Unripe tomatoes represent an agri-food waste resulting from industrial by-processing products of tomatoes, yielding products with a high content of bioactive compounds with potential nutraceutical properties. The food-matrix biological properties are attributed to the high steroidal glycoalkaloid (SGA) content. Among them, α-tomatine is the main SGA reported in unripe green tomatoes.
- green tomatoes
- glycoalkaloids
- α-tomatine
1. Introduction
2. Unripe Green Tomatoes as a Source of Glycoalkaloids
SGAs are a class of secondary metabolites produced by some plants in the Solanaceae family, such as tomatoes, potatoes, and eggplants. They are distributed in different botanical parts, which include the leaves, flowers, stems, roots, and unripe fruits [19][20]. Tomatine was firstly isolated by Fontaine et al., in tomato leaves and represents the main SGA isolated from the whole tomato plant [20][21]. Tomatine was originally isolated as a mixture of two SGAs, α-tomatine and dehydrotomatine, which differ based on a double bond in the aglycon moiety across positions 5 and 6. However, the chemical composition of tomatine commercial standards were characterized as a mixture of 90% α-tomatine and 10% dehydrotomatine in 1994 [21][22]. Therefore, all the studies conducted up to that year on tomatine actually referred to a mixture of the two compounds, presumably with the ratio 9/1. α-tomatine (C50H83NO21; molecular weight: 1034.2) is characterized by a nitrogen-containing spirostanic aglycone, called tomatidine (C27H45NO2; molecular weight: 415.7), attached to a tetrasaccharide unit in position 3β (S configuration). It is referred to as lycotetraose, which consists of two D-glucose and single D-galactose and D-xylose units [22][23]. The chemical structure is reported in Figure 1. α-tomatine belongs to the class of phytoanticipins, which are natural antimicrobial compounds that protect the plant against potential pathogens as fungi [23][24]. Although α-tomatine represents the most abundant tomato SGA, several secondary metabolites have been identified, both as biosynthetic precursors and as products of α-tomatine metabolism in the plant. The chemical diversity of α-tomatine derivatives is derived from some chemical modifications, such as isomerization, hydroxylation, acetylation, and glycosylation [24][25]. α-tomatine is present in all tomato parts, and the fruit concentration depends on the stage of maturity. Indeed, this compound is accumulated in unripe and green tomatoes, whereas during fruit maturation, it drastically decreases [25][26]. During the transition from green to red fruit, it is metabolized to esculeoside A, which represents the main mature tomato SGA [24][25].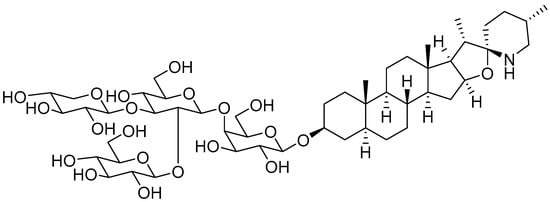
3. α-Tomatine Extraction from Green Tomatoes
3. α-Tomatine Extraction from Green Tomatoes
4. α-Tomatine Analysis Methods
5. Nutraceutical Potential of Green Tomatoes
5.1. Antiviral, Antifungal, and Antibiotic Activity
SGAs are produced by plants as a defense against bacteria, fungi, viruses, and insects [65][102]. It is thus not surprising that the healthy properties of green tomatoes’ alkaloids are a consequence of the antibiotic power of these secondary metabolites. Leaves and immature green fruit extracts of Californian Solanum lycopersicum L. display antimicrobial activity against several bacteria (Salmonella enterica, Staphylococcus aureus, and Escherichia coli K12) [66][81]. Interestingly, the extract does not affect the growth of the beneficial bacteria Lactobacillus acidophilus, Lactobacillus rhamnosus, and Lactobacillus reuteri, which are part of the human gut microbiota [66][81]. A-tomatine affects the membrane permeability of many crop-infesting fungi by sequestering ergosterols, one of the main components of fungal membranes [67][103]. Ergosterol sequestration disrupts the membrane bilayer, ultimately causing the leakage of cell components, osmotic stress, and cell death [27][68][44,104]. Among SGAs, α-tomatine has the highest bactericidal activity against bacteria and fungi [67][103]. α-tomatine, isolated from young leaves of Lycopersicon Pimpinellifolium, showed activity against the pathogen Fusarium caereleum (IC50 = 460 µM). Further, α-tomatine included in bacterial Petri dishes completely inhibits the growth of fungal species, such as Candida albicans (α-tomatine-enriched extracts of green tomatoes, leaves, and stems) [66][81]; Fusarium oxisporum (IC50 = 40 μM); and Cladosporium fulvum, as well as the spore germination of Paecilomyces Fumosoreus (IC50 = 500 μM), and partially reduced of 45% the spore germination of Beauveria brassiana (IC50 = 1 mM) [69][70][82,83]. In the fungal pathogen Fusarium, the damage caused by this compound increases reactive oxygen species (ROS) production and leads to fungal programmed cell death [71][105].5.2. Anti-Inflammatory Effects
Several articles have reported the anti-inflammatory effects of pure SGAs and green tomatoes’ extracts. Extracts obtained from the locular gel and serum of Solanum lycopersicum L. var. “Camone” (respectively, containing 61.7 ± 0.9 mg of α-tomatine/kg of FW of locular gel and 12.5 ± 0.5 mg of α-tomatine/kg of FW of serum) significantly reduce inflammation in humans, decreasing the blood inflammatory cytokine count, systolic pressure, heart rate, and aorta thickness [36][46]. The supplementation of 1–2% dietary tomato powder containing α-tomatine ameliorates hemato-immunological and antioxidant clinical parameters in rabbits [72][86]. Pure α-tomatine inhibits the production of the proinflammatory cytokines IL-1β, IL-6, and TNF-α in LPS-stimulated macrophages by preventing IκB degradation and ERK phosphorylation [73][107]. In agreement with these reports, α-tomatine has been shown to inhibit the expression of Cox-2 and iNOS and decrease the production of prostaglandin E2 (PGE2) in murine LPS-stimulated macrophages. Furthermore, α-tomatine exerts a powerful antihistaminic effect [74][108].5.3. Anti-Aging Effects
Green tomatoes and SGAs have shown promising anti-aging effects in many tissues, including the bones, brain, and muscles. A diet supplementation with a green tomato extract from “Korean chal tomato” (containing tomatidine in the amount of 1.06 ± 0.11 mg of tomatidine/100 g of dry weight) improved bone mineral density and overall bone quality in ovariectomizes rats, a model of postmenopausal osteoporosis [54][58]. The aglycone tomatidine inhibits osteoclastogenesis and reduces estrogenic deficiency-induced bone mass loss [75][90] through a mechanism that has not been fully elucidated, but that probably involves the modulation of the p53 and MAPK signaling pathways [76][109].
