Drastic climate changes over the years have triggered environmental challenges for wild plants and crops due to fluctuating weather patterns worldwide. This has caused different types of stressors, responsible for a decrease in plant life and biological productivity, with consequent food shortages, especially in areas under threat of desertification. Nanotechnology-based approaches have great potential in mitigating environmental stressors, thus fostering a sustainable agriculture. Zinc oxide nanoparticles (ZnO NPs) have demonstrated to be biostimulants as well as remedies to both environmental and biotic stresses. Their administration in the early sowing stages, i.e., seed priming, proved to be effective in improving germination rate, seedling and plant growth and in ameliorating the indicators of plants’ well-being. Seed nano-priming acts through several mechanisms such as enhanced nutrients uptake, improved antioxidant properties, ROS accumulation and lipid peroxidation. The target for seed priming by ZnO NPs is mostly crops of large consumption or staple food, in order to meet the increased needs of a growing population and the net drop of global crop frequency, due to climate changes and soil contaminations.
- seed priming
- ZnO NPS
- crops
- stress alleviation
1. Introduction
2. Seed Nano-Priming Mechanism NPs Entry
Seed priming by ZnO NPs may alleviate plants’ abiotic and biotic stress, act as a biostimulant causing an increase in germination rate, seedling and plant growth and overall fresh weight, and improve the biomass and photosynthetic machinery. The effects of seed priming are implemented through several mechanisms. NPs affect the germination and vigor of plants by the stimulation and improvement of seed metabolic rate, vigor index and seedling characteristics [48], especially in the case of ZnO NPs priming [49]. Furthermore, seed nano-priming plays a role in nutrient uptake. This is particularly important, since poor nutrient uptake negatively affects the whole growth process, including root formation, seedling growth, flowering, and fruit formation [50,51][50][51]. Implementing nutrient delivery by conventional management systems may not be efficient, thus opening the way to nano-priming technology [52,53][52][53]. In fact, NPs may improve nutrient uptake by altering the metabolism of seeds, for instance, by a steep rise in α-amylase activity via gibberellic acid [54], the breakdown of stored starch, and stimulation of the release of growth regulators, ultimately fostering the growth and productivity of plants. This can also be monitored by the activity of enzymes like α-amylase, which has a steep increase upon seeding. Management of the antioxidant system is crucial to a plant’s well-being, since it prevents potential cellular molecule damage by maintaining ROS homeostasis [55]. Enzymatic and non-enzymatic antioxidant enzymes participate in the ROS management system: catalase (CAT), peroxidase (POX), ascorbate-peroxidase (APX), superoxide-dismutase (SOD), phenylalanine ammonia lyase (PAL), glutathione, and glutathione