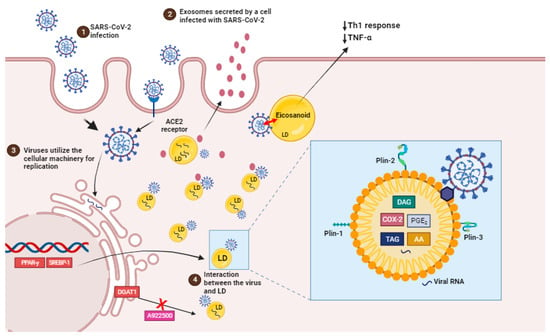
Figure 2. SARS-CoV-2 infection induces lipid droplet formation. (1) SARS-CoV-2 infection, via ACE2 receptor, can increase the activation of important transcription factors, such as SREBP1 and PPAR-gamma, leading to an increased induction of LDs biogenesis and the production of eicosanoids which modulate the host’s immune response. (2) EVs containing viral cargo are released into the extracellular environment. (3) Virus utilie the ER machinery for replication. (4) LDs interact with virus and support SARS-CoV-2 replication (box). Pharmacological inhibition and the genetic knockdown of SREBPs and DGAT1 inhibitor (A922500) can reduce SARS-CoV-2 replication and LD biogenesis. In detail, viral particles interact with LD, which is exhibiting structural proteins, such as PLIN-1, PLIN-2 and PLIN-3, and viral RNA. AA, arachidonic acid; COX-2, cyclooxygenase; DAG, diacylglycerol; PGE2, prostaglandin E2; TAG, triacylglycerol.
4. Impact of SARS-CoV-2 Infection in the Cellular Lipid Metabolism
Recent data suggest that lipid domains, known as lipid droplets, play an important role in SARS-CoV-2 replication and the synthesis of inflammatory mediators during the disease as well as in other viral infections such as dengue HCV, DENV, and rotavirus.
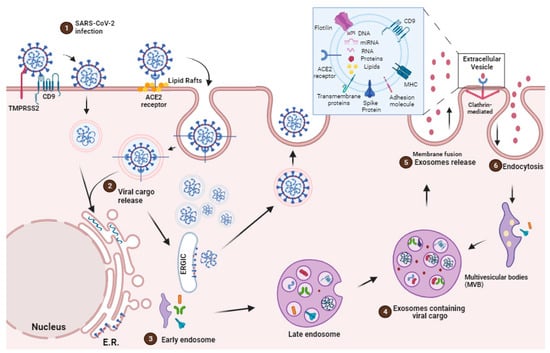
The virus’s cellular tropism for lipid metabolism cell machinery suggests that the virus may exploit endogenous lipid materials of different forms, such as lipoproteins and exosomes, as “Trojan horses” to facilitate immune evasion in their systemic spreading [40].
LDs are intracellular structures that contain triglycerides, cholesterol esters, and enzymes involved in lipid synthesis and storage (Figure 2, box). The expression of proteins linked with lipid metabolism and de novo lipid synthesis is modified during SARS-CoV-2 infection, as well as the pathways involved in lipid uptake, such as CD36 and the primary transcriptional factors involved in lipogenesis, including (peroxisome proliferator-activated receptor) PPARγ and SREBP-1 (Figure 2) [17,102].
SREBP is a transcription factor family that regulates lipid homeostasis by directing the expression of a wide range of fatty acid (SREBP1) and cholesterol (SREBP2) metabolic enzymes [102]. SREBP isoforms have been shown to increase during SARS-CoV-2 infection. Their activation is linked to the immunological response via the increased assembly of the inflammasome complex, which results in the production of IL-1 [103].
Beyond their direct role in viral replication, LDs have also been associated with the establishment of a pro-inflammatory state in COVID-19. In human monocytes infected in vitro with SARS-CoV-2, the accumulation of LDs leads to the upregulation of lipid metabolism-related genes and the release of pro-inflammatory mediators such as leukotrienes (LTB4 and cysLT), chemokines (IL-8 and CXCL10), and inflammatory cytokines (IL-6, TNF, and IL-10). These inflammatory mediators amplify the immune response, leading to the recruitment and activation of immune cells at the site of infection and thereby contributing to COVID-19 development [
104,
105].
In fact, LDs are also sites of compartmentalized synthesis of eicosanoids during inflammatory conditions. Eicosanoids are inflammatory mediators that are derived from the breakdown of arachidonic acid (AA). Phospholipase A2 (PLA2) cleaves LDs and membrane phospholipids to produce AA. AA can be converted into prostaglandins (PGs), thromboxanes (TXs), or leukotrienes (LTs) by cyclooxygenases (COX) or lipoxygenases (LPX), which have regulatory roles in immunological homeostasis. PGE
2, TXB
2 and leukotriene B4 (LTB
4) levels were higher in COVID-19 patients’ bronchoalveolar lavage fluid than in healthy controls. Also, eicosanoid levels were positively correlated with cytokines (IL-1, IL-6, TNF-, IL-12p70, IL-22, and IFN-2) and chemokines (CCL2, CCL11, CXCL9) [
18]. PGE
2, the most prevalent PG, is a key mediator in a variety of physiological processes, particularly the development and regulation of inflammation [
106].
