
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos Ocampo-López | -- | 2819 | 2024-01-14 01:10:47 | | | |
| 2 | Peter Tang | Meta information modification | 2819 | 2024-01-15 03:53:06 | | |
Video Upload Options
Waste printed circuit boards (WPCBs), constituting approximately 10% of all electronic waste (e-waste), are particularly intriguing due to their high content of valuable metals and rare earth elements.
1. Significance of Waste Printed Circuit Boards (WPCBs)
2. Composition of WPCBs
|
Classification |
Percent (%) |
Element |
|---|---|---|
|
Major elements |
5–20 |
Fe, Cu, Al |
|
Intermediate elements |
0.1–10 |
Zn, Sn, Ni, Pb, Cr |
|
Minor elements |
<0.1 |
Ag, Au, Ba, Bi, Cd, Ga, Ge, In, Mn, Pb, Pd, Ta, Ti |
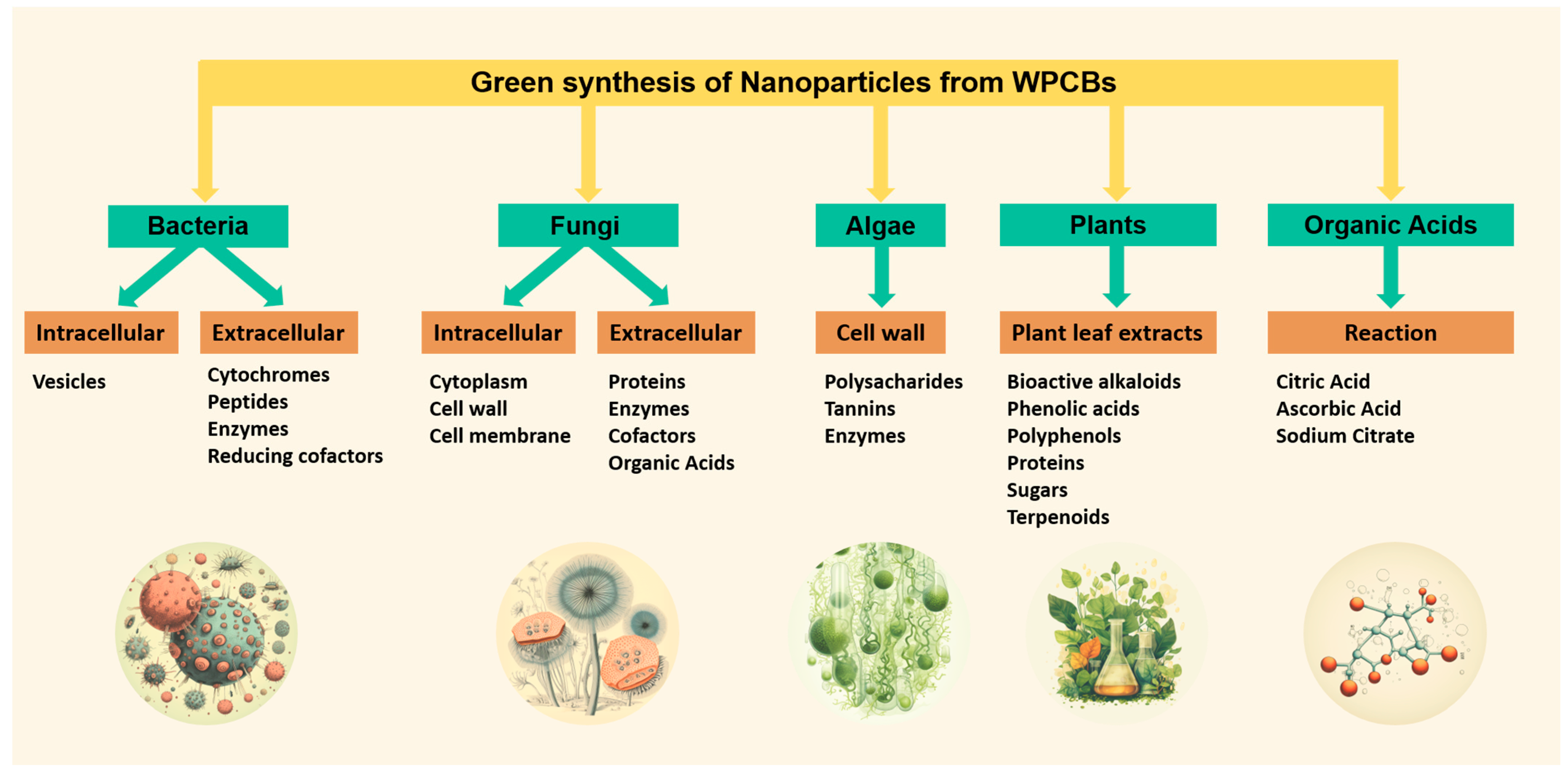
3. Green Organic Acids
4. Plants
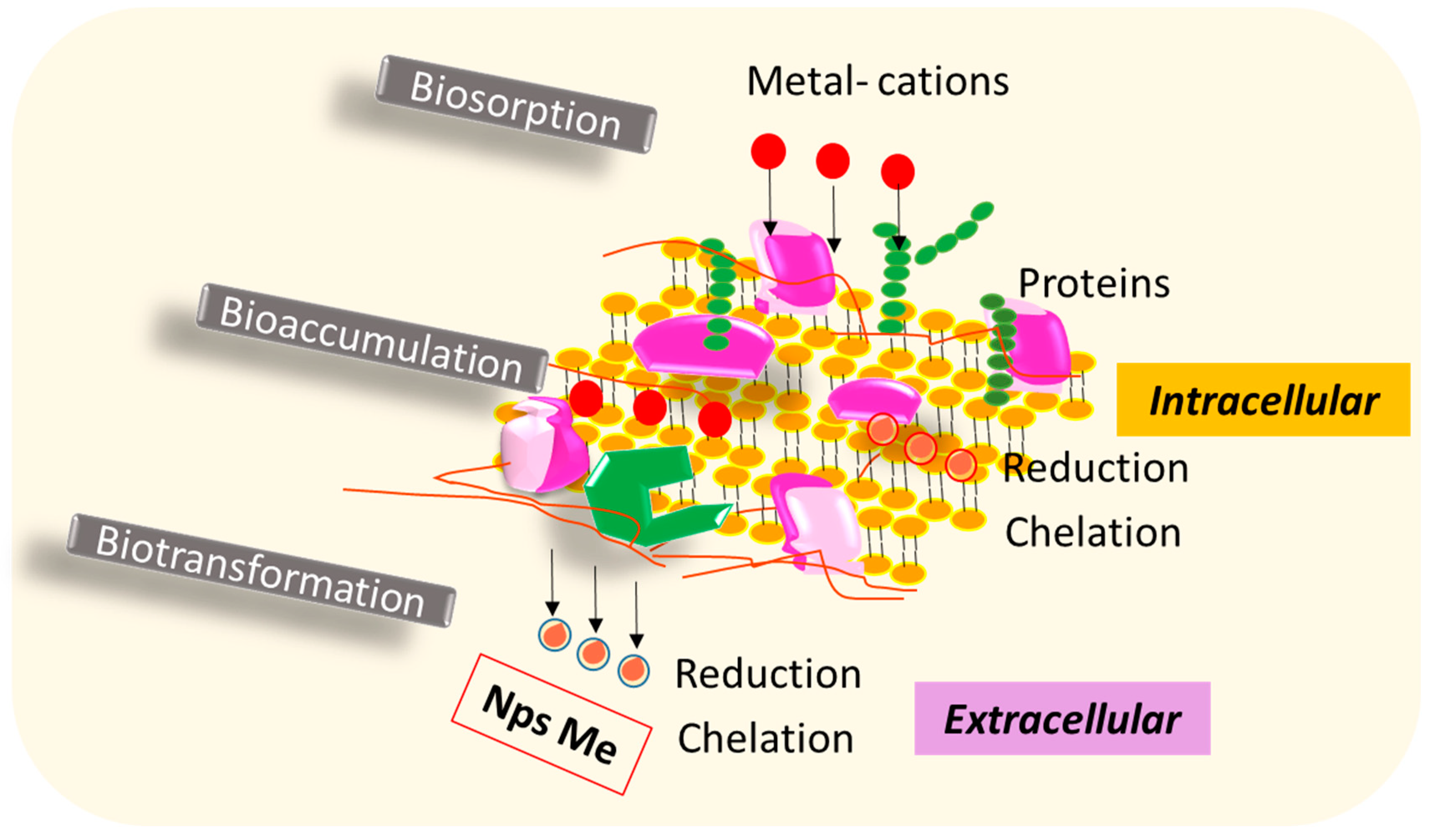
5. Bacteria
6. Fungi
7. Algae
|
Algae |
Highlights |
Element |
Nanoparticle Size |
Reference |
|---|---|---|---|---|
|
Sargassum spp. |
The microwave-assisted synthe-sis (MAS) of AgNPs using Sar-gassum spp. biomass involves the reduction of Ag+ ions by diverse organic compounds present in the macroalgae extract, including polysaccharides, proteins, poly-phenols, and flavonoids, through redox reactions. |
Ag |
10 to 175 nm. Average size of 36.43 nm |
[43] |
|
Rhizoclonium hieroglyphicum, Lyngbya majuscule and Spirulina subsalsa |
