
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marilena Carbone | -- | 3661 | 2024-01-10 11:57:02 | | | |
| 2 | Peter Tang | Meta information modification | 3661 | 2024-01-11 03:53:07 | | |
Video Upload Options
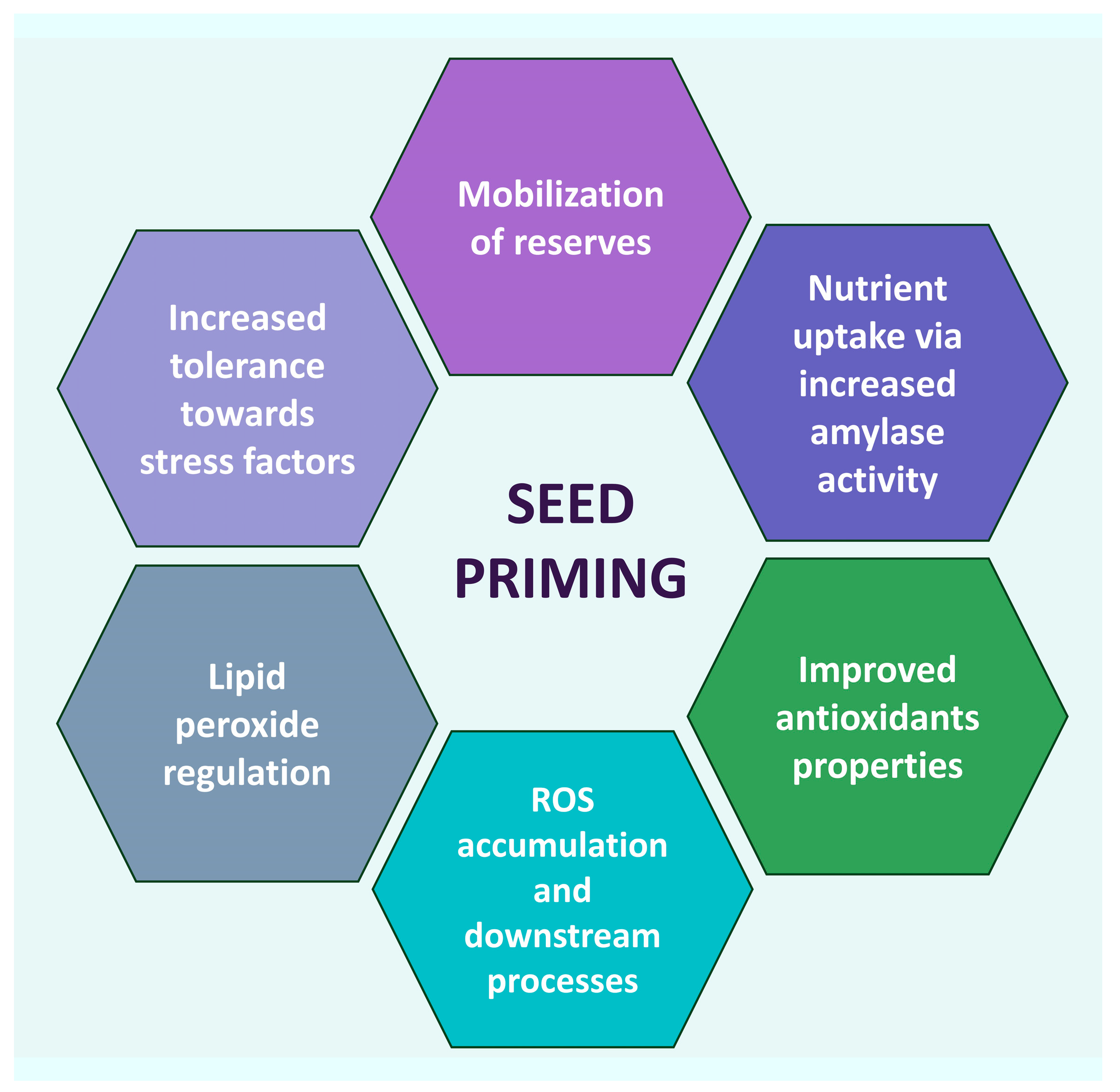
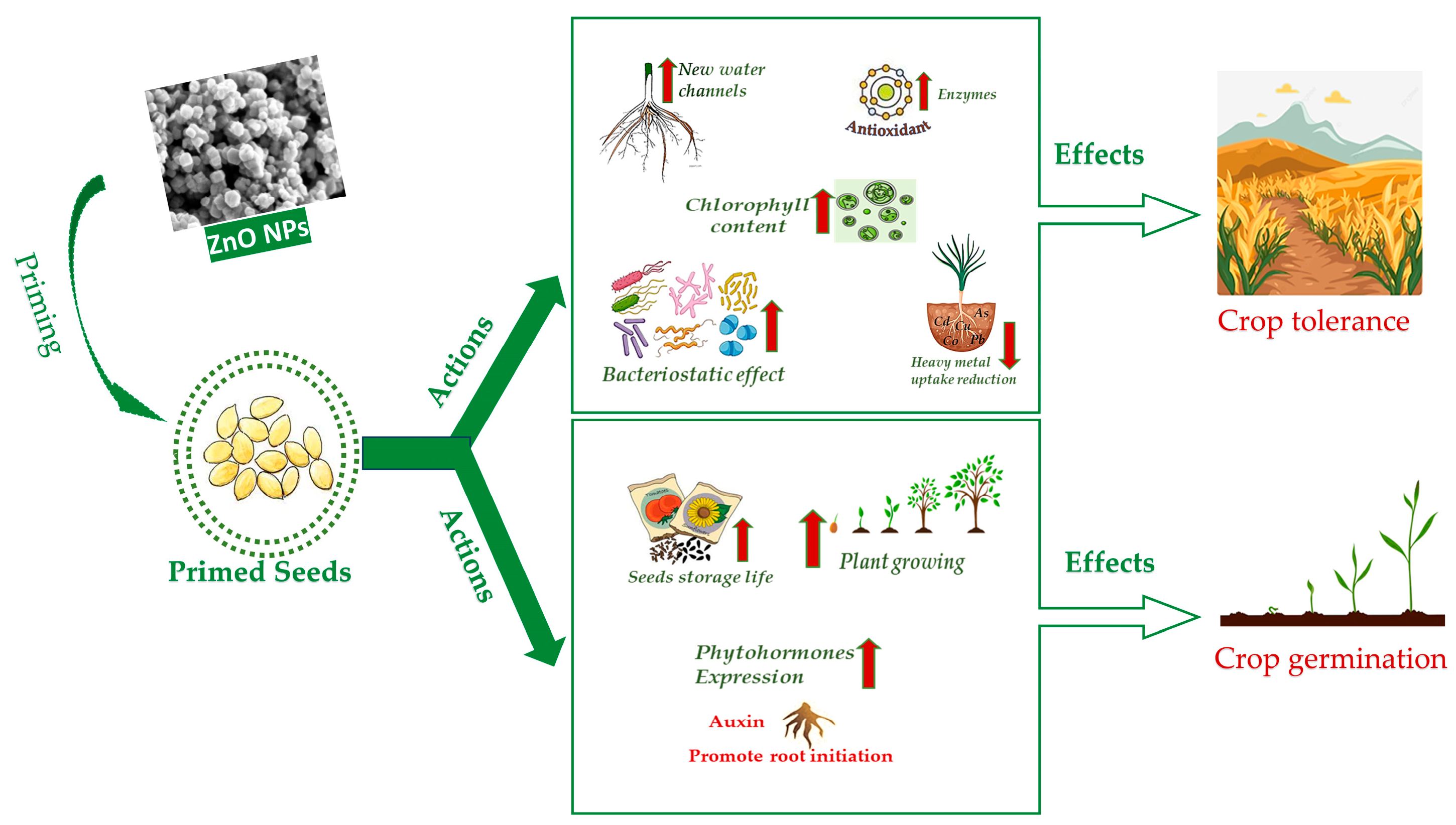
Drastic climate changes over the years have triggered environmental challenges for wild plants and crops due to fluctuating weather patterns worldwide. This has caused different types of stressors, responsible for a decrease in plant life and biological productivity, with consequent food shortages, especially in areas under threat of desertification. Nanotechnology-based approaches have great potential in mitigating environmental stressors, thus fostering a sustainable agriculture. Zinc oxide nanoparticles (ZnO NPs) have demonstrated to be biostimulants as well as remedies to both environmental and biotic stresses. Their administration in the early sowing stages, i.e., seed priming, proved to be effective in improving germination rate, seedling and plant growth and in ameliorating the indicators of plants’ well-being. Seed nano-priming acts through several mechanisms such as enhanced nutrients uptake, improved antioxidant properties, ROS accumulation and lipid peroxidation. The target for seed priming by ZnO NPs is mostly crops of large consumption or staple food, in order to meet the increased needs of a growing population and the net drop of global crop frequency, due to climate changes and soil contaminations.
