
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Patrícia Elaine de Almeida | -- | 3215 | 2024-01-15 18:36:53 | | | |
| 2 | Lindsay Dong | Meta information modification | 3215 | 2024-01-16 01:16:14 | | |
Video Upload Options
Extracellular vesicles (EVs) have a significant impact on the pathophysiological processes associated with various diseases such as tumors, inflammation, and infection. They exhibit molecular, biochemical, and entry control characteristics similar to viral infections. Viruses, on the other hand, depend on host metabolic machineries to fulfill their biosynthetic requirements. Due to potential advantages such as biocompatibility, biodegradation, and efficient immune activation, EVs have emerged as potential therapeutic targets against the SARS-CoV-2 infection.
1. Introduction
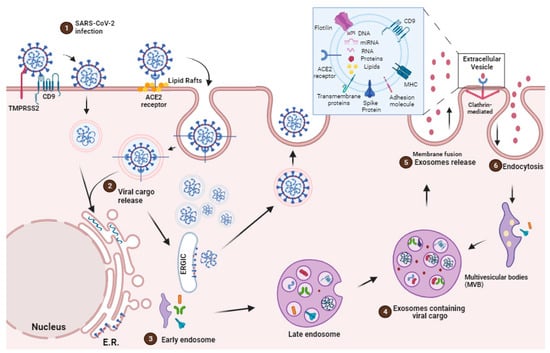
2. EVs Origins, Secretion and Communication
3. EVs as Potential Role for Immune System Evasion during SARS-CoV-2 Infection
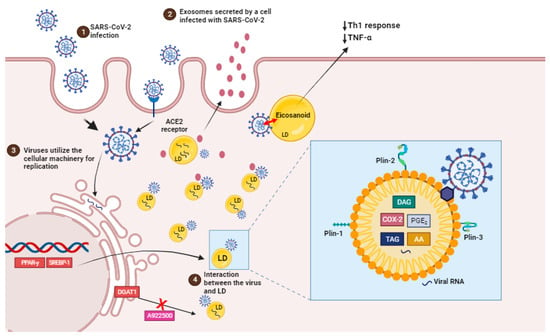
4. Impact of SARS-CoV-2 Infection in the Cellular Lipid Metabolism
Recent data suggest that lipid domains, known as lipid droplets, play an important role in SARS-CoV-2 replication and the synthesis of inflammatory mediators during the disease as well as in other viral infections such as dengue HCV, DENV, and rotavirus.
The virus’s cellular tropism for lipid metabolism cell machinery suggests that the virus may exploit endogenous lipid materials of different forms, such as lipoproteins and exosomes, as “Trojan horses” to facilitate immune evasion in their systemic spreading [28].
LDs are intracellular structures that contain triglycerides, cholesterol esters, and enzymes involved in lipid synthesis and storage (Figure 2, box). The expression of proteins linked with lipid metabolism and de novo lipid synthesis is modified during SARS-CoV-2 infection, as well as the pathways involved in lipid uptake, such as CD36 and the primary transcriptional factors involved in lipogenesis, including (peroxisome proliferator-activated receptor) PPARγ and SREBP-1 (Figure 2) [12][66].
SREBP is a transcription factor family that regulates lipid homeostasis by directing the expression of a wide range of fatty acid (SREBP1) and cholesterol (SREBP2) metabolic enzymes [66]. SREBP isoforms have been shown to increase during SARS-CoV-2 infection. Their activation is linked to the immunological response via the increased assembly of the inflammasome complex, which results in the production of IL-1 [67].
5. EVs as Therapeutic Target
6. Conclusions
References
- World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 10 October 2023).
- Kumar, D.; Manuel, O.; Natori, Y.; Egawa, H.; Grossi, P.; Han, S.H.; Fernández-Ruiz, M.; Humar, A. COVID-19: A Global Transplant Perspective on Successfully Navigating a Pandemic. Am. J. Transplant. 2020, 20, 1773–1779.
- Prather, B.K.A.; Wang, C.C.; Schooley, R.T. R 1422. Science 2020, 368, 1422–1424.
- van Doremalen, N.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; Lloyd-Smith, J.O.; et al. Correspondance Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567.
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. An Animal Model of Inhaled Vitamin E Acetate and EVALI-like Lung Injury. N. Engl. J. Med. 2020, 382, 1177–1179.
- Bailey, J.; Blankson, J.N.; Wind-Rotolo, M.; Siliciano, R.F. Mechanisms of HIV-1 Escape from Immune Responses and Antiretroviral Drugs. Curr. Opin. Immunol. 2004, 16, 470–476.
