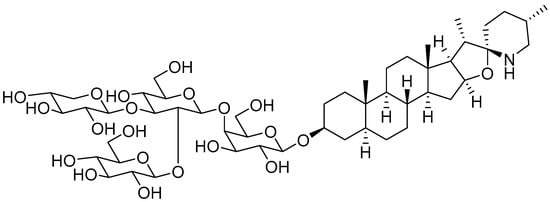
3. α-Tomatine Extraction from Green Tomatoes
3. α-Tomatine Extraction from Green Tomatoes
4. α-Tomatine Analysis Methods
5. Nutraceutical Potential of Green Tomatoes
5.1. Antiviral, Antifungal, and Antibiotic Activity
SGAs are produced by plants as a defense against bacteria, fungi, viruses, and insects [102][65]. It is thus not surprising that the healthy properties of green tomatoes’ alkaloids are a consequence of the antibiotic power of these secondary metabolites. Leaves and immature green fruit extracts of Californian Solanum lycopersicum L. display antimicrobial activity against several bacteria (Salmonella enterica, Staphylococcus aureus, and Escherichia coli K12) [81][66]. Interestingly, the extract does not affect the growth of the beneficial bacteria Lactobacillus acidophilus, Lactobacillus rhamnosus, and Lactobacillus reuteri, which are part of the human gut microbiota [81][66]. A-tomatine affects the membrane permeability of many crop-infesting fungi by sequestering ergosterols, one of the main components of fungal membranes [103][67]. Ergosterol sequestration disrupts the membrane bilayer, ultimately causing the leakage of cell components, osmotic stress, and cell death [44,104][27][68]. Among SGAs, α-tomatine has the highest bactericidal activity against bacteria and fungi [103][67]. α-tomatine, isolated from young leaves of Lycopersicon Pimpinellifolium, showed activity against the pathogen Fusarium caereleum (IC50 = 460 µM). Further, α-tomatine included in bacterial Petri dishes completely inhibits the growth of fungal species, such as Candida albicans (α-tomatine-enriched extracts of green tomatoes, leaves, and stems) [81][66]; Fusarium oxisporum (IC50 = 40 μM); and Cladosporium fulvum, as well as the spore germination of Paecilomyces Fumosoreus (IC50 = 500 μM), and partially reduced of 45% the spore germination of Beauveria brassiana (IC50 = 1 mM) [82,83][69][70]. In the fungal pathogen Fusarium, the damage caused by this compound increases reactive oxygen species (ROS) production and leads to fungal programmed cell death [105][71].5.2. Anti-Inflammatory Effects
Several articles have reported the anti-inflammatory effects of pure SGAs and green tomatoes’ extracts. Extracts obtained from the locular gel and serum of Solanum lycopersicum L. var. “Camone” (respectively, containing 61.7 ± 0.9 mg of α-tomatine/kg of FW of locular gel and 12.5 ± 0.5 mg of α-tomatine/kg of FW of serum) significantly reduce inflammation in humans, decreasing the blood inflammatory cytokine count, systolic pressure, heart rate, and aorta thickness [46][36]. The supplementation of 1–2% dietary tomato powder containing α-tomatine ameliorates hemato-immunological and antioxidant clinical parameters in rabbits [86][72]. Pure α-tomatine inhibits the production of the proinflammatory cytokines IL-1β, IL-6, and TNF-α in LPS-stimulated macrophages by preventing IκB degradation and ERK phosphorylation [107][73]. In agreement with these reports, α-tomatine has been shown to inhibit the expression of Cox-2 and iNOS and decrease the production of prostaglandin E2 (PGE2) in murine LPS-stimulated macrophages. Furthermore, α-tomatine exerts a powerful antihistaminic effect [108][74].5.3. Anti-Aging Effects
Green tomatoes and SGAs have shown promising anti-aging effects in many tissues, including the bones, brain, and muscles. A diet supplementation with a green tomato extract from “Korean chal tomato” (containing tomatidine in the amount of 1.06 ± 0.11 mg of tomatidine/100 g of dry weight) improved bone mineral density and overall bone quality in ovariectomizes rats, a model of postmenopausal osteoporosis [58][54]. The aglycone tomatidine inhibits osteoclastogenesis and reduces estrogenic deficiency-induced bone mass loss [90][75] through a mechanism that has not been fully elucidated, but that probably involves the modulation of the p53 and MAPK signaling pathways [109][76].