The reduction of gold particles is attributed to the presence of cellular reductases, with proteins such as cysteine serving to stabilize the nanoparticles. |
Au |
<20 nm |
[44] |
|
Chlorella vulgaris |
Carboxylate groups on the surface of Chlorella vulgaris cells capture metal ions, which are subsequently reduced to silver nanoparticles by reductase enzymes. |
Ag |
Average size 10.95 nm |
[45] |
References
- Duman, G.M.; Kongar, E. ESG Modeling and Prediction Uncertainty of Electronic Waste. Sustainability 2023, 15, 11281.
- Tatariants, M.; Yousef, S.; Skapas, M.; Juskenas, R.; Makarevicius, V.; Lukošiūtė, S.-I.; Denafas, G. Industrial Technology for Mass Production of SnO2 Nanoparticles and PbO2 Microcube/Microcross Structures from Electronic Waste. J. Clean. Prod. 2018, 203, 498–510.
- Yousef, S.; Tatariants, M.; Makarevičius, V.; Lukošiūtė, S.-I.; Bendikiene, R.; Denafas, G. A Strategy for Synthesis of Copper Nanoparticles from Recovered Metal of Waste Printed Circuit Boards. J. Clean. Prod. 2018, 185, 653–664.
- Zielonka, A.; Klimek-Ochab, M. Fungal Synthesis of Size-Defined Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 043001.
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal Bioleaching of WPCBs Using Aspergillus niger: Observation, Optimization and Kinetics. J. Environ. Manag. 2018, 217, 775–787.