1. Introduction
2. Seed Nano-Priming Mechanism NPs Entry
3. Application to Different Crops
4. ZnO NPs Seed Priming against Abiotic Stresses
|
Application |
Type of Seed |
Doses |
Effects |
Ref. |
|---|---|---|---|---|
|
Drought stress alleviation |
Rice |
0; 5; 10; 15; 25; 50 ppm |
Agronomic profile yield |
[81] |
|
Wheat |
40; 80; 120; 160 ppm |
Chlorophyll content and plant nutrients |
[82] |
|
|
Wheat |
10 mg/L |
Reduced DNA damage |
[83] |
|
|
Salt stress alleviation |
Sorghum bicolor |
5; 10 mg/L |
Increased Fresh weights |
[84] |
|
Wheat |
50 mg/L |
Increased plant growth, grain yield and macronutrients |
[85] |
|
|
Wheat |
50 mg/L |
Improved germination |
[86] |
|
|
Rapeseed |
25; 50; 100 mg/L |
Increased vigor indexes |
[87] |
|
|
Cu stress alleviation |
Wheat |
20 ppm |
Improved growth |
[86] |
|
Co stress alleviation |
Maize |
500 mg/L |
Improved plant growth, biomass and photosynthetic machinery |
[88] |
|
Pb stress alleviation |
Basella alba |
200 mg/L |
Increased seed germination, seedling and roots growth, seed vigor; reduced Pb uptake |
[89] |
|
Cd stress alleviation |
Rice |
0; 25; 50; 100 mg/L |
Improved early growth and related physio-biochemical attributes |
[90] |
|
Arsenic stress alleviation |
V. mungo (bean) |
50; 100; 150; 200 mg/L |
Increased germination by modulation of metabolic pathways |
[91] |
|
Biotic stress counteraction |
Chickpea |
0.25; 0.50; 0.75; 1.0 µg/mL 1000 mg/kg |
Antifungal Activity |
[92] |
|
Reduction in disease indices |
[93] |
|||
|
Seed storage |
V. radiata (bean) |
1000 ppm |
Increased germination percentage |
[94] |
|
Chickpea |
100 ppm |
increased seed storage life; decreased disease incidence |
[95] |
|
|
Crop improvement |
Wheat |
5; 10; 15; 20 ppm |
Increased plant growth |
[96] |
|
Oryza sativa (rice) |
10 µmol |
Seedling vigor; increased plant chlorophyll content; nutrients uptake |
[97] |
|
|
Tomato |
100 ppm |
Improved germination process |
[98] |
|
|
Cotton |
400 ppm |
Increased germination and seed parameters |
[99] |
|
|
Wheat |
10 mgL−1 |
Increased germination, vigor index and chlorophyll content |
[100] |
|
|
V. faba (bean) |
50, 100 mgL−1 |
Increased biometric parameters, chlorophyll content |
[101] |
|
ZnO NPs Source |
Reagents and Conditions |
Characterization |
Size and Purity |
Refs. |
|---|---|---|---|---|
|
Purchased |
Not Reported |
20–30 nm 98% purity level |
[81] |
|
|
Purchased |
UV–Vis, TEM, XRD * |
Not reported—99,99%purity level |
[82] |
|
|
Purchased |
Not Reported |
Not Reported |
[83] |
|
|
Phyto-Synthesis |
Zn(NO3)2 ·6H2O, Agathosma betulina, 80 °C, calcination 600 °C |
UV–Vis, TEM, XRD, FTIR, SEM |
20–30 nm |
[84] |
|
Purchased |
Not reported |
<50 nm |
[85] |
|
|
Purchased |
Not reported |