5.4. Anti-Tumoral Effects
Red tomato extracts from the fruits of variants of Solanum lycopersicum L. (var. Sancheri premium, Yoyo, Chobok Power, and Rokusanmaru) have only subtle growth inhibitory effects in several in vitro cell culture models (breast cancer (MCF-7), colon cancer (HT-29), gastric cancer (AGS), hepatocarcinoma (HepG2), and liver cancer (Chag)). However, the corresponding extracts from unripe green fruit (α-tomatine content ranging from 5.75 ± 0.29 mg of α-tomatine/100 g of FW to 31.40 ± 1.97 mg of α-tomatine/100 g of FW) inhibit the growth of several human cancer lines, such as the MCF-7, HT-29, AGS, HepG2, and Chag lines [52,78][44][77].5.5. Pharmacokinetics and Toxicological Aspects of Glycoalkaloids and Green Tomato Extracts
The correlation between in vitro pharmacological activities and possible beneficial effects on human health is strictly correlated to the study of α-tomatine pharmacokinetics (e.g., absorption, distribution, biotransformation, and excretion). Although α-tomatine toxicology and the in vivo fate of other SGAs (e.g., α-solanine, α-chaconine) have been extensively studied [118][78], there are few data about the pharmacokinetics of tomato SGAs. For many years, α-tomatine was considered a molecule with low bioavailability. It is stable at 37 °C under acidic conditions that mimic the pH of the stomach. Furthermore, α-tomatine and cholesterol form insoluble complexes that are eliminated through feces [102][65].6. Conclusions
Waste products of the tomato industry represent a rich, natural source of α-tomatine, and the recycling of this waste represents an appealing research field to develop innovative nutraceutical products. It is thus not surprising that over-the-counter products containing green tomato extracts are starting to become popular. Among their secondary metabolites, α-tomatine exhibits significant biological activities on human health. The health-beneficial properties of pure tomato compounds (e.g., α-tomatine and tomatidine) and Solanum lycopersicum L. extracts in several diseases have been discussed. Besides its antioxidant power, α-tomatine-containing extracts show interesting antimicrobial, anti-inflammatory, anti-aging, and anti-tumoral activities. In vitro, the cellular and molecular mechanisms involved in green tomato pharmacological activities have been identified and proven to involve the modulation of several metabolic patterns.References
- Garcia-Marti, M.; Simal-Gandara, J. Chapter 5: Chemical and Biological Valorization of Tomato Waste. In Agri-Food Waste Valorisation; Royal Society of Chemistry: London, UK, 2023; Volume 78, p. 326.
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton, S.K.; Schwartz, S.J. Lycopene from Heat-Induced Cis-Isomer-Rich Tomato Sauce Is More Bioavailable than from All-Trans-Rich Tomato Sauce in Human Subjects. Br. J. Nutr. 2007, 98, 140–146.
- Carillo, P.; D’Amelia, L.; Dell’Aversana, E.; Faiella, D.; Cacace, D.; Giuliano, B.; Morrone, B. Eco-Friendly Use of Tomato Processing Residues for Lactic Acid Production in Campania. Chem. Eng. Trans. 2018, 64, 223–228.
- Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials 2021, 14, 4581.
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A.P.C. Characterization of Tomato Processing By-Product for Use as a Potential Functional Food Ingredient: Nutritional Composition, Antioxidant Activity and Bioactive Compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160.