reductase. Seed priming affects the antioxidant enzyme system of plants at several levels. It was observed that the activity of antioxidant enzymes increased at various degrees, depending on the type of plant and on the specific priming, though the exact action pathway was not quite fully disclosed. Some correlations were hypothesized, though. For instance, H2O2 radicals were significantly reduced in tomato, cucumber, and pea nano-primed seeds due to increased SOD and CAT activity [56,57,58][56][57][58]. On the other hand, H2O2 is responsible for carbonylating proteins, initiating and changing kinase transduction pathways; therefore, it can interfere with the expression of multiple genes in the germination process [59]. Seed nano-priming plays a role in ROS and lipid peroxide regulation. Seeds accumulated in the seed coat induce ROS accumulation and activate a number of downstream processes [60]. ROS are produced in plants’ chloroplasts, mitochondria, and peroxisomes as a by-product during aerobic metabolism [61,62][61][62]. They may irreversibly damage DNA, but they also act as signaling molecules that allow plants to grow normally and mitigate abiotic stresses [63,64][63][64]. Increased ROS accumulation in plant cells upon seed nano-priming helps to break the bonds among the polysaccharides in the cell wall of seed endosperm, thus facilitating quick and healthy seed germination [65]. It triggers processes of breaking seed dormancy and stimulating seed germination [66] through the activation of the synthesis of gibberellic and abscisic acid [67,68][67][68]. The lowering of ROS by NPs may result in a large rise in antioxidant enzyme levels. This may help increasing tolerance towards stress factors, such as salinity and drought [69,70][69][70]. Heavy metals affect crop growth development and production, hampering plant growth progressions. However, in general, NPs regulate plant physiological and biochemical parameters to reduce their detrimental effects [71]. Several studies indicate a connection between NPs priming and decreases in toxic metal accumulations by stimulating antioxidant enzyme activities and decreasing ROS and lipid peroxidation [72]. A scheme of many nano-priming mechanisms is summarized in Figure 1.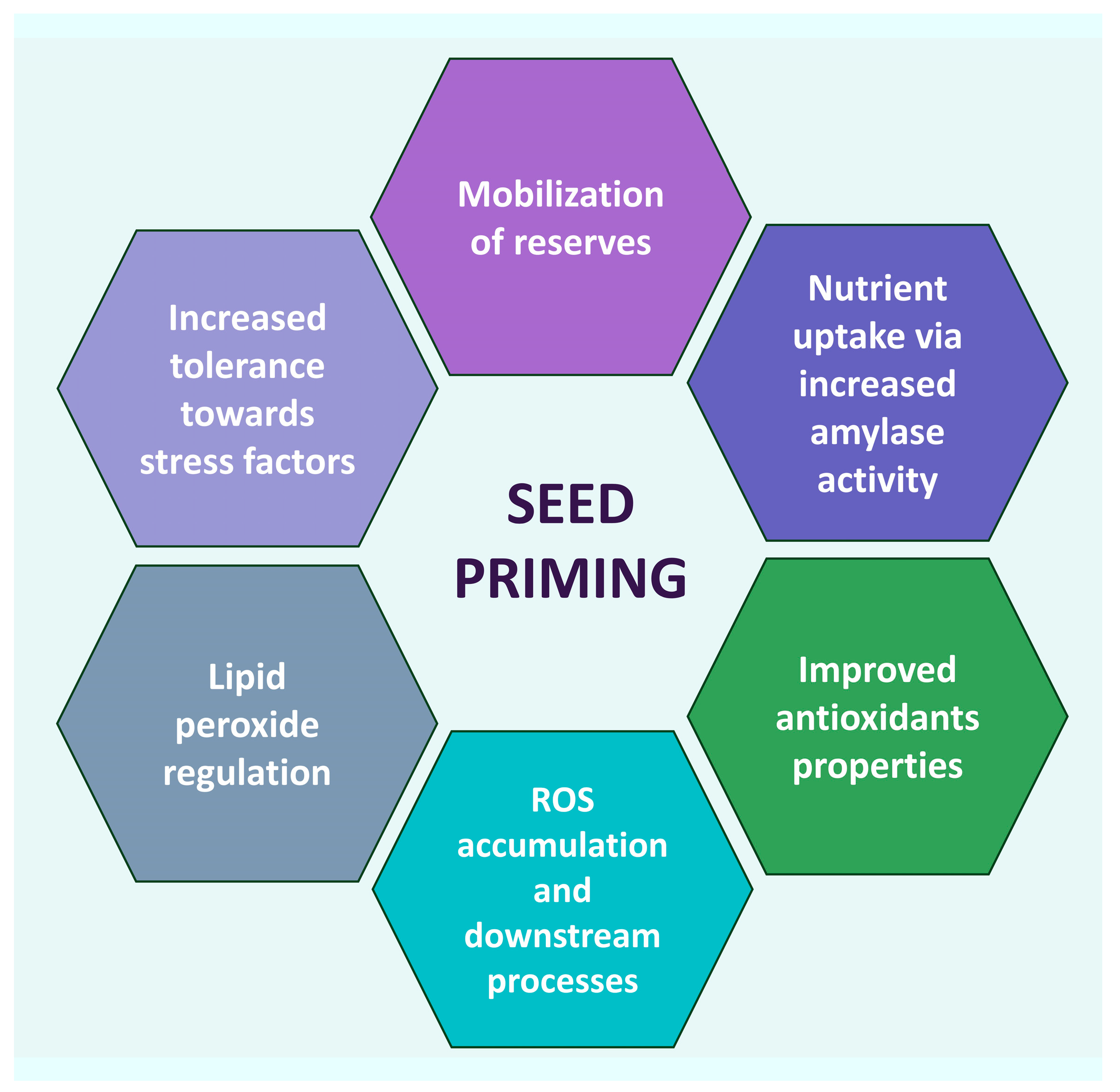
3. Application to Different Crops
Nano-priming techniques are deemed beneficial for improving crop yields, frequencies and seedling and plant well-being. In this framework, great effort has been devoted to promote and support nanotechnologies in agriculture under different flagships such as the Nano Mission [78] of the Indian government to encourage private-sector investment and support the growth and commercial application of nanotechnology. Broad, systematic and low-cost applications of NPs on fields are general requirements for sustainable agricultural stimulation. The success of seed nano-priming as a form of biostimulation and an agent of contrast against abiotic and stresses varies based on the species of plants and the characteristics of treating agents. However, ZnO NPs appear to be