- Yaashikaa, P.R.; Priyanka, B.; Senthil Kumar, P.; Karishma, S.; Jeevanantham, S.; Indraganti, S. A Review on Recent Advancements in Recovery of Valuable and Toxic Metals from E-Waste Using Bioleaching Approach. Chemosphere 2022, 287, 132230.
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782.
- Tatariants, M.; Yousef, S.; Sakalauskaitė, S.; Daugelavičius, R.; Denafas, G.; Bendikiene, R. Antimicrobial Copper Nanoparticles Synthesized from Waste Printed Circuit Boards Using Advanced Chemical Technology. Waste Manag. 2018, 78, 521–531.
- Priya, A.; Hait, S. Extraction of Metals from High Grade Waste Printed Circuit Board by Conventional and Hybrid Bioleaching Using Acidithiobacillus Ferrooxidans. Hydrometallurgy 2018, 177, 132–139.
- Rao, M.D.; Singh, K.K. Sequential recovery of copper, nickel, gold, silver and zinc from WPCBs of obsolete mobile phones through hydrometallurgical recycling. Mater. Today Proc. 2023, in press.
- Huang, T.; Zhu, J.; Huang, X.; Ruan, J.; Xu, Z. Assessment of Precious Metals Positioning in Waste Printed Circuit Boards and the Economic Benefits of Recycling. Waste Manag. 2022, 139, 105–115.
- Xia, D.; Charpentier, N.M.; Maurice, A.A.; Brambilla, A.; Yan, Q.; Gabriel, J.C.P. Sustainable route for Nd recycling from waste electronic components featured with unique element-specific sorting enabling simplified hydrometallurgy. Chem. Eng. J. 2022, 441, 135886.