- Joyner, A.S.; Willis, J.R.; Crowe, J.E.; Aiken, C. Maturation-Induced Cloaking of Neutralization Epitopes on Hiv-1 Particles. PLoS Pathog. 2011, 7, e1002234.
- Lazarevic, I.; Banko, A.; Miljanovic, D.; Cupic, M. Immune-Escape Hepatitis B Virus Mutations Associated with Viral Reactivation upon Immunosuppression. Viruses 2019, 11, 778.
- Rydell, G.E.; Prakash, K.; Norder, H.; Lindh, M. Hepatitis B Surface Antigen on Subviral Particles Reduces the Neutralizing Effect of Anti-HBs Antibodies on Hepatitis B Viral Particles in Vitro. Virology 2017, 509, 67–70.
- Weber, F.; Haller, O. Viral Suppression of the Interferon System. Biochimie 2007, 89, 836–842.
- Yewdell, J.W.; Hill, A.B. Viral Interference with Antigen Presentation. Nat. Immunol. 2002, 3, 1019–1025.
- Dias da Silva Gomes, S.; Soares, V.C.; Ferreira, A.C.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Teixeira, L.; da Silva, M.A.N.; Barreto, E.; Mattos, M.; et al. Lipid Droplets Fuel SARS-CoV-2 Replication and Production of Inflammatory Mediators. PLoS Pathog. 2020, 16, e1009127.
- Chen, P.; Wu, M.; He, Y.; Jiang, B.; He, M.L. Metabolic Alterations upon SARS-CoV-2 Infection and Potential Therapeutic Targets against Coronavirus Infection. Signal Transduct. Target. Ther. 2023, 8, 237.
- Altan-Bonnet, N. Lipid Tales on Viral Replication and Transmission Nihal. Trends Cell Biol. 2017, 27, 201–203.
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-like Vesicles, and Apoptotic Bodies. J. Neurooncol. 2013, 1, 1–11.
- Hoen, E.N.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular Vesicles and Viruses: Are They Close Relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161.
- Simons, M.; Raposo, G. Exosomes—Vesicular Carriers for Intercellular Communication. Curr. Opin. Cell Biol. 2009, 21, 575–581.
- Mueller, S.K.; Nocera, A.L.; Bleier, B.S. Exosome Function in Aerodigestive Mucosa. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 269–277.
- Kowal, J.; Tkach, M.; Théry, C.; Kowal, J.; Tkach, M.; Biogenesis, C.T. Biogenesis and Secretion of Exosomes To Cite This Version: HAL Id: Inserm-02452742. Curr. Opin. Cell Biol. 2014, 29, 116–125.
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collén, A.; Sunnerhagen, P.; et al. Identification of RNA-Binding Proteins in Exosomes Capable of Interacting with Different Types of RNA: RBP-Facilitated Transport of RNAs into Exosomes. PLoS ONE 2018, 13, e0195969.
- Mathieu, M.; Martin-jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nature Cell Biol. 2019, 21, 9–17.
- Gould, S.J.; Booth, A.M.; Hildreth, J.E.K. The Trojan Exosome Hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597.
- Izquierdo-Useros, N.; Lorizate, M.; McLaren, P.J.; Telenti, A.; Kräusslich, H.G.; Martinez-Picado, J. HIV-1 Capture and Transmission by Dendritic Cells: The Role of Viral Glycolipids and the Cellular Receptor Siglec-1. PLoS Pathog. 2014, 10, e1004146.
- Elrashdy, F.; Aljaddawi, A.A.; Redwan, E.M.; Uversky, V.N. On the Potential Role of Exosomes in the COVID-19 Reinfection/Reactivation Opportunity. J. Biomol. Struct. Dyn. 2020, 39, 5831–5842.
- Borowiec, B.M.; Volponi, A.A.; Mozdziak, P.; Kempisty, B.; Dyszkiewicz-Konwińska, M. Small Extracellular Vesicles and Covid19—Using the “Trojan Horse” to Tackle the Giant. Cells 2021, 10, 3383.
- Tsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-Mediated MRNA Delivery in Vivo Is Safe and Can Be Used to Induce SARS-CoV-2 Immunity. J. Biol. Chem. 2021, 297, 101266.