very efficient and versatile among nanoparticles, for seed nano-priming purposes, since they are suitable for targeting different types of crops worldwide. Besides this, their production is low cost and can be achieved with environmentally friendly methods [79]. Crops which were successfully tested for ZnO NPs nano-priming include produce grown in localized areas, as well as global harvests. Large consumption cereals are a primary target for improved production, in dry, salty and contaminated soils, especially since cereal crops play an important role in satisfying daily calorie intake in the developing world. However, the Zn concentration in grain is inherently very low, particularly when grown on Zn-deficient soils. Rice alone (Oryza sativa L.) is one of the major staples, feeding more than half of the world’s population. It is grown in more than 100 countries, predominantly in Asia [80]. Hence, rice, wheat, maize, sorghum, chickpeas and several varieties of faba beans (lupine, vigna mungo, vigna radiata and vicia faba) were recently probed for seed priming with ZnO NPs. However, tests are also being performed on different types of edibles, such as spinach (basella alba) and tomatoes. Finally, textile fibers (cotton) and vegetables for animal feed and industrial oil production (rapeseed, Brassica napus L.) were also investigated for ZnO NPs seed priming.4. ZnO NPs Seed Priming against Abiotic Stresses
The efficacy of seed priming is dose dependent, and investigations may be carried out in vitro, in pot or in field. In the following sections, a summary of the latest research is reported on the effects of ZnO NPs priming on the most widespread crops worldwide. Drought, soil salinity and heavy metal and arsenic accumulation are listed among abiotic stresses as well as improper seed storage. In addition, action against biotic stresses is considered in association with biotic remedies. The sheer biostimulating effect of ZnO NPs is also reported. Details are mentioned for the major effects, doses and mechanisms when specified in the original papers. The ZnO NPs used in the experiments were of two types, commercial or purposely synthetized. Information on ZnO NPs preparation and characterization methods was reported where available. Figure 2 summarizes the main actions of ZnO NPs seed priming and the consequent effects on crops. The type of stress, ZnO NPs doses, target plants and major benefits of seed priming are reported in Table 1, whereas the type of ZnO NPs and the synthesis and characterization methods (if available) are reported in Table 2.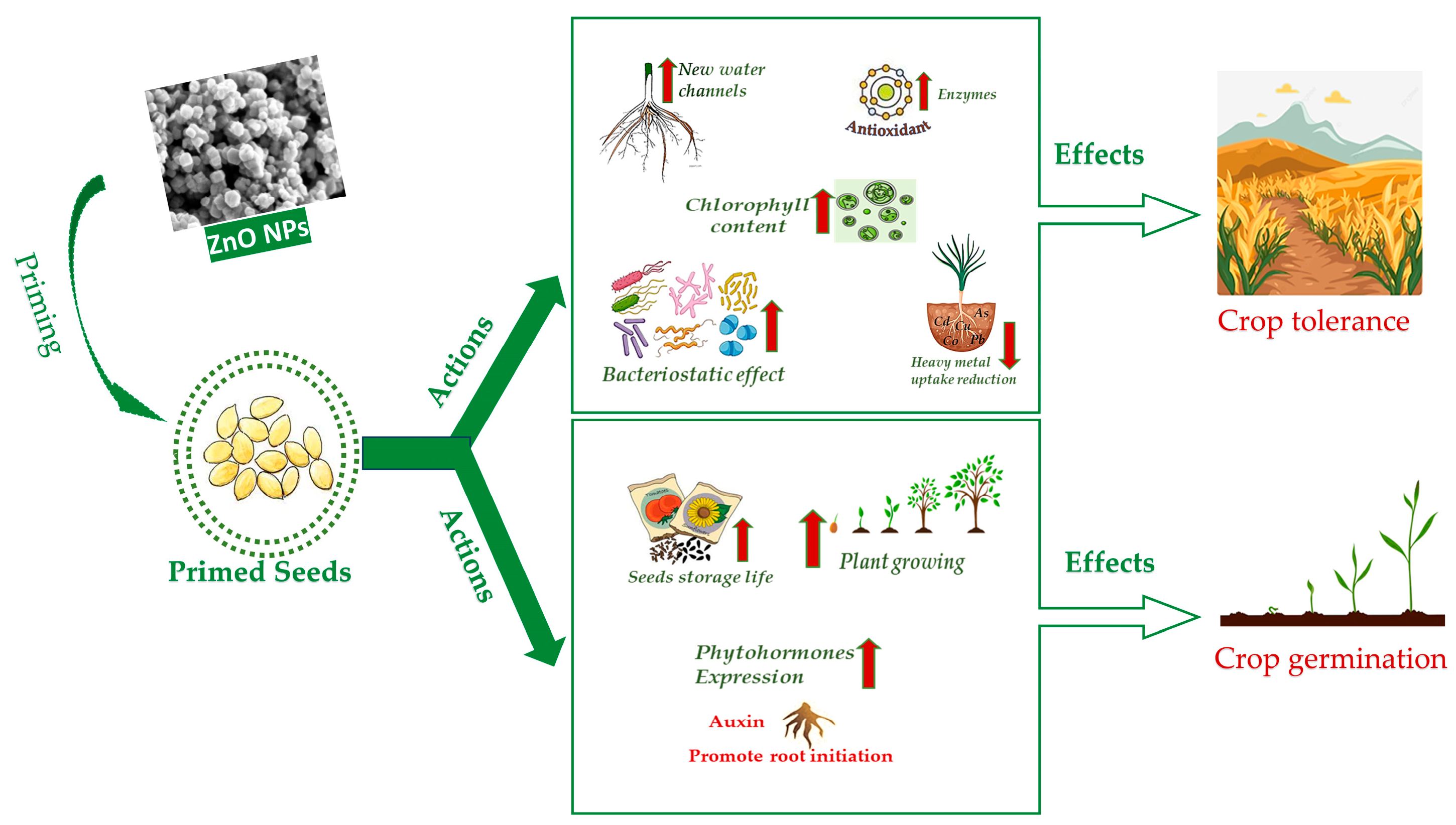
|
Application |
Type of Seed |
|---|
|
ZnO NPs Source | Doses |
Reagents and Conditions Effects |
Ref. |
||||||
|---|---|---|---|---|---|---|---|---|---|
Characterization |
Size and Purity |
Refs. |
|||||||
|
Drought stress alleviation |
Rice |
0; 5; 10; 15; 25; 50 ppm |
Agronomic profile yield |
||||||
|
Purchased |
Not Reported |
20–30 nm 98% purity level |
[81] |
||||||
[ | ] |
Wheat |
40; 80; 120; 160 ppm |
||||||
|
Purchased | Chlorophyll content and plant nutrients |