- Escorcia-Díaz, D.; García-Mora, S.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review. Nanomaterials 2023, 13, 2586.
- Ravi, B.; Duraisamy, P.; Marimuthu, T. A Novel Integrated Circular Economy Approach in Green Synthesis of Copper Oxide Nanoparticles from Waste Printed Circuit Boards and Utilization of Its Residue for Preparation of Carbon Engulfed Nano Polymer Membrane. J. Clean. Prod. 2023, 383, 135457.
- Caldas, M.P.K.; Martins, T.A.G.; de Moraes, V.T.; Tenório, J.A.S.; Espinosa, D.C.R. Synthesis of Ag Nanoparticles from Waste Printed Circuit Board. J. Environ. Chem. Eng. 2021, 9, 106845.
- Punjabi, K.; Mehta, S.; Yedurkar, S.; Jain, R.; Mukherjee, S.; Kale, A.; Deshpande, S. Extracellular Synthesis of Silver Nanoparticle by Pseudomonas Hibiscicola—Mechanistic Approach. Adv. Nano Res. 2018, 6, 81–92.
- Shokri, A.; Pahlevani, F.; Levick, K.; Cole, I.; Sahajwalla, V. Synthesis of Copper-Tin Nanoparticles from Old Computer Printed Circuit Boards. J. Clean. Prod. 2017, 142, 2586–2592.
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707.
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. Kona 2020, 37, 224–232.
- Martins, T.A.G.; Falconi, I.B.A.; Pavoski, G.; de Moraes, V.T.; Galluzzi Baltazar, M.d.P.; Espinosa, D.C.R. Green Synthesis, Characterization, and Application of Copper Nanoparticles Obtained from Printed Circuit Boards to Degrade Mining Surfactant by Fenton Process. J. Environ. Chem. Eng. 2021, 9, 106576.
- Abdelbasir, S.M.; Rayan, D.A.; Ismail, M.M. Synthesis of Cu and CuO Nanoparticles from E-Waste and Evaluation of Their Antibacterial and Photocatalytic Properties. Res. Sq. 2023, 30, 89690–89704.
- Rana, S.; Mishra, P.; Wahid, Z.; Thakur, S.; Pant, D.; Singh, L. Microbe-Mediated Sustainable Bio-Recovery of Gold from Low-Grade Precious Solid Waste: A Microbiological Overview. J. Environ. Sci. 2020, 89, 47–64.
- Zhu, X.; Nie, C.; Ni, Y.; Zhang, T.; Li, B.; Wang, D.; Qu, S.; Qiao, F.; Lyu, X.; Qiu, J.; et al. Advanced Utilization of Copper in Waste Printed Circuit Boards: Synthesis of Nano-Copper Assisted by Physical Enrichment. J. Hazard. Mater. 2021, 401, 123294.
- Alexandre-Franco, M.F.; Fernández-González, C.; Reguero-Padilla, G.; Cuerda-Correa, E.M. Olive-Tree Polyphenols and Urban Mining. A Greener Alternative for the Recovery of Valuable Metals from Scrap Printed Circuit Boards. Environ. Res. 2022, 214, 114112.
- Antunes Filho, S.; dos Santos, M.S.; dos Santos, O.A.L.; Backx, B.P.; Soran, M.-L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060.
- Ettadili, F.E.; Aghris, S.; Laghrib, F.; Farahi, A.; Saqrane, S.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Recent Advances in the Nanoparticles Synthesis Using Plant Extract: Applications and Future Recommendations. J. Mol. Struct. 2022, 1248, 131538.
- Nithya, R.; Sivasankari, C.; Thirunavukkarasu, A.; Selvasembian, R. Novel Adsorbent Prepared from Bio-Hydrometallurgical Leachate from Waste Printed Circuit Board Used for the Removal of Methylene Blue from Aqueous Solution. Microchem. J. 2018, 142, 321–328.
- Priya, B.; Sobiya, S.; Kshayalakshmi, S.; Umamaheshwari, S.; Priyadharshini, M.; Rathi Devi, P. Phyto-Mediated Synthesis of Copper Nanoparticles by Cassia Auriculata and Its Characterization with Reference to E-Waste Management. Int. J. Environ. Agric. Biotechnol. 2017, 2, 808–811.