- Maisto, M.; Annunziata, G.; Schiano, E.; Piccolo, V.; Iannuzzo, F.; Santangelo, R.; Ciampaglia, R.; Tenore, G.C.; Novellino, E.; Grieco, P. Potential Functional Snacks: Date Fruit Bars Supplemented by Different Species of Lactobacillus spp. Foods 2021, 10, 1760.
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010.
- Patel, A.H.; Sharma, H.P. Vaishali Physiological Functions, Pharmacological Aspects and Nutritional Importance of Green Tomato- a Future Food. Crit. Rev. Food Sci. Nutr. 2023, 1–23.
- El Mashad, H.M.; Zhao, L.; Zhang, R.; Pan, Z. Tomato. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–131. ISBN 9780128141397.
- Lu, S.; Chen, S.; Li, H.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; et al. Sustainable Valorization of Tomato Pomace (Lycopersicon esculentum) in Animal Nutrition: A Review. Animals 2022, 12, 3294.
- Faria-Silva, C.; de Sousa, M.; Carvalheiro, M.C.; Simões, P.; Simões, S. Alpha-Tomatine and the Two Sides of the Same Coin: An Anti-Nutritional Glycoalkaloid with Potential in Human Health. Food Chem. 2022, 391, 133261.
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 2367453.
- Periago, M.J.; Martínez-Valverde, I.; Chesson, A.; Provan, G. Phenolic Compounds, Lycopene and Antioxidant Activity in Commercial Varieties of Tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330.
- Kozukue, N.; Friedman, M. Tomatine, Chlorophyll, β-Carotene and Lycopene Content in Tomatoes during Growth and Maturation. J. Sci. Food Agric. 2003, 83, 195–200.
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of Phytochemicals Present in Tomato. J. Food Sci. Technol. 2018, 55, 2833–2849.
- Hernández-Ortega, M.; Ortiz-Moreno, A.; Hernández-Navarro, M.D.; Chamorro-Cevallos, G.; Dorantes-Alvarez, L.; Necoechea-Mondragón, H. Antioxidant, Antinociceptive, and Anti-Inflammatory Effects of Carotenoids Extracted from Dried Pepper (Capsicum annuum L.). J. Biomed Biotechnol. 2012, 2012, 524019.
- Subramoniam, A.; Asha, V.V.; Nair, S.A.; Sasidharan, S.P.; Sureshkumar, P.K.; Rajendran, K.N.; Karunagaran, D.; Ramalingam, K. Chlorophyll Revisited: Anti-Inflammatory Activities of Chlorophyll a and Inhibition of Expression of TNF-α Gene by the Same. Inflammation 2012, 35, 959–966.
- Maisto, M.; Iannuzzo, F.; Schiano, E.; Ciampaglia, R.; Labanca, A.; Montesano, D.; Piccolo, V.; Rossi, P.; Tenore, G.C. Effects of Fortified Laying Hen Diet with Moringa Oleifera Leaves and Goji Berries on Cholesterol and Carotenoid Egg Content. Foods 2022, 11, 3156.
- Zhang, Q.; Liu, D.; Cui, Y.; Xu, T.; Liu, X.; Liu, K.; Wang, Q.; Li, A.; Zhao, P.; Cheng, Z. Bioactivities and chemical profiling comparison and metabolomic variations of polyphenolics and steroidal glycoalkaloids in different parts of Solanum nigrum L. Phytochemical Analysis. 2023, 1–19.
- Fontaine, T.; Irving, G. Isolation and Partial Characterization of Crystalline Tomatine, an Antibiotic Agent from the Tomato Plant. Arch Biochem. 1948, 18, 467–475.
- Friedman, M.; Levin, C.E.; Mcdonald, G.M. α-Tomatine Determination in Tomatoes by HPLC Using Pulsed Amperometric Detection. Food Chem. 1994, 42, 1959–1964.
- Cárdenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. The Bitter Side of the Nightshades: Genomics Drives Discovery in Solanaceae Steroidal Alkaloid Metabolism. Phytochemistry 2015, 113, 24–32.
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419.
- Iijima, Y.; Watanabe, B.; Sasaki, R.; Takenaka, M.; Ono, H.; Sakurai, N.; Umemoto, N.; Suzuki, H.; Shibata, D.; Aoki, K. Steroidal Glycoalkaloid Profiling and Structures of Glycoalkaloids in Wild Tomato Fruit. Phytochemistry 2013, 95, 145–157.
- Friedman, M. Tomato Glycoalkaloids: Role in the Plant and in the Diet. J. Agric. Food Chem. 2002, 50, 5751–5780.
- Dzakovich, M.P.; Hartman, J.L.; Cooperstone, J.L. A High-Throughput Extraction and Analysis Method for Steroidal Glycoalkaloids in Tomato. Front. Plant Sci. 2020, 11, 767.
- Nepal, B.; Stine, K.J. Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems. Processes 2019, 7, 513.
- Monteiro, T.; Pinheiro, M.A. Lycopersicon Esculentum Miller: From by-Products towards Neuroprotection. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2013.
- Pardini, A.; Consumi, M.; Leone, G.; Bonechi, C.; Tamasi, G.; Sangiorgio, P.; Verardi, A.; Rossi, C.; Magnani, A. Effect of Different Post-Harvest Storage Conditions and Heat Treatment on Tomatine Content in Commercial Varieties of Green Tomatoes. J. Food Compos. Anal. 2021, 96, 103735.
- Koh, E.; Kaffka, S.; Mitchell, A.E. A Long-Term Comparison of the Influence of Organic and Conventional Crop Management Practices on the Content of the Glycoalkaloid α-Tomatine in Tomatoes. J. Sci. Food Agric. 2013, 93, 1537–1542.
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical Investigation and Antioxidant Properties of Unripe Tomato Cultivars (Solanum lycopersicum L.). Food Chem. 2023, 438, 137863.
- Ngo, T.H.; Park, J.; Jo, Y.D.; Jin, C.H.; Jung, C.H.; Nam, B.; Han, A.R.; Nam, J.W. Content of Two Major Steroidal Glycoalkaloids in Tomato (Solanum lycopersicum Cv. Micro-Tom) Mutant Lines at Different Ripening Stages. Plants 2022, 11, 2895.
- Taveira, M.; Ferreres, F.; Gil-Izquierdo, A.; Oliveira, L.; Valentão, P.; Andrade, P.B. Fast Determination of Bioactive Compounds from Lycopersicon Esculentum Mill. Leaves. Food Chem. 2012, 135, 748–755.
- Friedman, M.; Levin, C.E. Tomatine Content in Tomato and Tomato Products Determined by HPLC with Pulsed Amperometric Detection. J. Agric. Food Chem. 1995, 43, 1507–1511.
- Friedman, M.; Kozukue, N.; Harden, L.A. Preparation and Characterization of Acid Hydrolysis Products of the Tomato Glycoalkaloid R-Tomatine. J. Agric. Food Chem. 1998, 46, 2096–2101.
- Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. Var. “Camone” Tomato in Spontaneously Hypertensive Rats. Molecules 2020, 25, 3758.
- Itkin, M.; Rogachev, I.; Alkan, N.; Rosenberg, T.; Malitsky, S.; Masini, L.; Meir, S.; Iijima, Y.; Aoki, K.; de Vos, R.; et al. GLYCOALKALOID METABOLISM1 Is Required for Steroidal Alkaloid Glycosylation and Prevention of Phytotoxicity in Tomato. Plant Cell 2011, 23, 4507–4525.
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of Matrix Effects and Extraction Efficiencies of LC-MS/MS Methods as the Essential Part for Proper Validation of Multiclass Contaminants in Complex Feed. J. Agric. Food Chem. 2020, 68, 3868–3880.
