
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Luigetti | -- | 2207 | 2024-01-18 07:15:02 | | | |
| 2 | Lindsay Dong | Meta information modification | 2207 | 2024-01-19 03:27:29 | | |
Video Upload Options
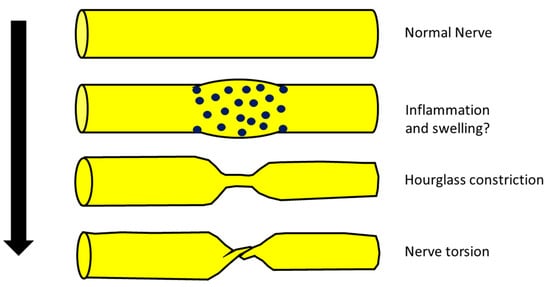
Neuralgic amyotrophy, also called Parsonage–Turner syndrome, in its classic presentation is a brachial plexopathy or a multifocal neuropathy, involving mainly motor nerves of the upper limb with a monophasic course. Recently, a new radiological entity was described, the hourglass constriction, which is characterized by a very focal constriction of a nerve, or part of it, usually associated with nerve thickening proximally and distally to the constriction. Another condition, which is similar from a radiological point of view to hourglass constriction, is nerve torsion. The pathophysiology of neuralgic amyotrophy, hourglass constriction and nerve torsion is still poorly understood, and a generic role of inflammation is proposed for all these conditions.
1. Introduction
2. NA, Hourglass Constriction and Nerve Torsion
- (a)
- (b)
- (c)
-
From a neurophysiological point of view, the typical picture of NA is the main or exclusive involvement of motor fibers, even in the case of mixed nerve involvement. On the other hand, hourglass constriction/nerve torsion usually affects both motor and sensory fibers at the same time and with the same severity. Motor fibers are exclusively included only in the case of pure motor nerve involvement (e.g., PIN and AIN) [11][12].
- (d)
3. Best Diagnostic Approach for These Conditions
3.1. Neuroimaging
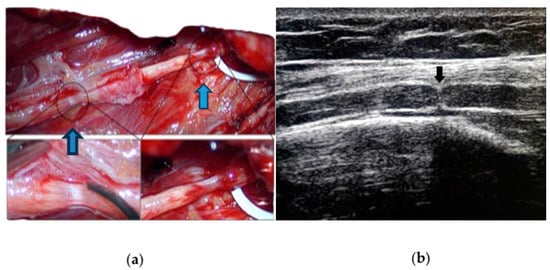
3.2. Neurophysiology
3.3. Differential Diagnosis of Acute Mononeuropathies and Related Examinations
4. Best Treatment Approach for These Conditions
References
- Smith, C.C.; Bevelaqua, A.C. Challenging pain syndromes: Parsonage-Turner syndrome. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 265–277.
- Van Eijk, J.J.; Groothuis, J.T.; Van Alfen, N. Neuralgic amyotrophy: An update on diagnosis, pathophysiology, and treatment. Muscle Nerve 2016, 53, 337–350.
- Seror, P. Neuralgic amyotrophy: An update. Jt. Bone Spine 2017, 2, 153–158.
- Farr, E.; D’Andrea, D.; Franz, C.K. Phrenic Nerve Involvement in Neuralgic Amyotrophy (Parsonage-Turner Syndrome). Sleep Med. Clin. 2020, 15, 539–543.
- Glorioso, D.; Palestini, R.; Cuccagna, C.; Lauretti, L.; Padua, L. Nerve Torsion as a Pattern of Parsonage-Turner Syndrome: Literature Review and Two Representative Cases. J. Clin. Med. 2023, 12, 4542.
- Sneag, D.B.; Urban, C.; Li, T.Y.; Colucci, P.G.; Pedrick, E.G.; Nimura, C.A.; Feinberg, J.H.; Milani, C.J.; Tan, E.T. Hourglass-like constrictions on MRI are common in electromyography-confirmed cases of neuralgic amyotrophy (Parsonage-Turner syndrome): A tertiary referral center experience. Muscle Nerve 2023. early view.
