Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maciej Stanisław Walędziak | -- | 1296 | 2024-01-19 22:56:55 | | | |
| 2 | Fanny Huang | Meta information modification | 1296 | 2024-01-22 07:26:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jędrysik, M.; Wyszomirski, K.; Różańska-Walędziak, A.; Grosicka-Maciąg, E.; Walędziak, M.; Chełstowska, B. GLP-1 Biomarkers in the Development of Metabolic Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/54138 (accessed on 03 March 2026).
Jędrysik M, Wyszomirski K, Różańska-Walędziak A, Grosicka-Maciąg E, Walędziak M, Chełstowska B. GLP-1 Biomarkers in the Development of Metabolic Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/54138. Accessed March 03, 2026.
Jędrysik, Malwina, Krzysztof Wyszomirski, Anna Różańska-Walędziak, Emilia Grosicka-Maciąg, Maciej Walędziak, Beata Chełstowska. "GLP-1 Biomarkers in the Development of Metabolic Disorders" Encyclopedia, https://encyclopedia.pub/entry/54138 (accessed March 03, 2026).
Jędrysik, M., Wyszomirski, K., Różańska-Walędziak, A., Grosicka-Maciąg, E., Walędziak, M., & Chełstowska, B. (2024, January 19). GLP-1 Biomarkers in the Development of Metabolic Disorders. In Encyclopedia. https://encyclopedia.pub/entry/54138
Jędrysik, Malwina, et al. "GLP-1 Biomarkers in the Development of Metabolic Disorders." Encyclopedia. Web. 19 January, 2024.
Copy Citation
Metabolic illnesses, including obesity and type 2 diabetes, have become worldwide epidemics that have an effect on public health. Clinical investigations and further exploration of these mechanisms could lead to innovative, effective, and personalized treatment strategies for individuals. It is important to screen biomarkers in previous studies to discover what is missing. Glucagon-like peptide-1′s role in insulin secretion and glucose control highlights its diagnostic and therapeutic potential. In validating these biomarkers, it will be easier to reflect pathophysiological processes, and clinicians will be able to better assess disease severity, monitor disease progression, and tailor treatment strategies.
diabetes
obesity
GLP-1
biomarkers
1. Introduction
Type 2 diabetes (T2D) and obesity are common civilian diseases and two interconnected metabolic disorders that have reached epidemic proportions worldwide. Roughly 90% of all instances of diabetes are type 2, making it the most common form. Insulin resistance is a characteristic that causes the body to react to insulin less effectively. Rising blood glucose levels prevent insulin from working properly, which leads to the release of more insulin. This may cause the pancreas to gradually wear out in some type 2 diabetic people, which will lead to the body producing less and less insulin and even higher blood sugar levels (hyperglycemia) [1][2]. Additionally, diabetes has a substantial burden on individuals and healthcare systems due to its high prevalence, economic costs, and increased risk of complications, and comorbidities. For instance, cardiovascular diseases, such as coronary artery disease and stroke, are complications of diabetes, leading to significant morbidity and mortality [3]. Chronic kidney disease, another complication of diabetes, contributes to end-stage renal disease and requires costly treatments like dialysis or transplantation [4][5]. Biomarker identification is crucial as it enables early detection, accurate diagnosis, and monitoring of disease progression and treatment response. Scientific examples include circulating microRNAs and glycated hemoglobin (HbA1c) as diagnostic and prognostic markers [6][7][8]. This method has some limitations, such as individual variability in the relationship between average blood glucose levels and HbA1c. Some people may have higher or lower HbA1c levels for a given average glucose concentration due to factors such as age, race, and genetic differences. Relying solely on HbA1c without considering other indicators may lead to misinterpretation. However, by identifying new reliable biomarkers, healthcare professionals can enhance risk assessment, tailor treatment strategies, and ultimately mitigate the burden of metabolic disorders on public health.
Obesity, on the other hand, is a condition characterized by excessive accumulation of body fat understood as the accumulation of triacylglycerols in adipocytes, which results in an increased number of those cells and thus higher body weight in patients [1][2]. Obesity has also become a global epidemic, presenting a significant public health challenge in recent years. Defined as an excess accumulation of body fat, obesity is a complex multifactorial disorder influenced by genetic, environmental, and behavioral factors [9][10]. Understanding the etiology and consequences of and potential interventions for obesity is of the utmost importance in addressing this pressing health issue. The pathogenesis of obesity involves a dysregulation of energy balance, characterized by an imbalance between energy intake and expenditure [11]. Adipose tissue is now understood to be an active endocrine organ that secretes a variety of bioactive chemicals known as adipokines. It was previously thought to be a passive energy (triacylglycerides) storage organ, but this view is changing. These adipokines are essential for maintaining insulin sensitivity, energy homeostasis, inflammation, and appetite regulation [12]. To effectively combat obesity and its associated health complications, it is essential to identify reliable biomarkers that can aid in early detection, risk assessment, and monitoring of treatment outcomes.