- Sonawane, P.D.; Gharat, S.A.; Jozwiak, A.; Barbole, R.; Heinicke, S.; Almekias-Siegl, E.; Meir, S.; Rogachev, I.; Connor, S.E.O.; Giri, A.P.; et al. A BAHD-Type Acyltransferase Concludes the Biosynthetic Pathway of Non-Bitter Glycoalkaloids in Ripe Tomato Fruit. Nat. Commun. 2023, 14, 4540.
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071.
- Kozukue, N.; Kozukue, E.; Yamashita, H.; Fujii, S. Alpha-Tomatine Purification and Quantification in Tomatoes by HPLC. J. Food Sci. 1994, 6, 1211–1212.
- Vaananen, T.; Kuronen, P.; Pehu, E. Comparison of Commercial Solid-Phase Extraction Sorbents for the Sample Preparation of Potato Glycoalkaloids. J. Chromatogr. A 2000, 869, 301–305.
- Choi, K.M.; Lee, Y.S.; Shin, D.M.; Lee, S.; Yoo, K.S.; Lee, M.K.; Lee, J.H.; Kim, S.Y.; Lee, Y.M.; Hong, J.T.; et al. Green Tomato Extract Attenuates High-Fat-Diet-Induced Obesity through Activation of the AMPK Pathway in C57BL/6 Mice. J. Nutr. Biochem. 2013, 24, 335–342.
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kim, H.J.; Lee, I.S.; Byun, J.O.; Kozukue, N. Tomatine-Containing Green Tomato Extracts Inhibit Growth of Human Breast, Colon, Liver, and Stomach Cancer Cells. J. Agric. Food Chem. 2009, 57, 5727–5733.
- Taveira, M.; Sousa, C.; Valentão, P.; Ferreres, F.; Teixeira, J.P.; Andrade, P.B. Neuroprotective Effect of Steroidal Alkaloids on Glutamate-Induced Toxicity by Preserving Mitochondrial Membrane Potential and Reducing Oxidative Stress. J. Steroid Biochem. Mol. Biol. 2014, 140, 106–115.
- Caprioli, G.; Cahill, M.G.; James, K.J. Mass Fragmentation Studies of α-Tomatine and Validation of a Liquid Chromatography LTQ Orbitrap Mass Spectrometry Method for Its Quantification in Tomatoes. Food Anal. Methods 2014, 7, 1565–1571.
- Leonardi, C.; Ambrosino, P.; Esposito, F.; Fogliano, V. Antioxidative Activity and Carotenoid and Tomatine Contents in Different Typologies of Fresh Consumption Tomatoes. J. Agric. Food Chem. 2000, 48, 4723–4727.
- Abbasi-Parizad, P.; Salvino, R.A.; Passera, A.; Follador, A.R.V.; Cosentino, C.; Jucker, C.; Savoldelli, S.; Bacenetti, J.; Casati, P.; Scaglia, B. Development of a Cascade Production System Finalized to the Extraction of All-Tomatine-Rich Fraction Using the Tomato Cannery Waste as Feedstock. J. Clean Prod. 2023, 401, 136743.
- Roddick, J.G.; Melchers, G. Steroidal glycoalkaloid content of potato, tomato and their somatic hybrids. Theor. Appl. Genet. 1985, 70, 655–660.
- Drummer, O.H. Chromatographic screening techniques in systematic toxicological analysis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 1–2, 27–45.
- Klein- Júnior, L.C.; Heyden, Y.V.; Henriques, A.T. Enlarging the bottleneck in the analysis of alkaloids: A review on sample preparation in herbal matrices. Trends Anal. Chem. 2016, 80, 66–82.
- Bushway, R.J.; Perkins, B.; Paradis, L.R.; Vanderpan, S. High-Performance Liquid Chromatographic Determination of the Tomato Glycoalkaloid, Tomatine, in Green and Red Tomatoes. J. Agric. Food Chem. 1994, 42, 2824–2829.
