Microvesicles or ectosomes, on the other hand, are larger EVs (200–500 nm) believed to bud directly from the plasma membrane
[32][40][41][47,54,55]. Similar to exosomes, the generation of microvesicles involves the active participation of several protein factors. These factors encompass Ca
2+-dependent aminophospholipid translocases, namely flippases and floppases, sphingomyelinase 2 (nSMase2), scramblases, and calpain. They collectively orchestrate the rearrangement of phospholipids, membrane curvature, and actin cytoskeleton reorganization, ultimately resulting in the extracellular formation of microvesicles
[42][43][44][45][56,57,58,59]. Apoptotic bodies or oncosomes are fragments of cells undergoing programmed cell death, representing the largest EVs with a diameter ranging from 1 to 5 μm
[32][41][42][46][47,55,56,60].
EVs from eukaryotic cells play a vital role in both local and systemic cellular communication, serving as intracellular mediators upon release into the extracellular space
[21][26]. Since EVs have the same membrane orientation as cells, they expose on their surface the extracellular domains of transmembrane proteins that can bind to nearby or long-distance targets. These EVs transfer their bioactive cargo to target cells, thereby influencing and altering their behaviors
[47][61].
The uptake of EVs is influenced by several factors, encompassing their dimensions, surface composition (lipids, glycans, proteins), pH conditions, temperature, and oxidative/hypoxic environment
[35][8]. The nature of both the donor and recipient cells can modulate this process
[48][63]. EVs are secreted through exocytosis or in multivesicular endosomes (MVEs), and they interact with target cells through ligand/receptor signaling at the plasma membrane
[21][26]
The cargo of EVs, which includes lipids, DNA, RNA and proteins, can alter the transcription and signaling activity of recipient cells, thereby regulating their phenotype and function
[35][8]. The protein composition of various types of EVs largely reflects that of the parent cells, exhibiting a notable enrichment of specific molecules. These include adhesion molecules, membrane-trafficking proteins, cytoskeleton components, heat-shock proteins, cytoplasmic enzymes, signal transduction molecules, cytokines, chemokines, proteinases, and cell-specific antigens
[49][66]. They originate from both immune and non-immune cells, which play a vital role in immune system regulation. EVs also possess the capacity to either facilitate immune stimulation or suppress it, thereby influencing the development of inflammatory, autoimmune, and infectious diseases.
3. EVs as Potential Role for Immune System Evasion during SARS-CoV-2 Infection
Viruses have developed intricate strategies to counteract the innate immune response, including interfering with antigen presentation, disrupting interferon signaling, and producing “decoy” sub-viral particles that bind to neutralizing antibodies (nAbs), thereby reducing their effective concentration available for neutralizing infectious virions
[6][7][8][9][10][11][11,12,13,14,15,16].
The virions consist of a structural spike glycoprotein, an M-membrane protein (a type III transmembrane glycoprotein), an N-nucleocapsid protein (present within the phospholipid bilayer), and non-structural proteins
[50][51][76,77]. Approximately one-third of the RNA sequence encodes four fundamental structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. The remaining two-thirds of the viral genome consists of ORF1a and ORF1b, which encode non-structural replicase/transcriptase proteins
[52][78].
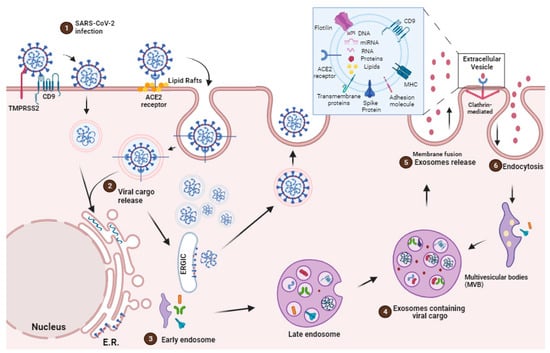
SARS-CoV-2 enters the host through the respiratory tract and infects the cell mainly via the angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 is a type I integral membrane protein involved in the renin–angiotensin system, which is found in the kidney, testis, intestine, lung, retina, cardiovascular system, adipose tissue, and central nervous system
[53][80]. Once SARS-CoV-2 is internalized into the cell cytoplasm, its lipid bilayers are dismantled by lysosomal enzymes. Subsequently, SARS-CoV-2 utilizes the host cell’s RNA polymerase to replicate its viral single-stranded RNA, thereby increasing the viral load within the host cell
[54][81] (
Figure 1).