The intricate interplay of metabolic illnesses is shown by the link between diabetes and obesity. Obesity and diabetes, especially type 2 diabetes, are commonly linked in both directions, creating a synergistic hub of metabolic dysregulation. Insulin resistance, a condition wherein cells in the body lose their sensitivity to insulin and blood glucose levels rise, is a feature shared by both disorders [1][3]. Obesity, especially visceral adiposity, is a significant risk factor for insulin resistance and type 2 diabetes. The similarities lie in the underlying mechanisms of inflammation and metabolic dysfunction, wherein adipose tissue plays a central role [11][12]. Despite these shared aspects, differences exist in the temporal progression and manifestation of these disorders. Biomarkers are measurable indicators that can reflect the presence, progression, or response to treatment of a disease. In the context of T2D and obesity, biomarkers hold immense potential for improving early detection, risk stratification, and personalized treatment approaches [1]. Firstly, T2D is often diagnosed in the late stages, after significant damage has occurred, leading to increased morbidity and mortality rates. Additionally, obesity, a major risk factor for T2D, is a complex condition with diverse underlying mechanisms and phenotypes. Biomarkers specific to obesity can aid in understanding its heterogeneity, allowing for targeted interventions and personalized approaches [1][3]. Secondly, biomarkers play a crucial role in monitoring disease progression and assessing treatment response. Traditional clinical parameters, such as blood glucose levels or body mass index, have limitations in capturing the multifaceted aspects of T2D and obesity [2]. Lastly, biomarkers have the potential to facilitate the development of innovative treatment therapies. Moreover, they can help identify possible treatment targets by clarifying the biological processes and mechanisms involved in T2D and obesity [12]. Notably, markers such as glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), monocyte chemoattractant protein-1 (MCP-1), and insulin-like growth factor-binding protein 7 (IGFBP-7), while implicated in both diabetes and obesity, may exhibit distinct patterns and quantitative variations at different stages. Gaining an understanding of the complex roles played by these indicators in the complex landscapes of obesity and diabetes can help identify commonalities and differences between these related metabolic disorders [1][3][7][11][12][13].
2. GLP-1 Biomarkers in the Development of Metabolic Disorders
GLP-1 is a biomarker that has been extensively studied and identified as a potential factor associated with the development of metabolic disorders, including T2D and obesity. GLP-1 is an incretin hormone secreted by the intestinal L-cells in response to food intake. It plays a crucial role in regulating glucose homeostasis and insulin secretion.
2.1. GLP-1 and Its Impaired Function in T2D Patients
In individuals with type 2 diabetes, there is often a deficiency or impaired function of GLP-1, leading to reduced insulin secretion and impaired glucose control (Figure 1) [13][14]. Numerous scientific studies have investigated the role of GLP-1 in metabolic disorders. For example, a study by Drucker et al. demonstrated that GLP-1 receptor agonists such as exenatide and liraglutide improve glycemic control and promote weight loss in patients with type 2 diabetes [15]. These agonists mimic the action of endogenous GLP-1 and enhance insulin secretion, suppress glucagon release, delay gastric emptying, and promote satiety [16][17]. Another study by Nauck et al. showed that GLP-1 receptor agonists not only improve glycemic control but also have beneficial effects on cardiovascular outcomes, reducing the risk of major adverse cardiovascular events [18].
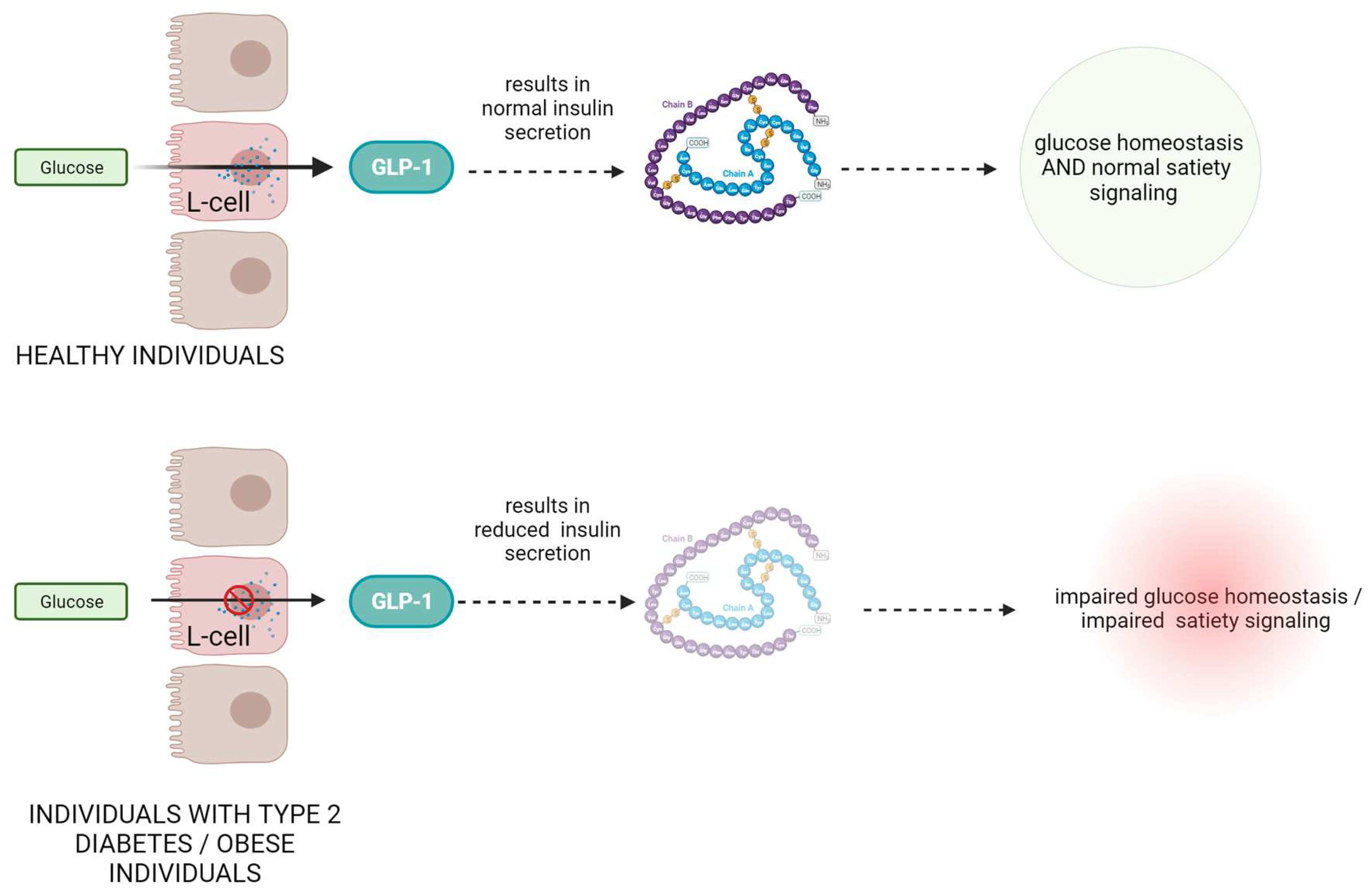