[82 |
] |
||||||
UV–Vis, TEM, XRD * |
Not reported—99,99%purity level |
[82] |
Wheat |
10 mg/L |
Reduced DNA damage |
[83] |
|||
|
Purchased |
Not Reported |
Not Reported |
Salt stress alleviation |
Sorghum bicolor |
Zn(NO3)2 ·6H2O, Agathosma betulina, 80 °C, calcination 600 °C |
5; 10 mg/L |
UV–Vis, TEM, XRD, FTIR, SEM | ||
50 mg/L |
Improved germination |
[86] |
|||||||
|
Rapeseed |
|||||||||
] | |||||||||
[ | ] |
20–30 nm |
[84] |
25; 50; 100 mg/L |
Increased vigor indexes |
[87] |
|||
|
Purchased |
36 nm |
[90] |
|||||||
|
Phyto-Synthesis |
Zn(ac)2 ·2H2O V. mungo extract, 70 °C |
UV–Vis, SEM, XRD |
30–80 nm |
[91] |
|||||
|
Biogenic synthesis |
Zn(ac)2 ·2H2O, T. Harzanium, 40 °C, calcination 500 °C |
UV–Vis, FTIR, TEM, SEM, EDX, XRD |
5–27 nm, agglomerated |
[92 | |||||
Wheat | |||||||||
10 mgL−1 |
Increased germination, vigor index and chlorophyll content |
[100] |
|||||||
|
Phyto-Synthesis | Increased Fresh weights |
[84] |
|||||||
|
Wheat |
50 mg/L |
Increased plant growth, grain yield and macronutrients |
Not reported [85] |
||||||
<50 nm | [85] |
Wheat |
|||||||
|
Purchased |
Not reported |
<100 nm |
[86] |
||||||
|
Precipitation |
ZnSO4·7H2O, NaOH, drying 100 °C |
XRD, IR, SEM, TEM |
~20 nm |
[87] |
Cu stress alleviation |
||||
|
Purchased |
Wheat |
20 ppm |
Improved growth |
XRD, EDX, TEM, SEM [ |
~20 nm-99% purity 86] |
||||
[ | ] |
Co stress alleviation |
|||||||
|
“Chemical” |
Maize |
ZnSO4, citrate, 70 °C 500 mg/L |
DLS, TEM Improved plant growth, biomass and photosynthetic machinery |
spherical shape ~ 193 nm High purity [88] |
|||||
[ | ] ** |
Pb stress alleviation |
Basella alba |
200 mg/L |
Increased seed germination, seedling and roots growth, seed vigor; reduced Pb uptake |
[89] |
|||
|
Purchased |
SEM, TEM * |
Cd stress alleviation |
Rice |
0; 25; 50; 100 mg/L |
Improved early growth and related physio-biochemical attributes |
[90] |
|||
|
Arsenic stress alleviation |
V. mungo (bean) |
50; 100; 150; 200 mg/L |
Increased germination by modulation of metabolic pathways |
[91] |
|||||
] |
Biotic stress counteraction |
Chickpea |
|||||||
|
Purchased | 0.25; 0.50; 0.75; 1.0 µg/mL 1000 mg/kg |
XRD Antifungal Activity |
15–25 nm [92] |
||||||
[ | ] |
Reduction in disease indices |
|||||||
|
Precipitation |
[93] |
||||||||
|
Zn(NO3)2 ·6H2O, NaOH, 55 °C, vacuum 60 °C |
SEM, EDAX, TEM, Raman |
Narrow rods— 80–150 nm |
[94] |
Seed storage |
|||||
|
Wet chemical synthesis | V. radiata (bean) |
Trichoderm asperellum |
1000 ppm |
UV–Vis, SEM, TEM, FTIRIncreased germination percentage |
[ |
spherical shape, 33.4 nm 94] |
|||
[ | ] | ** |
Chickpea |
100 ppm |
increased seed storage life; decreased disease incidence |
||||
|
Precipitation |
Zn(ac)2 ·2H2O, KOH, 60 °C, dried at 60 °C |
UV-Vis, XRD, SEM, FTIR |
[95] |
||||||
Hexagonal shape | 20 nm |
[96] |
Crop improvement |
Wheat |
5; 10; 15; 20 ppm |
Increased plant growth |
|||
|
Biogenic synthesis |
Zn(NO3)2, ZnSO4, ZnCl2 fungi, from arid field | [ | 96] |
||||||
DLS, TEM-EDX, zeta potential |
10 nm |
[97] ** |
Oryza sativa (rice) |
10 µmol |
|||||
|