- Troyer, Z.; Alhusaini, N.; Tabler, C.O.; Schlatzer, D.M.; Tilton, J.C.; Sweet, T.; Carias, L.; Inacio, K.; De Carvalho, L.; King, C.L.; et al. Extracellular Vesicles Carry SARS-CoV-2 Spike Protein and Serve as Decoys for Neutralizing Antibodies Zach. J. Extracell. Vesicles 2021, 10, e12112.
- Lam, S.M.; Huang, X.; Shui, G. Neurological Aspects of SARS-CoV-2 Infection: Lipoproteins and Exosomes as Trojan Horses. Trends Endocrinol. Metab. 2022, 33, 554–568.
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420.
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles. Traffic 2011, 12, 1659–1668.
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 ( MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 8, 1535750.
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247.
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral Sphingomyelinases Control Extracellular Vesicles Budding from the Plasma Membrane. J. Extracell. Vesicles 2017, 6, 1378056.
- Liu, Y.J.; Wang, C. A Review of the Regulatory Mechanisms of Extracellular Vesicles-Mediated Intercellular Communication. Cell Commun. Signal. 2023, 21, 77.
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Rome, L.H.; Burnette, D.T.; Coffey, R.J.; Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; et al. Reassessment of Exosome Composition Article Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18.
- Hurley, J.H. ESCRTs Are Everywhere. EMBO J. 2015, 34, 2398–2407.
- Zhang, J.; Kumar, S.; Jayachandran, M.; Hernandez, L.P.H.; Wang, S.; Wilson, E.M.; Lieske, J.C. Excretion of Urine Extracellular Vesicles Bearing Markers of Activated Immune Cells and Calcium/Phosphorus Physiology Differ between Calcium Kidney Stone Formers and Non-Stone Formers. BMC Nephrol. 2021, 22, 204.
- Jayachandran, M.; Yuzhakov, S.V.; Kumar, S.; Larson, N.B.; Enders, F.T.; Milliner, D.S.; Rule, A.D.; Lieske, J.C. Specific Populations of Urinary Extracellular Vesicles and Proteins Differentiate Type 1 Primary Hyperoxaluria Patients without and with Nephrocalcinosis or Kidney Stones. Orphanet J. Rare Dis. 2020, 15, 319.
- Heijnen, H.F.G.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived from Exocytosis of Multivesicular Bodies and α-Granules. Blood 1999, 94, 3791–3799.
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M.; Hospital, M.G. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188.
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and Release of Arrestin Domain-Containing Protein 1-Mediated Microvesicles (ARMMs) at Plasma Membrane by Recruitment of TSG101 Protein. Proc. Natl. Acad. Sci. USA 2012, 109, 4146–4151.
- Wang, X.; Melino, G.; Shi, Y. Actively or Passively Deacidified Lysosomes Push β-Coronavirus Egress. Cell Death Dis. 2021, 12, 12–14.
- Li, B.; Antonyak, M.A.; Zhang, J.; Cerione, R.A. RhoA Triggers a Specific Signaling Pathway That Generates Transforming Microvesicles in Cancer Cells. Oncogene 2012, 31, 4740–4749.
- Yang, J.; Gould, S.J. The Cis -Acting Signals That Target Proteins to Exosomes and Microvesicles. Biochem. Soc. Trans 2013, 41, 277–282.
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue kinetics. Br. J. Cancer 1972, 26, 239–257.
- Karnas, E.; Dudek, P.; Zuba-Surma, E.K. Stem Cell-Derived Extracellular Vesicles as New Tools in Regenerative Medicine—Immunomodulatory Role and Future Perspectives. Front. Immunol. 2023, 14, 1120175.
- Petroni, D.; Fabbri, C.; Babboni, S.; Menichetti, L.; Basta, G.; Del Turco, S. Extracellular Vesicles and Intercellular Communication: Challenges for In Vivo Molecular Imaging and Tracking. Pharmaceutics 2023, 15, 1639.
- Robbins, P.D.; Morelli, A.E. Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14, 195–208.
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448.
- Zhang, H.; Lv, P.; Jiang, J.; Liu, Y.; Yan, R.; Shu, S.; Hu, B.; Xiao, H.; Cai, K.; Yuan, S.; et al. Advances in Developing ACE2 Derivatives against SARS-CoV-2. Lancet Microbe 2023, 4, e369–e378.
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20.
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474.
- Perrier, A.; Bonnin, A.; Desmarets, L.; Danneels, A.; Goffard, A.; Rouillé, Y.; Dubuisson, J.; Belouzard, X.S. Cro The C-Terminal Domain of the MERS Coronavirus M Protein Contains a Trans -Golgi Network Localization Signal. J. Biol. Chem. 2019, 294, 14406–14421.