Figure 1. The role and mechanism of action of GLP-1 protein in healthy subjects and in patients with type II diabetes and obesity.
2.2. Dysregulated GLP-1 Secretion in Obese Patients
Moreover, GLP-1 has also been found to play a role in obesity. In obese individuals, GLP-1 secretion is often dysregulated, leading to reduced GLP-1 levels and impaired satiety signaling [19]. Research by Ard et al. demonstrated that administration of GLP-1 to obese individuals resulted in reduced food intake and increased feelings of fullness [20].
2.3. GLP-1 as a Therapeutic Hope
Additionally, GLP-1-based therapies have shown promising results in clinical trials. The LEADER trial, conducted by Marso et al. demonstrated that the GLP-1 receptor agonist liraglutide reduced the risk of major adverse cardiovascular events and cardiovascular mortality in patients with type 2 diabetes [21]. Another study by Davies et al. showed that exenatide, a GLP-1 receptor agonist, led to significant improvements in glycemic control and weight reduction in obese individuals with type 2 diabetes [22]. Further, they emphasize the potential of GLP-1 as an important biomarker for early diagnostics and therapeutic intervention in metabolic disorders [23][24][25][26].
References
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146.
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251, Erratum in Lancet 2017, 389, 2192.
- The Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222.
- Hoogeveen, E.K. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442.
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diabetes Rep. 2016, 16, 87.
- Chevillet, J.R.; Lee, I.; Briggs, H.A.; He, Y.; Wang, K. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules 2014, 19, 6080–6105.
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723.
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S15–S33.
- The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27.
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88.
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics 2015, 33, 673–689.
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355.
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705.
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102.
- Drucker, D.J. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003, 26, 2929–2940.
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165.
- Derosa, G.; Maffioli, P. GLP-1 Agonists Exenatide and Liraglutide: A Review about Their Safety and Efficacy. Curr. Clin. Pharmacol. 2012, 7, 214–228.
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; El Aziz, M.A.; Drucker, D.J. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017, 136, 849–870.
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3–36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463.
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2821–2839.
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. Drug Ther. Bull. 2016, 54, 311–322.
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE diabetes randomized clinical trial. JAMA J. Am. Med. Assoc. 2015, 314, 687–699.
- Vilsbøll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012, 344, d7771.
- Li, C.; Luo, J.; Jiang, M.; Wang, K. The Efficacy and Safety of the Combination Therapy with GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Front. Pharmacol. 2022, 13, 838277.
- Nauck, M.; Weinstock, R.S.; Umpierrez, G.E.; Guerci, B.; Skrivanek, Z.; Milicevic, Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care 2014, 37, 2149–2158.
- Sun, F.; Wu, S.; Wang, J.; Guo, S.; Chai, S.; Yang, Z.; Li, L.; Zhang, Y.; Ji, L.; Zhan, S. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: A systematic review and network meta-analysis. Clin. Ther. 2015, 37, 225–241.e8.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
643
Revisions:
2 times
(View History)
Update Date:
22 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