The SARS-CoV-2 pathogenicity is highlighted by the entry of the virus through ACE2 receptors, cleavage of the complex, and activation of the S-protein by TMPRSS 2
[55][82]. According to structural analyses, the spike protein of SARS-CoV (SARS-S) contacts the apex of subunit I of the ACE2 catalytic domain. Once attached to ACE2 by SARS-CoV, the ectodomain of ACE2 is cleaved, which is accompanied by endocytosis of the transmembrane domain into the cell and sometimes internalized as an intact molecule
[56][83]. The internalization and virus particle–host cell fusion is essential for virus entry
[57][84] (
Figure 1).
In the pathogenesis of COVID-19, cells that express ACE2 and CD9 can transfer these viral receptors to other cells via EVs, making recipient cells more susceptible for SARS-CoV-2 infection. Recent investigations have shed light not only on the presence of ACE2 within EVs but also on the capacity of the EVs to transfer ACE2 across various cell types
[58][85]. In this context, SARS-CoV-2 gains access to target cells through binding to exosomal ACE2. However, recent studies have underscored the virus’s strong dependence on endocytic mechanisms with the ability to undergo swift endocytosis in cells that express high levels of ACE2
[59][86] (
Figure 1).
Hence, an alternative mechanism for EV-mediated viral entry involves one of the most highly expressed surface proteins on EVs, tetraspanin CD9
[60][75]. Research has indicated that CD9 collaborates with TMPRSS2 in cleaving viral fusion glycoproteins, expediting the entry of coronaviruses
[61][74], such as MERS-CoV
[62][87], into lung cells. These findings suggest that CD9 and other tetraspanins on the exosomal surface may serve as mediators in SARS-CoV-2 infection
[61][74] (
Figure 1).
Many factors have been associated with both altered ACE2 expression and COVID-19 severity and progression, including age, sex, ethnicity, medication, and several comorbidities, such as cardiovascular disease and metabolic syndrome. Although ACE2 is widely distributed in various human tissues and many of its determinants have been well recognized, ACE2-expressing organs do not equally participate in COVID-19 pathophysiology, implying that other mechanisms are involved in orchestrating cellular infection, resulting in tissue damage
[61][74].
Thus, one of the immune evasion strategies employed by SARS-CoV-2 involves the release of exosomes
[63][88], which can carry viruses, viral proteins, and genetic material. Many studies have suggested the role of released SARS-CoV-2-loaded exosomes and other EVs as potential mechanisms for COVID-19 infection relapse
[25][64][30,89]. The composition of plasma exosomes varies with the severity of the disease, with mild disease-associated vesicles modulating antigen-specific CD4 T cell responses and severe disease-associated vesicles linked to chronic inflammation
[65][90]. Therefore, analyzing exosome components provides valuable insights into different disease states.
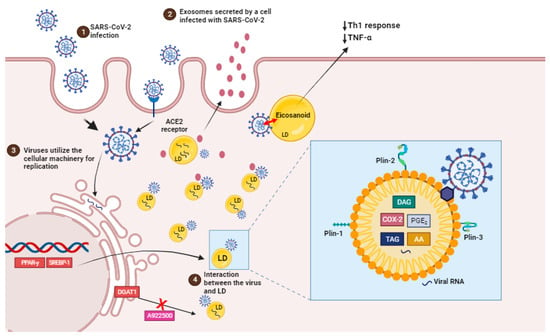
The virus co-opts the host’s lipid metabolism to optimize its replication dynamics. By targeting the host cells’ LDs, the principal reservoir of neutral lipids, the SARS-CoV-2 acquires energy substrates crucial for supporting its replication cycles
[12][17] (
Figure 2).