<100 nm |
[86] |
|
|
Precipitation |
ZnSO4·7H2O, NaOH, drying 100 °C |
XRD, IR, SEM, TEM |
~20 nm |
[87] |
|
Purchased |
XRD, EDX, TEM, SEM |
~20 nm-99% purity |
[88] |
|
|
“Chemical” |
ZnSO4, citrate, 70 °C |
DLS, TEM |
spherical shape ~ 193 nm High purity |
[89] ** |
|
Purchased |
SEM, TEM * |
36 nm |
[90] |
|
|
Phyto-Synthesis |
Zn(ac)2 ·2H2O V. mungo extract, 70 °C |
UV–Vis, SEM, XRD |
30–80 nm |
[91] |
|
Biogenic synthesis |
Zn(ac)2 ·2H2O, T. Harzanium, 40 °C, calcination 500 °C |
UV–Vis, FTIR, TEM, SEM, EDX, XRD |
5–27 nm, agglomerated |
[92] |
|
Purchased |
XRD |
15–25 nm |
[93] |
|
|
Precipitation |
Zn(NO3)2 ·6H2O, NaOH, 55 °C, vacuum 60 °C |
SEM, EDAX, TEM, Raman |
Narrow rods— 80–150 nm |
[94] |
|
Wet chemical synthesis |
Trichoderm asperellum |
UV–Vis, SEM, TEM, FTIR |
spherical shape, 33.4 nm |
[95] ** |
|
Precipitation |
Zn(ac)2 ·2H2O, KOH, 60 °C, dried at 60 °C |
UV-Vis, XRD, SEM, FTIR |
Hexagonal shape 20 nm |
[96] |
|
Biogenic synthesis |
Zn(NO3)2, ZnSO4, ZnCl2 fungi, from arid field |
DLS, TEM-EDX, zeta potential |
10 nm |
[97] ** |
|
Phyto-Synthesis |
Zn(ac)2 ·2H2O, Coriandrum sativum, 70 °C, calcination 500 °C |
UV–Vis, FTIR, TEM, SEM, XRD |
spherical shape 30 nm |
[98] |
|
Precipitation |
Zn(NO3)2 ·6H2O, NaOH, 55 °C, Dried at 60 °C |
UV –Vis, FTIR, TEM, SEM |
spherical shape 36 nm |
[99] |
|
Purchased |
UV –Vis, XRD, TEM |
spherical shape 20–30 nm |
[100] |
|
|
Precipitation |
Zn(NO3)2 ·6H2O, NaOH 40 °C–60 °C |
XRD, FTIR, SEM, TGA |
58–60 nm |
[101] |
* data provided by the manufacturer; ** and references therein.
5. ZnO NPs Seed Priming against Biotic Stresses
References
- United Nations General Assembly, Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://sdgs.un.org/2030agenda (accessed on 3 December 2023).
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76.
- Zhu, P.; Burney, J.; Chang, J.; Jin, Z.; Mueller, N.D.; Xin, Q.; Xu, J.; Yu, L.; Makowski, D.; Ciais, P. Warming reduces global agricultural production by decreasing cropping frequency and yields. Nat. Clim. Chang. 2022, 12, 1016–1023.
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57.
- Pimentel, D.; Burgess, M. Soil erosion threatens food production. Agriculture 2013, 3, 443–463.
- Khan, Z.; Fan, X.; Khan, M.N.; Khan, M.A.; Zhang, K.; Fu, Y.; Shen, H. The toxicity of heavy metals and plant signaling facilitated by biochar application: Implications for stress mitigation and crop production. Chemosphere 2022, 308, 136466.
- Chen, L.; Beiyuan, J.; Hu, W.; Zhang, Z.; Duan, C.; Cui, Q.; Zhu, X.; He, H.; Huang, X.; Fang, L. Phytoremediation of potentially toxic elements (PTEs) contaminated soils using alfalfa (Medicago sativa L.): A comprehensive review. Chemosphere 2022, 293, 133577.