- Figueiredo-González, M.; Valentão, P.; Andrade, P.B. Tomato Plant Leaves: From by-Products to the Management of Enzymes in Chronic Diseases. Ind. Crop. Prod. 2016, 94, 621–629.
- Nirmala, F.S.; Lee, H.; Kim, J.S.; Ha, T.; Jung, C.H.; Ahn, J. Green Tomato Extract Prevents Bone Loss in Ovariectomized Rats, a Model of Osteoporosis. Nutrients 2020, 12, 3210.
- Wolfender, J.L. HPLC in Natural Product Analysis: The Detection Issue. Planta Med. 2009, 75, 719–734.
- Moco, S.; Bino, R.J.; Vorst, O.; Verhoeven, H.A.; De Groot, J.; Van Beek, T.A.; Vervoort, J.; Ric De Vos, C.H. A Liquid Chromatography-Mass Spectrometry-Based Metabolome Database for Tomato. Plant. Physiol. 2006, 141, 1205–1218.
- Kozukue, N.; Kim, D.S.; Choi, S.H.; Mizuno, M.; Friedman, M. Isomers of the Tomato Glycoalkaloids α-Tomatine and Dehydrotomatine: Relationship to Health Benefits. Molecules 2023, 28, 3621.
- Cichon, M.J.; Riedl, K.M.; Wan, L.; Thomas-Ahner, J.M.; Francis, D.M.; Clinton, S.K.; Schwartz, S.J. Plasma Metabolomics Reveals Steroidal Alkaloids as Novel Biomarkers of Tomato Intake in Mice. Mol. Nutr. Food Res. 2017, 61, 1700241.
- Cooperstone, J.L.; Tober, K.L.; Riedl, K.M.; Teegarden, M.D.; Cichon, M.J.; Francis, D.M.; Schwartz, S.J.; Oberyszyn, T.M. Tomatoes Protect against Development of UV-Induced Keratinocyte Carcinoma via Metabolomic Alterations. Sci. Rep. 2017, 7, 5106.
- Dzakovich, M.P.; Goggans, M.L.; Thomas-Ahner, J.M.; Moran, N.E.; Clinton, S.K.; Francis, D.M.; Cooperstone, J.L.; Cooperstone, J. Transcriptomics and Metabolomics Reveal Tomato Consumption Alters Hepatic Xenobiotic Metabolism and Induces Steroidal Alkaloid Metabolite Accumulation in Mice. Mol. Nutr. Food Res. 2023, 16, 2300239.
- Yamanaka, T.; Vincken, J.P.; De Waard, P.; Sanders, M.; Takada, N.; Gruppen, H. Isolation, Characterization, and Surfactant Properties of the Major Triterpenoid Glycosides from Unripe Tomato Fruits. J. Agric. Food Chem. 2008, 56, 11432–11440.
- Rial, C.; Gómez, E.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Ecological Relevance of the Major Allelochemicals in Lycopersicon Esculentum Roots and Exudates. J. Agric. Food Chem. 2018, 66, 4638–4644.
- Bachmann, H.; Autzen, S.; Frey, U.; Wehr, U.; Rambeck, W.; McCormack, H.; Whitehead, C.C. The Efficacy of a Standardised Product from Dried Leaves of Solanum Glaucophyllum as Source of 1,25-Dihydroxycholecalciferol for Poultry. Br. Poult. Sci. 2013, 54, 642–652.
- Altria, K.D.; Marsh, A.; Sänger-van de Griend, C. Capillary Electrophoresis for the Analysis of Small-Molecule Pharmaceuticals. Electrophoresis 2006, 27, 2263–2282.
- Bailly, C. The steroidal alkaloids α-tomatine and tomatidine: Panorama of their mode of action and pharmacological properties. Steroids 2021, 176, 108933.