- Senapati, S.; Banerjee, P.; Bhagavatula, S.; Kushwaha, P.P.; Kumar, S. Contributions of Human ACE2 and TMPRSS2 in Determining Host–Pathogen Interaction of COVID-19. J. Genet. 2021, 100, 12.
- Cabal, A.B.S.; Wu, T.Y. Recombinant Protein Technology in the Challenging Era of Coronaviruses. Processes 2022, 10, 946.
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20.
- Wang, J.; Chen, S.; Bihl, J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxid. Med. Cell. Longev. 2020, 2020, 4213541.
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 Infects Cells after Viral Entry via Clathrin-Mediated Endocytosis. J. Biol. Chem. 2021, 296, 100306.
- Fozouni, P.; Son, S.; Díaz de León Derby, M.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-Free Detection of SARS-CoV-2 with CRISPR-Cas13a and Mobile Phone Microscopy. Cell 2021, 184, 323–333.
- Xia, X.; Yuan, P.; Zheng, J.C. Emerging Roles of Extracellular Vesicles in COVID—Edged Sword? Immunology 2021, 163, 416–430.
- Earnest, J.T.; Hantak, M.P.; Li, K.; Mccray, P.B.; Perlman, S.; Gallagher, T. The Tetraspanin CD9 Facilitates MERS- Coronavirus Entry by Scaffolding Host Cell Receptors and Proteases. PLoS Pathog. 2017, 13, e1006546.
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. SARS-CoV-2: A Master of Immune Evasion. Biomedicines 2022, 10, 1339.
- Horn, M.D.; MacLean, A.G. Extracellular Vesicles as a Means of Viral Immune Evasion, CNS Invasion, and Glia-Induced Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 695899.
- Pesce, E.; Manfrini, N.; Cordiglieri, C.; Santi, S.; Bandera, A.; Gobbini, A.; Gruarin, P.; Favalli, A.; Bombaci, M.; Cuomo, A.; et al. Exosomes Recovered From the Plasma of COVID-19 Patients Expose SARS-CoV-2 Spike-Derived Fragments and Contribute to the Adaptive Immune Response. Front. Immunol. 2022, 12, 785941.
- Soares, V.C.; Dias, S.S.G.; Santos, J.C.; Azevedo-Quintanilha, I.G.; Moreira, I.B.G.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; da Silva, M.A.N.; Barreto-Vieira, D.F.; et al. Inhibition of the SREBP Pathway Prevents SARS-CoV-2 Replication and Inflammasome Activation. Life Sci. Alliance 2023, 6, e202302049.
- Li, Y.; Xu, S.; Jiang, B.; Cohen, R.A.; Zang, M. Activation of Sterol Regulatory Element Binding Protein and NLRP3 Inflammasome in Atherosclerotic Lesion Development in Diabetic Pigs. PLoS ONE 2013, 8, e67532.
- Fintelman-Rodrigues, N.; Sacramento, C.Q.; Lima, C.R.; da Silva, F.S.; Ferreira, A.; Mattos, M.; de Freitas, C.S.; Soares, V.C.; da Silva Gomes Dias, S.; Temerozo, J.R.; et al. Atazanavir Inhibits SARS-CoV-2 Replication and pro-Inflammatory Cytokine Production. bioRxiv 2020.
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/Chemokine-Receptor System. Cytokine Growth Factor Rev. 2020, 53, 25–32.
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000.
- Kong, M.; Zhang, H.; Cao, X.; Mao, X.; Lu, Z. Higher Level of Neutrophil-to-Lymphocyte Is Associated with Severe COVID-19. Epidemiol. Infect. 2020, 148, e139.
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martínez-Colón, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A Single-Cell Atlas of the Peripheral Immune Response in Patients with Severe COVID-19. Nat. Med. 2020, 26, 1070–1076.
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients with COVID-19. Nat. Med. 2020, 26, 842–844.
- Chatel-Chaix, L.; Bartenschlager, R. Dengue Virus- and Hepatitis C Virus-Induced Replication and Assembly Compartments: The Enemy Inside—Caught in the Web. J. Virol. 2014, 88, 5907–5911.
- Lee, J.Y.; Cortese, M.; Haselmann, U.; Tabata, K.; Romero-Brey, I.; Funaya, C.; Schieber, N.L.; Qiang, Y.; Bartenschlager, M.; Kallis, S.; et al. Spatiotemporal Coupling of the Hepatitis C Virus Replication Cycle by Creating a Lipid Droplet- Proximal Membranous Replication Compartment. Cell Rep. 2019, 27, 3602–3617.e5.