Phyto-Synthesis | Seedling vigor; increased plant chlorophyll content; nutrients uptake |
Zn(ac)2 ·2H2O, Coriandrum sativum, 70 °C, calcination 500 °C |
[97] |
||||||
UV–Vis, FTIR, TEM, SEM, XRD |
spherical shape 30 nm |
[98] |
Tomato |
||||||
|
Precipitation | 100 ppm |
Zn(NO3)2 ·6H2O, NaOH, 55 °C, Dried at 60 °C |
Improved germination process |
[98] |
|||||
UV –Vis, FTIR, TEM, SEM |
spherical shape 36 nm |
[99] |
Cotton |
400 ppm |
Increased germination and seed parameters |
||||
|
Purchased |
UV –Vis, XRD, TEM |
[99 |
spherical shape 20–30 nm |
[100] |
|||||
|
Precipitation |
Zn(NO3)2 ·6H2O, NaOH 40 °C–60 °C |
XRD, FTIR, SEM, TGA |
58–60 nm |
[101] |
V. faba (bean) |
50, 100 mgL−1 |
Increased biometric parameters, chlorophyll content |
[101] |
* data provided by the manufacturer; ** and references therein.
5. ZnO NPs Seed Priming against Biotic Stresses
References
- United Nations General Assembly, Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://sdgs.un.org/2030agenda (accessed on 3 December 2023).
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76.
- Zhu, P.; Burney, J.; Chang, J.; Jin, Z.; Mueller, N.D.; Xin, Q.; Xu, J.; Yu, L.; Makowski, D.; Ciais, P. Warming reduces global agricultural production by decreasing cropping frequency and yields. Nat. Clim. Chang. 2022, 12, 1016–1023.
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57.
- Pimentel, D.; Burgess, M. Soil erosion threatens food production. Agriculture 2013, 3, 443–463.
- Khan, Z.; Fan, X.; Khan, M.N.; Khan, M.A.; Zhang, K.; Fu, Y.; Shen, H. The toxicity of heavy metals and plant signaling facilitated by biochar application: Implications for stress mitigation and crop production. Chemosphere 2022, 308, 136466.
- Chen, L.; Beiyuan, J.; Hu, W.; Zhang, Z.; Duan, C.; Cui, Q.; Zhu, X.; He, H.; Huang, X.; Fang, L. Phytoremediation of potentially toxic elements (PTEs) contaminated soils using alfalfa (Medicago sativa L.): A comprehensive review. Chemosphere 2022, 293, 133577.
- Okey-Onyesolu, C.F.; Hassanisaadi, M.; Bilal, M.; Barani, M.; Rahdar, A.; Iqbal, J.; Kyzas, G.Z. Nanomaterials as Nanofertilizers and Nanopesticides: An Overview. ChemistrySelect 2021, 33, 8645–8663.
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 738, 140240.
- Prajapati, R.; Kataria, S.; Jain, M. Seed priming for alleviation of heavy metal toxicity in plants: An overview. Plant Sci. Today 2020, 7, 308–313.
- Filippov, P.; Tanou, G.; Molassiotis, A.; Fotopoulos, V. Plant Acclimation to Environmental Stress Using Priming Agents; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27.
- Méndez, A.A.; Pena, L.B.; Benavides, M.P.; Gallego, S.M. Priming with NO controls redox state and prevents cadmium-induced general up-regulation of methionine sulfoxide reductase gene family in Arabidopsis. Biochimie 2016, 131, 128–136.
- Demecsová, L.; Zelinová, V.; Liptáková, Ľ.; Valentovičová, K.; Tamás, L. Indole-3-butyric acid priming reduced cadmium toxicity in barley root tip via NO generation and enhanced glutathione peroxidase activity. Planta 2020, 252, 1–16.
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752.