- Sneag, D.B.; Rancy, S.K.; Wolfe, S.W.; Lee, S.C.; Kalia, V.; Lee, S.K.; Feinberg, J.H. Brachial plexitis or neuritis? MRI features of lesion distribution in Parsonage-Turner syndrome. Muscle Nerve 2018, 58, 359–366.
- McGraw, I. Isolated spontaneous posterior interosseous nerve palsy: A review of aetiology and management. J. Hand Surg. Eur. Vol. 2019, 44, 310–316.
- Sigamoney, K.V.; Rashid, A.; Ng, C.Y. Management of Atraumatic Posterior Interosseous Nerve Palsy. J. Hand Surg. Am. 2017, 42, 826–830.
- Rubin, D.I. Neuralgic amyotrophy: Clinical features and diagnostic evaluation. Neurologist 2001, 7, 350–356.
- Shi, M.; Qi, H.; Ding, H.; Chen, F.; Xin, Z.; Zhao, Q.; Guan, S.; Shi, H. Electrophysiological examination and high frequency ultrasonography for diagnosis of radial nerve torsion and compression. Medicine 2018, 97, e9587.
- Granata, G.; Coraci, D.; Erra, C.; Tsukamoto, H.; Padua, L.; Luigetti, M. Posterior interosseous nerve syndrome due to radioulnar joint cyst. Muscle Nerve. 2013, 48, 842–843.
- Pan, Y.W.; Wang, S.; Tian, G.; Li, C.; Tian, W.; Tian, M. Typical brachial neuritis (Parsonage-Turner syndrome) with hourglass-like constrictions in the affected nerves. J. Hand Surg. Am. 2011, 36, 1197–1203.
- Deng, H.; Lu, B.; Yin, C.; Xu, Y.; Ding, Y.; Mi, Y.; Xu, P. The Effectiveness of Ultrasonography in the Diagnosis of Spontaneous Hourglasslike Constriction of Peripheral Nerve in the Upper Extremity. World Neurosurg. 2020, 134, e103–e111.
- Heiling, B.; Waschke, A.; Ceanga, M.; Grimm, A.; Witte, O.W.; Axer, H. Not your average Saturday night palsy-High resolution nerve ultrasound resolves rare cause of wrist drop. Clin. Neurol. Neurosurg. 2018, 172, 160–161.
- Lee, C.H.; Jeon, T.; Kim, C.U. Value of ultrasound in diagnosis of radial nerve palsy with hourglass-like constrictions without extrinsic compression: A case report. J. Hand Surg. Eur. Vol. 2018, 43, 445–447.
- Kim, D.H.; Sung, D.H.; Chang, M.C. Diagnosis of Hourglass-Like Constriction Neuropathy of the Radial Nerve Using High-Resolution Magnetic Resonance Neurography: A Report of Two Cases. Diagnostics 2020, 10, 232.
- Sneag, D.B.; Saltzman, E.B.; Meister, D.W.; Feinberg, J.H.; Lee, S.K.; Wolfe, S.W. MRI bullseye sign: An indicator of peripheral nerve constriction in parsonage-turner syndrome. Muscle Nerve 2017, 56, 99–106.
- Chan, J.K.; Kennett, R.; Smith, G. Posterior interosseous nerve palsy in rheumatoid arthritis: Case report and literature review. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, e556–e560.
- Ochi, K.; Horiuchi, Y.; Tazaki, K.; Nishi, K.; Kawashima, H.; Yabe, H. Spontaneous anterior interosseous nerve palsy with Churg-Strauss syndrome. Mod. Rheumatol. 2010, 20, 514–517.
- Khadilkar, S.V.; Patil, S.B.; Shetty, V.P. Neuropathies of leprosy. J. Neurol. Sci. 2021, 420, 117288.
- Sindic, C.J. Infectious neuropathies. Curr. Opin. Neurol. 2013, 26, 510–515.
- Silva, F.; Pinto, C.; Barbosa, A.; Borges, T.; Dias, C.; Almeida, J. New insights in cryoglobulinemic vasculitis. J. Autoimmun. 2019, 105, 102313.