- Okey-Onyesolu, C.F.; Hassanisaadi, M.; Bilal, M.; Barani, M.; Rahdar, A.; Iqbal, J.; Kyzas, G.Z. Nanomaterials as Nanofertilizers and Nanopesticides: An Overview. ChemistrySelect 2021, 33, 8645–8663.
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 738, 140240.
- Prajapati, R.; Kataria, S.; Jain, M. Seed priming for alleviation of heavy metal toxicity in plants: An overview. Plant Sci. Today 2020, 7, 308–313.
- Filippov, P.; Tanou, G.; Molassiotis, A.; Fotopoulos, V. Plant Acclimation to Environmental Stress Using Priming Agents; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27.
- Méndez, A.A.; Pena, L.B.; Benavides, M.P.; Gallego, S.M. Priming with NO controls redox state and prevents cadmium-induced general up-regulation of methionine sulfoxide reductase gene family in Arabidopsis. Biochimie 2016, 131, 128–136.
- Demecsová, L.; Zelinová, V.; Liptáková, Ľ.; Valentovičová, K.; Tamás, L. Indole-3-butyric acid priming reduced cadmium toxicity in barley root tip via NO generation and enhanced glutathione peroxidase activity. Planta 2020, 252, 1–16.
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752.
- Ghiyasi, M.; Rezaee Danesh, Y.; Amirnia, R.; Najafi, S.; Mulet, J.M.; Porcel, R. Foliar Applications of ZnO and Its Nanoparticles Increase Safflower (Carthamus tinctorius L.) Growth and Yield under Water Stress. Agronomy 2023, 13, 192.
- Bruce, T.J.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608.
- Arnott, A.; Galagedara, L.; Thomas, R.; Cheema, M.; Sobze, J.-M. The potential of rock dust nanoparticles to improve seed germination and seedling vigor of native species: A review. Sci. Total Environ. 2021, 775, 145139.
- Kumar, S.P.J.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668.
- Mickky, B.M. Seed Priming as a Strategy to Improve Wheat Productivity Under Abiotic Stress: Global Meta-analysis. J. Plant Growth Regul. 2022, 41, 1397–1410.
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture-recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254.
- Torre-Roche, R.D.L.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed biofortifcation by engineered nanomaterials: A pathway to alleviate malnutrition? J. Agri. Food Chem. 2020, 68, 12189–12202.
- Kapoor, P.; Dhaka, R.K.; Sihag, P.; Mehla, S.; Sagwal, V.; Singh, Y.; Langaya, S.; Balyan, P.; Singh, K.P.; Xing, B.; et al. Nanotechnology-enabled biofortification strategies for micronutrients enrichment of food crops: Current understanding and future scope. NanoImpact 2022, 26, 100407.
- Kah, M. Nanopesticides and Nanofertilizers: Emerging Contaminants or Opportunities for Risk Mitigation? Front. Chem. 2015, 3, 64.
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal Nanoparticles in Agriculture: A Review of Possible Use. Coatings 2022, 12, 1586.
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial Power of Cu/Zn Mixed Oxide Nanoparticles to Escherichia coli. Environ. Nanotechnol. Monit. Manag. 2017, 7, 97–102.
- Carbone, M.; Sabbatella, G.; Antonaroli, S.; Remita, H.; Orlando, V.; Biagioni, S.; Nucara, A. Exogenous Control over Intracellular Acidification: Enhancement via Proton Caged Compounds Coupled to Gold Nanoparticles. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2304–2307.
- Gontrani, L.; Donia, D.T.; Bauer, E.M.; Tagliatesta, P.; Carbone, M. Novel Synthesis of Zinc Oxide Nanoparticles from Type IV Deep Eutectic Solvents. Inorg. Chim. Acta 2023, 545, 121268.
- Gontrani, L.; Bauer, E.M.; Talone, A.; Missori, M.; Imperatori, P.; Tagliatesta, P.; Carbone, M. CuO Nanoparticles and Microaggregates: An Experimental and Computational Study of Structure and Electronic Properties. Materials 2023, 16, 4800.