- Ghiyasi, M.; Rezaee Danesh, Y.; Amirnia, R.; Najafi, S.; Mulet, J.M.; Porcel, R. Foliar Applications of ZnO and Its Nanoparticles Increase Safflower (Carthamus tinctorius L.) Growth and Yield under Water Stress. Agronomy 2023, 13, 192.
- Bruce, T.J.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608.
- Arnott, A.; Galagedara, L.; Thomas, R.; Cheema, M.; Sobze, J.-M. The potential of rock dust nanoparticles to improve seed germination and seedling vigor of native species: A review. Sci. Total Environ. 2021, 775, 145139.
- Kumar, S.P.J.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668.
- Mickky, B.M. Seed Priming as a Strategy to Improve Wheat Productivity Under Abiotic Stress: Global Meta-analysis. J. Plant Growth Regul. 2022, 41, 1397–1410.
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture-recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254.
- Torre-Roche, R.D.L.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed biofortifcation by engineered nanomaterials: A pathway to alleviate malnutrition? J. Agri. Food Chem. 2020, 68, 12189–12202.
- Kapoor, P.; Dhaka, R.K.; Sihag, P.; Mehla, S.; Sagwal, V.; Singh, Y.; Langaya, S.; Balyan, P.; Singh, K.P.; Xing, B.; et al. Nanotechnology-enabled biofortification strategies for micronutrients enrichment of food crops: Current understanding and future scope. NanoImpact 2022, 26, 100407.
- Kah, M. Nanopesticides and Nanofertilizers: Emerging Contaminants or Opportunities for Risk Mitigation? Front. Chem. 2015, 3, 64.
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal Nanoparticles in Agriculture: A Review of Possible Use. Coatings 2022, 12, 1586.
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial Power of Cu/Zn Mixed Oxide Nanoparticles to Escherichia coli. Environ. Nanotechnol. Monit. Manag. 2017, 7, 97–102.
- Carbone, M.; Sabbatella, G.; Antonaroli, S.; Remita, H.; Orlando, V.; Biagioni, S.; Nucara, A. Exogenous Control over Intracellular Acidification: Enhancement via Proton Caged Compounds Coupled to Gold Nanoparticles. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2304–2307.
- Gontrani, L.; Donia, D.T.; Bauer, E.M.; Tagliatesta, P.; Carbone, M. Novel Synthesis of Zinc Oxide Nanoparticles from Type IV Deep Eutectic Solvents. Inorg. Chim. Acta 2023, 545, 121268.
- Gontrani, L.; Bauer, E.M.; Talone, A.; Missori, M.; Imperatori, P.; Tagliatesta, P.; Carbone, M. CuO Nanoparticles and Microaggregates: An Experimental and Computational Study of Structure and Electronic Properties. Materials 2023, 16, 4800.
- Bauer, E.M.; Talone, A.; Imperatori, P.; Briancesco, R.; Bonadonna, L.; Carbone, M. The Addition of Co into CuO–ZnO Oxides Triggers High Antibacterial Activity and Low Cytotoxicity. Nanomaterials 2023, 13, 2823.
- Caminiti, R.; Carbone, M.; Panero, S.; Sadun, C. Conductivity and structure of poly(ethylene glycol) complexes using energy dispersive X-ray diffraction. J. Phys. Chem. B 1999, 103, 10348–10355.
- Carbone, M.; Aneggi, E.; Figueredo, F.; Susmel, S. NiO-Nanoflowers Decorating a Plastic Electrode for the Non-Enzymatic Amperometric Detection of H2O2 in Milk: Old Issue, New Challenge. Food Control 2022, 132, 108549.
- Carbone, M. Cu Zn Co Nanosized Mixed Oxides Prepared from Hydroxycarbonate Precursors. J. Alloys Compd. 2016, 688, 202–209.
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417.
- Carbone, M. Zn Defective ZnCo2O4 Nanorods as High Capacity Anode for Lithium Ion Batteries. J. Electroanal. Chem. 2018, 815, 151–157.
- Valentini, F.; Ciambella, E.; Boaretto, A.; Rizzitelli, G.; Carbone, M.; Conte, V.; Cataldo, F.; Russo, V.; Casari, C.S.; Chillura-Martino, D.F.; et al. Sensor Properties of Pristine and Functionalized Carbon Nanohorns. Electroanalysis 2016, 28, 2489–2499.
- Babazadeh, S.; Bisauriya, R.; Carbone, M.; Roselli, L.; Cecchetti, D.; Bauer, E.M.; Sennato, S.; Prosposito, P.; Pizzoferrato, R. Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles. Sensors 2021, 21, 6353.
- Carbone, M. CQDs@NiO: An Efficient Tool for CH4 Sensing. Appl. Sci. 2020, 10, 6251.
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-Down N-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249.
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008, 302, 1–17.
- Prasad, T.N.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Raja Reddy, K.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927.