Figure 2. SARS-CoV-2 infection induces lipid droplet formation. (1) SARS-CoV-2 infection, via ACE2 receptor, can increase the activation of important transcription factors, such as SREBP1 and PPAR-gamma, leading to an increased induction of LDs biogenesis and the production of eicosanoids which modulate the host’s immune response. (2) EVs containing viral cargo are released into the extracellular environment. (3) Virus utilie the ER machinery for replication. (4) LDs interact with virus and support SARS-CoV-2 replication (box). Pharmacological inhibition and the genetic knockdown of SREBPs and DGAT1 inhibitor (A922500) can reduce SARS-CoV-2 replication and LD biogenesis. In detail, viral particles interact with LD, which is exhibiting structural proteins, such as PLIN-1, PLIN-2 and PLIN-3, and viral RNA. AA, arachidonic acid; COX-2, cyclooxygenase; DAG, diacylglycerol; PGE2, prostaglandin E2; TAG, triacylglycerol.
4. Impact of SARS-CoV-2 Infection in the Cellular Lipid Metabolism
Recent data suggest that lipid domains, known as lipid droplets, play an important role in SARS-CoV-2 replication and the synthesis of inflammatory mediators during the disease as well as in other viral infections such as dengue HCV, DENV, and rotavirus.
The virus’s cellular tropism for lipid metabolism cell machinery suggests that the virus may exploit endogenous lipid materials of different forms, such as lipoproteins and exosomes, as “Trojan horses” to facilitate immune evasion in their systemic spreading [28][40].
LDs are intracellular structures that contain triglycerides, cholesterol esters, and enzymes involved in lipid synthesis and storage (Figure 2, box). The expression of proteins linked with lipid metabolism and de novo lipid synthesis is modified during SARS-CoV-2 infection, as well as the pathways involved in lipid uptake, such as CD36 and the primary transcriptional factors involved in lipogenesis, including (peroxisome proliferator-activated receptor) PPARγ and SREBP-1 (Figure 2) [12][66][17,102].
SREBP is a transcription factor family that regulates lipid homeostasis by directing the expression of a wide range of fatty acid (SREBP1) and cholesterol (SREBP2) metabolic enzymes [66][102]. SREBP isoforms have been shown to increase during SARS-CoV-2 infection. Their activation is linked to the immunological response via the increased assembly of the inflammasome complex, which results in the production of IL-1 [67][103].
Beyond their direct role in viral replication, LDs have also been associated with the establishment of a pro-inflammatory state in COVID-19. In human monocytes infected in vitro with SARS-CoV-2, the accumulation of LDs leads to the upregulation of lipid metabolism-related genes and the release of pro-inflammatory mediators such as leukotrienes (LTB4 and cysLT), chemokines (IL-8 and CXCL10), and inflammatory cytokines (IL-6, TNF, and IL-10). These inflammatory mediators amplify the immune response, leading to the recruitment and activation of immune cells at the site of infection and thereby contributing to COVID-19 development
[68][69][104,105].
In fact, LDs are also sites of compartmentalized synthesis of eicosanoids during inflammatory conditions. Eicosanoids are inflammatory mediators that are derived from the breakdown of arachidonic acid (AA). Phospholipase A2 (PLA2) cleaves LDs and membrane phospholipids to produce AA. AA can be converted into prostaglandins (PGs), thromboxanes (TXs), or leukotrienes (LTs) by cyclooxygenases (COX) or lipoxygenases (LPX), which have regulatory roles in immunological homeostasis. PGE
2, TXB
2 and leukotriene B4 (LTB
4) levels were higher in COVID-19 patients’ bronchoalveolar lavage fluid than in healthy controls. Also, eicosanoid levels were positively correlated with cytokines (IL-1, IL-6, TNF-, IL-12p70, IL-22, and IFN-2) and chemokines (CCL2, CCL11, CXCL9)
[13][18]. PGE
2, the most prevalent PG, is a key mediator in a variety of physiological processes, particularly the development and regulation of inflammation
[70][106].
Leukotrienes are thought to be implicated in COVID-19 patients’ tissues because of increased neutrophil infiltration and neutrophil/lymphocyte ratios
[71][108]. In addition, single-cell examination of COVID-19 patients’ bronchoalveolar immune cells and PBMCs reveals enhanced 5-lipoxygenase expression
[72][73][109,110].