- Miyanari, Y.; Atsuzawa, K.; Usuda, N.; Watashi, K.; Hishiki, T.; Zayas, M.; Bartenschlager, R.; Wakita, T.; Hijikata, M.; Shimotohno, K. The Lipid Droplet Is an Important Organelle for Hepatitis C Virus Production. Nat. Cell Biol. 2007, 9, 1089–1097.
- Laufman, O.; Perrino, J.; Andino, R. Viral Generated Inter-Organelle Contacts Redirect Lipid Flux for Genome Replication. Cell 2019, 178, 275–289.
- Lu, Y.; Zhou, T.; Xu, C.; Wang, R.; Feng, D.; Li, J.; Wang, X.; Kong, Y.; Hu, G.; Kong, X.; et al. Occludin Is a Target of Src Kinase and Promotes Lipid Secretion by Binding to BTN1a1 and XOR. PLoS Biol. 2022, 20, e3001518.
- Collot, M.; Fam, T.K.; Ashokkumar, P.; Faklaris, O.; Galli, T.; Danglot, L.; Klymchenko, A.S. Ultrabright and Fluorogenic Probes for Multicolor Imaging and Tracking of Lipid Droplets in Cells and Tissues. J. Am. Chem. Soc. 2018, 140, 5401–5411.
- Flaherty, S.E., III; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A.W., Jr. A Lipase-Independent Pathway of Lipid Release and Immune Modulation by Adipocytes. Science 2019, 363, 989–993.
- Amarasinghe, I.; Phillips, W.; Hill, A.F.; Cheng, L.; Helbig, K.J.; Willms, E.; Monson, E.A. Cellular Communication through extracellular vesicles and lipid droplets. J. Extracell Biol. 2023, 2, e77.
- Pocsfalvi, G.; Mammadova, R.; Ramos Juarez, A.P.; Bokka, R.; Trepiccione, F.; Capasso, G. COVID-19 and Extracellular Vesicles: An Intriguing Interplay. Kidney Blood Press. Res. 2020, 45, 661–670.
- Keller, M.D.; Ching, K.L.; Liang, F.X.; Dhabaria, A.; Tam, K.; Ueberheide, B.M.; Unutmaz, D.; Torres, V.J.; Cadwell, K. Decoy Exosomes Provide Protection against Bacterial Toxins. Nature 2020, 579, 260–264.
- Kuate, S.; Cinatl, J.; Doerr, H.W.; Überla, K. Exosomal Vaccines Containing the S Protein of the SARS Coronavirus Induce High Levels of Neutralizing Antibodies. Virology 2007, 362, 26–37.
- Catalano, M.; O’Driscoll, L. Inhibiting Extracellular Vesicles Formation and Release: A Review of EV Inhibitors. J. Extracell. Vesicles 2020, 9, 1703244.
- Zhou, X.; Zhang, W.; Yao, Q.; Zhang, H.; Dong, G.; Zhang, M.; Liu, Y.; Chen, J.K.; Dong, Z. Exosome Production and Its Regulation of EGFR during Wound Healing in Renal Tubular Cells. Am. J. Physiol. -Ren. Physiol. 2017, 312, F963–F970.
- Schneider, D.J.; Speth, J.M.; Penke, L.R.; Wettlaufer, S.H.; Swanson, J.A.; Peters-Golden, M. Mechanisms and Modulation of Microvesicle Uptake in a Model of Alveolar Cell Communication. J. Biol. Chem. 2017, 292, 20897–20910.
- Sánchez Berumen, G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular Vesicles: Mediators of Intercellular Communication in Tissue Injury and Disease. Cell Commun. Signal. 2021, 19, 104.
- Zheng, Y.; Tu, C.; Zhang, J.; Wang, J. Inhibition of Multiple Myeloma-derived Exosomes Uptake Suppresses the Functional Response in Bone Marrow Stromal Cell. Int. J. Oncol. 2019, 54, 1061–1070.
- Rosell, A.; Havervall, S.; Von Meijenfeldt, F.; Hisada, Y.; Aguilera, K.; Grover, S.P.; Lisman, T.; MacKman, N.; Thålin, C. Patients With COVID-19 Have Elevated Levels of Circulating Extracellular Vesicle Tissue Factor Activity That Is Associated With Severity and Mortality—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 878–882.