- Tavee, J. Peripheral neuropathy in sarcoidosis. J. Neuroimmunol. 2022, 368, 577864.
- Fritz, D.; van de Beek, D.; Brouwer, M.C. Clinical features, treatment and outcome in neurosarcoidosis: Systematic review and meta-analysis. BMC Neurol. 2016, 16, 220.
- Nobile-Orazio, E.; Cappellari, A.; Meucci, N.; Carpo, M.; Terenghi, F.; Bersano, A.; Priori, A.; Barbieri, S.; Scarlato, G. Multifocal motor neuropathy: Clinical and immunological features and response to IVIg in relation to the presence and degree of motor conduction block. J. Neurol. Neurosurg. Psychiatry 2002, 72, 761–766.
- Beadon, K.; Guimarães-Costa, R.; Léger, J.M. Multifocal motor neuropathy. Curr. Opin. Neurol. 2018, 31, 559–564.
- Lucchetta, M.; Padua, L.; Granata, G.; Luigetti, M.; Campagnolo, M.; Dalla Torre, C.; Coraci, D.; Sabatelli, M.; Briani, C. Nerve ultrasound findings in neuropathy associated with anti-myelin-associated glycoprotein antibodies. Eur. J. Neurol. 2015, 22, 193–202.
- Rison, R.A.; Beydoun, S.R. Paraproteinemic neuropathy: A practical review. BMC Neurol. 2016, 16, 13.
- Ricci, L.; Luigetti, M.; Florio, L.; Capone, F.; Di Lazzaro, V. Causes of chronic neuropathies: A single-center experience. Neurol. Sci. 2019, 40, 1611–1617.
- Gerischer, L.M.; Scheibe, F.; Nümann, A.; Köhnlein, M.; Stölzel, U.; Meisel, A. Acute porphyrias—A neurological perspective. Brain Behav. 2021, 11, e2389.
- Meuleman, J.; Timmerman, V.; Van Broeckhoven, C.; De Jonghe, P. Hereditary neuralgic amyotrophy. Neurogenetics 2001, 3, 115–118.
- Hannibal, M.C.; Ruzzo, E.K.; Miller, L.R.; Betz, B.; Buchan, J.G.; Knutzen, D.M.; Barnett, K.; Landsverk, M.L.; Brice, A.; LeGuern, E.; et al. SEPT9 gene sequencing analysis reveals recurrent mutations in hereditary neuralgic amyotrophy. Neurology 2009, 72, 1755–1759.
- van Alfen, N.; van Engelen, B.G.; Hughes, R.A. Treatment for idiopathic and hereditary neuralgic amyotrophy (brachial neuritis). Cochrane Database Syst. Rev. 2009, 2009, CD006976.
- Qi, W.; Shen, Y.; Qiu, Y.; Jiang, S.; Yu, Y.; Yin, H.; Xu, W. Surgical treatment of hourglass-like radial nerve constrictions. Neurochirurgie 2021, 67, 170–175.
- Hashizume, H.; Nishida, K.; Nanba, Y.; Shigeyama, Y.; Inoue, H.; Morito, Y. Non-traumatic paralysis of the posterior interosseous nerve. J. Bone Jt. Surg. Br. 1996, 78, 771–776.
- Vastamäki, M. Prompt interfascicular neurolysis for the successful treatment of hourglass-like fascicular nerve compression. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2002, 36, 122–124.
- Wu, P.; Yang, J.Y.; Chen, L.; Yu, C. Surgical and conservative treatments of complete spontaneous posterior interosseous nerve palsy with hourglass-like fascicular constrictions: A retrospective study of 41 cases. Neurosurgery 2014, 75, 250–257; discussion 257.
- Burns, J.; Lister, G.D. Localized constrictive radial neuropathy in the absence of extrinsic compression: Three cases. J. Hand Surg. Am. 1984, 9A, 99–103.
- Eder, M.; Schulte-Mattler, W.; Pöschl, P. Neurographic course Of Wallerian degeneration after human peripheral nerve injury. Muscle Nerve 2017, 56, 247–252.