Leukotrienes are thought to be implicated in COVID-19 patients’ tissues because of increased neutrophil infiltration and neutrophil/lymphocyte ratios [
108]. In addition, single-cell examination of COVID-19 patients’ bronchoalveolar immune cells and PBMCs reveals enhanced 5-lipoxygenase expression [
109,
110].
Furthermore, some viruses can employ LDs as a crucial site for viral component accumulation, assisting in the production of viral replication complexes [
113,
114,
115,
116]. It has been discovered that LDs can be used as platforms for the assembly and maturation of new virus particles just before release. SARS-CoV-2 proteins and ds-RNA are currently tightly connected with the LDs and, in some circumstances, colocalizing with LDs in an in vitro infection model, indicating a probable function for LDs in the SARS-CoV-2 replication cycle [
17].
During COVID-19, the role of extracellular vesicles (EVs) in lipid metabolism remained limited. The transmission of viral components, including RNA, from infected to uninfected cells has been related to EVs. This could potentially facilitate the spread of the virus within the host, assisting viral replication and disease progression.
In parallel, there is evidence that cells can release LDs into the extracellular space, such as LDs being secreted from milk duct cells into milk [
119] and LDs moving between epithelial cells in vitro (KB HeLa cells) [
120]. It was also recently proposed that LDs could be bundled within adipocyte-derived EVs, activating macrophages in surrounding adipose tissue [
121]. There is a strong synergy between these cellular particles based on their similarities and the increasing role of LDs in intercellular communication. Some data suggest a previously unknown overlap and potential interaction between EV and LDs [
122].
5. EVs as Therapeutic Target
EVs can act as a decoy for viruses [
69] and bacterial toxins [
68], suggesting a potential role as therapeutic agents. Before the COVID-19 pandemic, some research groups envisioned the use of exosomes as immunogenic components for the treatment of virus infections utilizing models of SARS coronavirus infection. Kuate et al. showed that EVs containing the SARS coronavirus spike S protein caused neutralizing antibody titers that were enhanced by priming with the SARS coronavirus spike vaccine [
142]. Thus, coronavirus EVs may be effective for delivering therapeutic substances and inducing immune cell responses in the patient. Drugs or biological modulators that decrease viral propagation and replication in infected cells can be delivered into the EVs [
142,
143]. EVs have potential advantages, such as cell origin, safety, and consistency, which distinguish them from other delivery techniques such as liposomes [
144].
Additionally, mRNAs, microRNAs, and DNA fragments carried by EVs can regulate gene expression in recipient cells [
146]. The diverse range of EVs and their cargo underscores the potential of utilizing EVs for therapeutic purposes, as they possess a repertoire of bioactive molecules with a combinatorial capacity that would be challenging to replicate artificially [
147]. As a result, miRNAs and lncRNAs extracted from exosomes could serve as biomarkers and potential drug carriers/delivery vehicles while acting as regulators of innate and acquired immunity through the stimulation of cytokine production, inflammatory responses, antigen presentation and lipid mediator synthesis.
The source of immunostimulatory exosomes for human antiviral vaccines is critical and must be further investigated [
148].
EVs, on the other hand, show significant potential in the diagnosis of COVID-19 and the prediction of mild and severe disease progression. SARS-CoV-2 infection produces thrombosis, and disease severity is closely linked with thrombosis development. Some clinical investigations show a large rise in circulating EVs carrying tissue factor (TF)/CD142 levels in COVID-19 patients as well as a strong connection with thrombosis, malignant disease development, and hospitalization length [
151].
6. Conclusions
The battle between viruses and their hosts has a long history. Whereas the immune system has evolved to protect from these pathogens, viruses have acquired clever evasion molecular mechanisms to avoid being detected and destroyed by the immune system. Given their extraordinarily high transmission rates and the possibility of recurrent outbreaks, there is an urgent need to find effective novel antiviral medicines for both treating present respiratory virus infections and preventing future outbreaks.
COVID-19 patients usually have dysregulated lipid profiles, which is associated with an adverse outcome of disease. As the virus is used to provide the building blocks for these vesicles and the viral envelope, they have developed sophisticated evasion strategies that explore lipid metabolism to replicate and avoid being recognized and destroyed by the immune system. Exosomes and respiratory viruses, such as coronavirus and other viruses, appear to make use of sorting complexes and cellular mechanisms that contribute to either maintain host homeostasis or allow viral replication, despite their distinct evolutionary origins. As a result, the development of exosome-based treatments, which include natural exosomes, nano-decoys, and antiviral-loaded exosomes, provide a range of treatment optimization choices that can successfully target, bind to, and limit the cellular absorption of various viruses.