- Jiang, W.; Struik, P.C.; Zhao, M.; van Keulen, H.; Fan, T.Q.; Stomph, T.J. Indices to screen for grain yield and grain zinc mass concentration in aerobic rice at different soil Zn levels. J. Life Sci. 2008, 55, 181–197.
- Rehman, H.; Aziz, T.; Farooq, M.; Wakeel, A.; Rengel, Z. Zinc nutrition in rice production systems: A review. Plant Soil. 2012, 361, 203–226.
- Latef, A.A.H.A.; Alhmad, M.F.A.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2017, 36, 60–70.
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85.
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585.
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250.
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828.
- Abbasi Khalaki, M.; Moameri, M.; Asgari Lajayer, B.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28.
- Itroutwar, P.D.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Raja, K.; Subramanian, K.S. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 2019, 39, 717–728.
- Srivastava, A.K.; Malhotra, S.K. Nutrient use efficiency in perennial fruit crops—A review. J. Plant Nutr. 2017, 40, 1928–1953.
- López-Valdez, F.; Miranda-Arámbula, M.; Ríos-Cortés, A.M.; Fernández-Luqueño, F.; De-la-Luz, V. Nanofertilizers and their controlled delivery of nutrients. In Agricultural Nanobiotechnology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 35–48.
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12.
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469.
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using photosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263.
- Dietz, K.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signalling. Plant Physiol. 2016, 171, 1535–1539.
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012, 7, 193–195.
- Bhardwaj, J.; Anand, A.; Nagarajan, S. Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol. Biochem. 2012, 57, 67–73.
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen peroxide signalling integrates with phytohormones during the germination of magneto primed tomato seeds. Sci. Rep. 2019, 9, 8814.
- El-Maarouf-Bouteau, H.; Meimoun, P.; Job, C.; Job, D.; Bailly, C. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant Sci. 2013, 4, 77.
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413.
- Sandalio, L.M.; Romero-Puertas, M.C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann. Bot. 2015, 116, 475–485.
- Huang, S.; Van Aken, O.; Schwarzlander, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physio. 2016, 171, 1551–1559.
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467.
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173.
- Chandrasekaran, U.; Luo, X.; Wang, Q.; Shu, K. Are there unidentified factors involved in the germination of nano primed seeds? Front. Plant Sci. 2020, 11, 832.
- Oracz, K.; Karpinski, S. Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 2016, 7, 864.
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032.
- Bailly, C.; Kranner, I. Analyses of reactive oxygen species and antioxidants in relation to seed longevity and germination. Methods Mol. Biol. 2011, 773, 343–367.
- Khan, M.N.; Li, Y.; Khan, Z.; Chen, L.; Liu, J.; Hu, J.; Wu, H.; Li, Z. Nanoceria seed priming improves salt tolerance in rapeseed. through modulating ROS homeostasis and α-amylase activities. J. Nanobiotechnol. 2021, 19, 276.
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.; Hoang, S.A.; Van Ha, C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 2022, 41, 364–375.
- Rajput, V.D.; Minkina, T.; Kumari, A.; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221.
- Ragab, G.; Saad-Allah, K. Seed priming with greenly synthesized sulfur nanoparticles enhances antioxidative defense machinery and restricts oxidative injury under manganese stress in Helianthus annuus (L.) seedlings. J. Plant Growth Regul. 2021, 40, 1894–1902.
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244.
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of nanoparticles and biosystems: Microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 2019, 9, 1365.
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafcking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 1–12.
- Li, J.H.; Santos-Otte, P.; Au, B.; Rentsch, J.; Block, S.; Ewers, H. Directed manipulation of membrane proteins by fuorescent magnetic nanoparticles. Nat. Commun. 2020, 11, 4259.
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; GardeaTorresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498.
- Bajpai, A.; Jadhav, K.; Muthukumar, M.; Kumar, S.; Srivatava, G. Use of nanotechnology in quality improvement of economically important agricultural crops. In Biogenic Nano-Particles and Their Use in Agro-Ecosystem; Ghorbanpour, M., Bhargava, P., Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2020; pp. 39–57.
- Donia, D.T.; Bauer, E.M.; Missori, M.; Roselli, L.; Cecchetti, D.; Tagliatesta, P.; Gontrani, L.; Carbone, M. Room Temperature Syntheses of ZnO and Their Structures. Symmetry 2021, 13, 733.
- Depar, N.; Rajpar, I.; Memon, M.Y.; Imtiaz, M.; Zia-ul-hassan. Mineral nutrient densities in some domestic and exotic rice genotypes. Pak. J. Agric. Agril. Eng. Vet. Sci. 2011, 27, 134–142.
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967.
- Abbas, S.F.; Bukhari, M.A.; Raza, M.A.S.; Abbasi, G.H.; Ahmad, Z.; Alqahtani, M.D.; Almutairi, K.F.; Abd_Allah, E.F.; Iqbal, M.A. Enhancing Drought Tolerance in Wheat Cultivars through Nano-ZnO Priming by Improving Leaf Pigments and Antioxidant Activity. Sustainability 2023, 15, 5835.