- Bauer, E.M.; Talone, A.; Imperatori, P.; Briancesco, R.; Bonadonna, L.; Carbone, M. The Addition of Co into CuO–ZnO Oxides Triggers High Antibacterial Activity and Low Cytotoxicity. Nanomaterials 2023, 13, 2823.
- Caminiti, R.; Carbone, M.; Panero, S.; Sadun, C. Conductivity and structure of poly(ethylene glycol) complexes using energy dispersive X-ray diffraction. J. Phys. Chem. B 1999, 103, 10348–10355.
- Carbone, M.; Aneggi, E.; Figueredo, F.; Susmel, S. NiO-Nanoflowers Decorating a Plastic Electrode for the Non-Enzymatic Amperometric Detection of H2O2 in Milk: Old Issue, New Challenge. Food Control 2022, 132, 108549.
- Carbone, M. Cu Zn Co Nanosized Mixed Oxides Prepared from Hydroxycarbonate Precursors. J. Alloys Compd. 2016, 688, 202–209.
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417.
- Carbone, M. Zn Defective ZnCo2O4 Nanorods as High Capacity Anode for Lithium Ion Batteries. J. Electroanal. Chem. 2018, 815, 151–157.
- Valentini, F.; Ciambella, E.; Boaretto, A.; Rizzitelli, G.; Carbone, M.; Conte, V.; Cataldo, F.; Russo, V.; Casari, C.S.; Chillura-Martino, D.F.; et al. Sensor Properties of Pristine and Functionalized Carbon Nanohorns. Electroanalysis 2016, 28, 2489–2499.
- Babazadeh, S.; Bisauriya, R.; Carbone, M.; Roselli, L.; Cecchetti, D.; Bauer, E.M.; Sennato, S.; Prosposito, P.; Pizzoferrato, R. Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles. Sensors 2021, 21, 6353.
- Carbone, M. CQDs@NiO: An Efficient Tool for CH4 Sensing. Appl. Sci. 2020, 10, 6251.
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-Down N-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249.
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008, 302, 1–17.
- Prasad, T.N.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Raja Reddy, K.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927.
- Jiang, W.; Struik, P.C.; Zhao, M.; van Keulen, H.; Fan, T.Q.; Stomph, T.J. Indices to screen for grain yield and grain zinc mass concentration in aerobic rice at different soil Zn levels. J. Life Sci. 2008, 55, 181–197.
- Rehman, H.; Aziz, T.; Farooq, M.; Wakeel, A.; Rengel, Z. Zinc nutrition in rice production systems: A review. Plant Soil. 2012, 361, 203–226.
- Latef, A.A.H.A.; Alhmad, M.F.A.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2017, 36, 60–70.
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85.
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585.
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250.
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828.
- Abbasi Khalaki, M.; Moameri, M.; Asgari Lajayer, B.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28.
- Itroutwar, P.D.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Raja, K.; Subramanian, K.S. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 2019, 39, 717–728.
- Srivastava, A.K.; Malhotra, S.K. Nutrient use efficiency in perennial fruit crops—A review. J. Plant Nutr. 2017, 40, 1928–1953.
- López-Valdez, F.; Miranda-Arámbula, M.; Ríos-Cortés, A.M.; Fernández-Luqueño, F.; De-la-Luz, V. Nanofertilizers and their controlled delivery of nutrients. In Agricultural Nanobiotechnology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 35–48.
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12.
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469.
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using photosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263.
- Dietz, K.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signalling. Plant Physiol. 2016, 171, 1535–1539.
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012, 7, 193–195.
- Bhardwaj, J.; Anand, A.; Nagarajan, S. Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol. Biochem. 2012, 57, 67–73.
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signalling integrates with phytohormones during the germination of magneto primed tomato seeds. Sci. Rep. 2019, 9, 8814.
- El-Maarouf-Bouteau, H.; Meimoun, P.; Job, C.; Job, D.; Bailly, C. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant Sci. 2013, 4, 77.
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413.
- Sandalio, L.M.; Romero-Puertas, M.C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann. Bot. 2015, 116, 475–485.
- Huang, S.; Van Aken, O.; Schwarzlander, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physio. 2016, 171, 1551–1559.