- Pawlowska, A.; Sadowski, Z. Biosynthesis of Copper Nanoparticles Using Aqueous Extracts of Aloe Vera and Geranium and Bioleaching Solutions. Solid State Phenom. 2017, 262, 193–196.
- Borthakur, A.; Singh, P. Microbial Biotechnology for Sustainable Electronic Waste. In Proceedings of the Asia-Pacific Conference on Biotechnology for Waste Conversion, Hong Kong, China, 5 December 2016; pp. 314–317.
- Subbaiyan, R.; Ganesan, A.; Sasikumar, B.; Rajendran, S.; Ramasubramanian, B. Synthesis and Characterization of Ferrous and Copper Nanoparticles from E-Waste Using Biological Reduction by Lichen-Associated Bacteria and Their Application in Antifouling Activity. Appl. Biochem. Biotechnol. 2023, 195, 3142–3155.
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599.
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 59361.
- Rozas, E.E.; Mendes, M.A.; Nascimento, C.A.O.; Espinosa, D.C.R.; Oliveira, R.; Oliveira, G.; Custodio, M.R. Bioleaching of Electronic Waste Using Bacteria Isolated from the Marine Sponge Hymeniacidon heliophila (Porifera). J. Hazard. Mater. 2017, 329, 120–130.
- Das, S.; Dong, B.; Ting, Y.P. Gold Biodissolution from Electronic Scrap and Biomineralization of Bacterial Gold Nanoparticles. Adv. Mat. Res. 2015, 1130, 668–672.
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.d.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 660582.
- Bindschedler, S.; Vu Bouquet, T.Q.T.; Job, D.; Joseph, E.; Junier, P. Chapter Two—Fungal Biorecovery of Gold from E-Waste; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: New York, NY, USA, 2017; Volume 99, pp. 53–81. ISBN 0065-2164.
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576.
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus Interaction: Review on Cellular Processes Underlying Heavy Metal Detoxification and Synthesis of Metal Nanoparticles. Chemosphere 2021, 274, 129976.
- Pineda-Vásquez, T.G.; Casas-Botero, A.E.; Ramírez-Carmona, M.E.; Torres-Taborda, M.M.; Soares, C.H.L.; Hotza, D. Biogeneration of Silica Nanoparticles from Rice Husk Ash Using Fusarium Oxysporum in Two Different Growth Media. Ind. Eng. Chem. Res. 2014, 53, 6959–6965.
- Ponduru, H.K.; Gurubilli, C.S.R.; Mudunuru, S.; Banisetti, D.K. Green Synthesis of Nano Particles from Biodegradable Waste Extracts and Their Applications. Int. J. Pharmacogn. Chem. 2023, 4, 46–54.
- Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolenčík, M. Mycosynthesis of Metal-Containing Nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and Their Application. Int. J. Mol. Sci. 2023, 24, 304.
- Pekkoh, J.; Ruangrit, K.; Kaewkod, T.; Tragoolpua, Y.; Hoijang, S.; Srisombat, L.; Wichapein, A.; Pathom-aree, W.; Kato, Y.; Wang, G.; et al. Innovative Eco-Friendly Microwave-Assisted Rapid Biosynthesis of Ag/AgCl-NPs Coated with Algae Bloom Extract as Multi-Functional Biomaterials with Non-Toxic Effects on Normal Human Cells. Nanomaterials 2023, 13, 2141.
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of Gold Using Cyanobacteria and an Eukaryotic Alga with Special Reference to Nanogold Formation—A Novel Phenomenon. J. Appl. Phycol. 2009, 21, 145–152.
- Soleimani, M.; Habibi-Pirkoohi, M. Biosynthesis of Silver Nanoparticles Using Chlorella Vulgaris and Evaluation of the Antibacterial Efficacy Against Staphylococcus Aureus. Avicenna J. Med. Biotechnol. 2017, 9, 120–125.
- Babu, S.; Jojo, L.; James, A.; Melethil, K.; Thomas, B. Metal-Based Catalysis for Biomass and Renewables Valorization- Current Status. Tetrahedron Green. Chem. 2023, 2, 100018.