Furthermore, some viruses can employ LDs as a crucial site for viral component accumulation, assisting in the production of viral replication complexes
[74][75][76][77][113,114,115,116]. It has been discovered that LDs can be used as platforms for the assembly and maturation of new virus particles just before release. SARS-CoV-2 proteins and ds-RNA are currently tightly connected with the LDs and, in some circumstances, colocalizing with LDs in an in vitro infection model, indicating a probable function for LDs in the SARS-CoV-2 replication cycle
[12][17].
During COVID-19, the role of extracellular vesicles (EVs) in lipid metabolism remained limited. The transmission of viral components, including RNA, from infected to uninfected cells has been related to EVs. This could potentially facilitate the spread of the virus within the host, assisting viral replication and disease progression.
In parallel, there is evidence that cells can release LDs into the extracellular space, such as LDs being secreted from milk duct cells into milk
[78][119] and LDs moving between epithelial cells in vitro (KB HeLa cells)
[79][120]. It was also recently proposed that LDs could be bundled within adipocyte-derived EVs, activating macrophages in surrounding adipose tissue
[80][121]. There is a strong synergy between these cellular particles based on their similarities and the increasing role of LDs in intercellular communication. Some data suggest a previously unknown overlap and potential interaction between EV and LDs
[81][122].
5. EVs as Therapeutic Target
EVs can act as a decoy for viruses
[82][69] and bacterial toxins
[83][68], suggesting a potential role as therapeutic agents. Before the COVID-19 pandemic, some research groups envisioned the use of exosomes as immunogenic components for the treatment of virus infections utilizing models of SARS coronavirus infection. Kuate et al. showed that EVs containing the SARS coronavirus spike S protein caused neutralizing antibody titers that were enhanced by priming with the SARS coronavirus spike vaccine
[84][142]. Thus, coronavirus EVs may be effective for delivering therapeutic substances and inducing immune cell responses in the patient. Drugs or biological modulators that decrease viral propagation and replication in infected cells can be delivered into the EVs
[84][85][142,143]. EVs have potential advantages, such as cell origin, safety, and consistency, which distinguish them from other delivery techniques such as liposomes
[86][144].
Additionally, mRNAs, microRNAs, and DNA fragments carried by EVs can regulate gene expression in recipient cells
[87][146]. The diverse range of EVs and their cargo underscores the potential of utilizing EVs for therapeutic purposes, as they possess a repertoire of bioactive molecules with a combinatorial capacity that would be challenging to replicate artificially
[88][147]. As a result, miRNAs and lncRNAs extracted from exosomes could serve as biomarkers and potential drug carriers/delivery vehicles while acting as regulators of innate and acquired immunity through the stimulation of cytokine production, inflammatory responses, antigen presentation and lipid mediator synthesis.
The source of immunostimulatory exosomes for human antiviral vaccines is critical and must be further investigated
[89][148].
EVs, on the other hand, show significant potential in the diagnosis of COVID-19 and the prediction of mild and severe disease progression. SARS-CoV-2 infection produces thrombosis, and disease severity is closely linked with thrombosis development. Some clinical investigations show a large rise in circulating EVs carrying tissue factor (TF)/CD142 levels in COVID-19 patients as well as a strong connection with thrombosis, malignant disease development, and hospitalization length
[90][151].
6. Conclusions
The battle between viruses and their hosts has a long history. Whereas the immune system has evolved to protect from these pathogens, viruses have acquired clever evasion molecular mechanisms to avoid being detected and destroyed by the immune system. Given their extraordinarily high transmission rates and the possibility of recurrent outbreaks, there is an urgent need to find effective novel antiviral medicines for both treating present respiratory virus infections and preventing future outbreaks.
COVID-19 patients usually have dysregulated lipid profiles, which is associated with an adverse outcome of disease. As the virus is used to provide the building blocks for these vesicles and the viral envelope, they have developed sophisticated evasion strategies that explore lipid metabolism to replicate and avoid being recognized and destroyed by the immune system. Exosomes and respiratory viruses, such as coronavirus and other viruses, appear to make use of sorting complexes and cellular mechanisms that contribute to either maintain host homeostasis or allow viral replication, despite their distinct evolutionary origins. As a result, the development of exosome-based treatments, which include natural exosomes, nano-decoys, and antiviral-loaded exosomes, provide a range of treatment optimization choices that can successfully target, bind to, and limit the cellular absorption of various viruses.