- Tam, C.C.; Nguyen, K.; Nguyen, D.; Hamada, S.; Kwon, O.; Kuang, I.; Gong, S.; Escobar, S.; Liu, M.; Kim, J.; et al. Antimicrobial Properties of Tomato Leaves, Stems, and Fruit and Their Relationship to Chemical Composition. BMC Complement. Med. Ther. 2021, 21, 229.
- Sandrock, R.W.; Vanetten, H.D.; Sandrock, W. Biochemistry and Cell Biology Fungal Sensitivity to and Enzymatic Degradation of the Phytoanticipin α-Tomatine. Phytopathology 1998, 88, 137–143.
- Blankemeyer, J.T.; White, J.B.; Stringer, B.K.; Friedman, M. Effect of c,Tomatine and Tomatidine on Membrane Potential of Frog Embryos and Active Transport of Ions in Frog Skin. Food Chem. Toxicol. 1997, 35, 639–646.
- Poprawski, T.J.; Greenberg, S.M.; Ciomperlik, M.A. Effect of Host Plant on Beauveria Bassiana-and Paecilomyces Fumosoroseus-Induced Mortality of Trialeurodes Vaporariorum (Homoptera: Aleyrodidae). Environ. Entomol. 2000, 29, 1048–1053.
- McKee, R.K. Affecting the Toxicity of Solanine and Related Alkaloids to Fusarium Caeruleum. Microbiology 1959, 20, 686–696.
- Ito, S.-I.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaal, F.F.; El-Sayed, M.A. α-Tomatine, the Major Saponin in Tomato, Induces Programmed Cell Death Mediated by Reactive Oxygen Species in the Fungal Pathogen Fusarium Oxysporum. FEBS Lett. 2007, 581, 3217–3222.
- Elwan, H.A.M.; Elnesr, S.S.; Mohany, M.; Al-Rejaie, S.S. The Effects of Dietary Tomato Powder (Solanum lycopersicum L.) Supplementation on the Haematological, Immunological, Serum Biochemical and Antioxidant Parameters of Growing Rabbits. J. Anim. Physiol. Anim. Nutr. 2019, 103, 534–546.
- Zhao, B.; Zhou, B.; Bao, L.; Yang, Y.; Guo, K. Alpha-Tomatine Exhibits Anti-Inflammatory Activity in Lipopolysaccharide-Activated Macrophages. Inflammation 2015, 38, 1769–1776.
- Kovacs, B.A.; Wakkary, J.A.; Goodfriend, L.; Rose, B. Isolation of an Antihistaminic Principle Resembling Tomatine from Crown Gall Tumors. Science 1964, 144, 295–296.
- Hu, B.; Sun, X.; Yang, Y.; Ying, Z.; Meng, J.; Zhou, C.; Jiang, G.; Li, S.; Wu, F.; Zhao, X.; et al. Tomatidine Suppresses Osteoclastogenesis and Mitigates Estrogen Deficiency-Induced Bone Mass Loss by Modulating TRAF6-Mediated Signaling. FASEB J. 2019, 33, 2574–2586.
- Yu, T.; Wu, Q.; You, X.; Zhou, H.; Xu, S.; He, W.; Li, Z.; Li, B.; Xia, J.; Zhu, H.; et al. Tomatidine Alleviates Osteoporosis by Downregulation of P53. Med. Sci. Monit. 2020, 26, e923996-1–e923996-9.
- Huang, H.; Chen, S.; Van Doren, J.; Li, D.; Farichon, C.; He, Y.; Zhang, Q.; Zhang, K.; Conney, A.H.; Goodin, S.; et al. α-Tomatine Inhibits Growth and Induces Apoptosis in HL-60 Human Myeloid Leukemia Cells. Mol. Med. Rep. 2015, 11, 4573–4578.
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk Assessment of Glycoalkaloids in Feed and Food, in Particular in Potatoes and Potato-Derived Products. EFSA J. 2020, 18, e06222.