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467.
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173.
- Chandrasekaran, U.; Luo, X.; Wang, Q.; Shu, K. Are there unidentified factors involved in the germination of nano primed seeds? Front. Plant Sci. 2020, 11, 832.
- Oracz, K.; Karpinski, S. Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 2016, 7, 864.
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032.
- Bailly, C.; Kranner, I. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. Methods Mol. Biol. 2011, 773, 343–367.
- Khan, M.N.; Li, Y.; Khan, Z.; Chen, L.; Liu, J.; Hu, J.; Wu, H.; Li, Z. Nanoceria seed priming improves salt tolerance in rapeseed. through modulating ROS homeostasis and α-amylase activities. J. Nanobiotechnol. 2021, 19, 276.
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.; Hoang, S.A.; Van Ha, C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 2022, 41, 364–375.
- Rajput, V.D.; Minkina, T.; Kumari, A.; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221.
- Ragab, G.; Saad-Allah, K. Seed priming with greenly synthesized sulfur nanoparticles enhances antioxidative defense machinery and restricts oxidative injury under manganese stress in Helianthus annuus (L.) seedlings. J. Plant Growth Regul. 2021, 40, 1894–1902.
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244.
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of nanoparticles and biosystems: Microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 2019, 9, 1365.
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafcking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 1–12.
- Li, J.H.; Santos-Otte, P.; Au, B.; Rentsch, J.; Block, S.; Ewers, H. Directed manipulation of membrane proteins by fuorescent magnetic nanoparticles. Nat. Commun. 2020, 11, 4259.
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; GardeaTorresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498.
- Bajpai, A.; Jadhav, K.; Muthukumar, M.; Kumar, S.; Srivatava, G. Use of nanotechnology in quality improvement of economically important agricultural crops. In Biogenic Nano-Particles and Their Use in Agro-Ecosystem; Ghorbanpour, M., Bhargava, P., Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2020; pp. 39–57.
- Donia, D.T.; Bauer, E.M.; Missori, M.; Roselli, L.; Cecchetti, D.; Tagliatesta, P.; Gontrani, L.; Carbone, M. Room Temperature Syntheses of ZnO and Their Structures. Symmetry 2021, 13, 733.
- Depar, N.; Rajpar, I.; Memon, M.Y.; Imtiaz, M.; Zia-ul-hassan. Mineral nutrient densities in some domestic and exotic rice genotypes. Pak. J. Agric. Agril. Eng. Vet. Sci. 2011, 27, 134–142.
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967.
- Abbas, S.F.; Bukhari, M.A.; Raza, M.A.S.; Abbasi, G.H.; Ahmad, Z.; Alqahtani, M.D.; Almutairi, K.F.; Abd_Allah, E.F.; Iqbal, M.A. Enhancing Drought Tolerance in Wheat Cultivars through Nano-ZnO Priming by Improving Leaf Pigments and Antioxidant Activity. Sustainability 2023, 15, 5835.
- El-Bassiouny, H.M.S.; Mahfouze, H.A.; Abdallah, M.M.S.; Bakry, B.A.; El-Enany, M.A.M. Physiological and Molecular Response of Wheat Cultivars to Titanium Dioxide or Zinc Oxide Nanoparticles under Water Stress Conditions. Int. J. Agron. 2022, 2022, 3806574.
- Rakgotho, T.; Ndou, N.; Mulaudzi, T.; Iwuoha, E.; Mayedwa, N.; Ajayi, R.F. Green-Synthesized Zinc Oxide Nanoparticles Mitigate Salt Stress in Sorghum bicolor. Agriculture 2022, 12, 597.
- Al-Salama, Y. Effect of Seed Priming with ZnO Nanoparticles and Saline Irrigation Water in Yield and Nutrients Uptake by Wheat. Plants Environ. Sci. Proc. 2022, 16, 37.
- Zaghdoud, C.; Nagaz, K. Zinc oxide nanoparticles enhances germination performance of wheat (Triticum durum L.) seeds under individual and combined salinity and copper treatments. J. Oasis Agric. Sustain. Dev. 2022, 4, 7–17.
- El-Badri, A.M.A.; Batool, M.; Mohamed, I.A.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J.; et al. Modulation of salinity impact on early seedling stage via nano-priming application of zinc oxide on rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392.
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021.
- Gupta, N.; Singh, P.M.; Sagar, V.; Pandya, A.; Chinnappa, M.; Kumar, R.; Bahadur, A. Seed Priming with ZnO and Fe3O4 Nanoparticles Alleviate the Lead Toxicity in Basella alba L. through Reduced Lead Uptake and Regulation of ROS. Plants 2022, 11, 2227.
- Wang, W.; Yamaji, N.; Ma, J.F. Molecular Mechanism of Cadmium Accumulation in Rice. In Cadmium Toxicity. Current Topics in Environmental Health and Preventive Medicine; Springer: Singapore, 2019.
- Banerjee, S.; Islam, J.; Mondal, S.; Saha, A.; Saha, B.; Sen, A. Proactive attenuation of arsenic-stress by nano-priming: Zinc Oxide Nanoparticles in Vigna mungo (L.) Hepper trigger antioxidant defense response and reduce root-shoot arsenic translocation. J. Hazard. Mater. 2023, 446, 130735.
- Farhana Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Haroon, U.; Ahmed, J.; Chaudhary, H.J.; Attia, H. ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt. J. Fungi 2022, 8, 753.
- Kashyap, D.; Siddiqui, Z.A. Effect of different inocula of Meloidogyne incognita and Pseudomonas syringae pv. pisi with and without Rhizobium leguminosarum on growth, chlorophyll, carotenoid and proline contents of pea. Indian Phytopathol. 2020, 73, 499–506.
- Sripathy, K.V.; Udayabhaskar, K.; Singh, C.; Ramesh, K.V.; Pal, G.; Kumar, A.; Jeevan Kumar, S.P.; Raja, D.K.; UmeshKamble, R.; Kumar, S.; et al. Interference of Nanoparticulates in seed invigoration of Green gram. Plant Physiol. Biochem. 2023, 195, 256–265.
- Das, G.; Dutta, P. Effect of Nanopriming with Zinc Oxide and Silver Nanoparticles on Storage of Chickpea Seeds and Management of Wilt Disease. J. Agr. Sci. Tech. 2022, 24, 213–226.
- Pirzada, T.; Chandio, W.A.; Talpur, M.M.A.; Ansari, A.M.; Kanhar, F.H. Synthesis, Characterization and Role of Zinc Oxide Nanoparticles in Wheat (Triticum indicum) Seeds Germination: Role of Zinc Oxide Nanoparticles in Wheat. Biol. Sci. PJSIR 2022, 65, 167–172.
- Adhikary, S.; Biswas, B.; Chakraborty, D.; Timsina, J.; Pal, S.; Tarafdar, J.C.; Banerjee, S.; Hossain, A.; Roy, S. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. 2022, 12, 7103.
- Asmat-Campos, D.; López-Medina, E.; Montes de Oca-Vásquez, G.; Gil-Rivero, E.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Villena-Zapata, L.; Gurreonero-Fernández, J.; Rafael-Amaya, R. ZnO Nanoparticles Obtained by Green Synthesis as an Alternative to Improve the Germination Characteristics of L. esculentum. Molecules 2022, 27, 2343.
- Eisa, H.M.; Barghouti, S.; Gillham, F.; Al-Saffy, M.T. Cotton Production Prospects for the Decade to 2005: A Global Overview; The World Bank: Washington, DC, USA, 1994.
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351.
- Carbone, M.; De Rossi, S.; Donia, D.T.; Di Marco, G.; Gustavino, B.; Roselli, L.; Tagliatesta, P.; Canini, A.; Gismondi, A. Biostimulants promoting growth of Vicia faba L. seedlings: Inulin coated ZnO nanoparticles. Chem. Biol.Technol. Agric. 2023, 10, 134.
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Shakeel, A. Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019.
- Bansal, V.; Ramanathan, R.; Bhargava, S.K. Fungus-mediated Biological Approaches Towards ‘Green’ Synthesis of Oxide Nanomaterials. Aust. J. Chem. 2011, 64, 279–293.