- El-Bassiouny, H.M.S.; Mahfouze, H.A.; Abdallah, M.M.S.; Bakry, B.A.; El-Enany, M.A.M. Physiological and Molecular Response of Wheat Cultivars to Titanium Dioxide or Zinc Oxide Nanoparticles under Water Stress Conditions. Int. J. Agron. 2022, 2022, 3806574.
- Rakgotho, T.; Ndou, N.; Mulaudzi, T.; Iwuoha, E.; Mayedwa, N.; Ajayi, R.F. Green-Synthesized Zinc Oxide Nanoparticles Mitigate Salt Stress in Sorghum bicolor. Agriculture 2022, 12, 597.
- Al-Salama, Y. Effect of Seed Priming with ZnO Nanoparticles and Saline Irrigation Water in Yield and Nutrients Uptake by Wheat. Plants Environ. Sci. Proc. 2022, 16, 37.
- Zaghdoud, C.; Nagaz, K. Zinc oxide nanoparticles enhances germination performance of wheat (Triticum durum L.) seeds under individual and combined salinity and copper treatments. J. Oasis Agric. Sustain. Dev. 2022, 4, 7–17.
- El-Badri, A.M.A.; Batool, M.; Mohamed, I.A.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J.; et al. Modulation of salinity impact on early seedling stage via nano-priming application of zinc oxide on rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392.
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021.
- Gupta, N.; Singh, P.M.; Sagar, V.; Pandya, A.; Chinnappa, M.; Kumar, R.; Bahadur, A. Seed Priming with ZnO and Fe3O4 Nanoparticles Alleviate the Lead Toxicity in Basella alba L. through Reduced Lead Uptake and Regulation of ROS. Plants 2022, 11, 2227.
- Wang, W.; Yamaji, N.; Ma, J.F. Molecular Mechanism of Cadmium Accumulation in Rice. In Cadmium Toxicity. Current Topics in Environmental Health and Preventive Medicine; Springer: Singapore, 2019.
- Banerjee, S.; Islam, J.; Mondal, S.; Saha, A.; Saha, B.; Sen, A. Proactive attenuation of arsenic-stress by nano-priming: Zinc Oxide Nanoparticles in Vigna mungo (L.) Hepper trigger antioxidant defense response and reduce root-shoot arsenic translocation. J. Hazard. Mater. 2023, 446, 130735.
- Farhana Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Haroon, U.; Ahmed, J.; Chaudhary, H.J.; Attia, H. ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt. J. Fungi 2022, 8, 753.
- Kashyap, D.; Siddiqui, Z.A. Effect of different inocula of Meloidogyne incognita and Pseudomonas syringae pv. pisi with and without Rhizobium leguminosarum on growth, chlorophyll, carotenoid and proline contents of pea. Indian Phytopathol. 2020, 73, 499–506.
- Sripathy, K.V.; Udayabhaskar, K.; Singh, C.; Ramesh, K.V.; Pal, G.; Kumar, A.; Jeevan Kumar, S.P.; Raja, D.K.; UmeshKamble, R.; Kumar, S.; et al. Interference of Nanoparticulates in seed invigoration of Green gram. Plant Physiol. Biochem. 2023, 195, 256–265.
- Das, G.; Dutta, P. Effect of Nanopriming with Zinc Oxide and Silver Nanoparticles on Storage of Chickpea Seeds and Management of Wilt Disease. J. Agr. Sci. Tech. 2022, 24, 213–226.
- Pirzada, T.; Chandio, W.A.; Talpur, M.M.A.; Ansari, A.M.; Kanhar, F.H. Synthesis, Characterization and Role of Zinc Oxide Nanoparticles in Wheat (Triticum indicum) Seeds Germination: Role of Zinc Oxide Nanoparticles in Wheat. Biol. Sci. PJSIR 2022, 65, 167–172.
- Adhikary, S.; Biswas, B.; Chakraborty, D.; Timsina, J.; Pal, S.; Tarafdar, J.C.; Banerjee, S.; Hossain, A.; Roy, S. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. 2022, 12, 7103.
- Asmat-Campos, D.; López-Medina, E.; Montes de Oca-Vásquez, G.; Gil-Rivero, E.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Villena-Zapata, L.; Gurreonero-Fernández, J.; Rafael-Amaya, R. ZnO Nanoparticles Obtained by Green Synthesis as an Alternative to Improve the Germination Characteristics of L. esculentum. Molecules 2022, 27, 2343.
- Eisa, H.M.; Barghouti, S.; Gillham, F.; Al-Saffy, M.T. Cotton Production Prospects for the Decade to 2005: A Global Overview; The World Bank: Washington, DC, USA, 1994.
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351.
- Carbone, M.; De Rossi, S.; Donia, D.T.; Di Marco, G.; Gustavino, B.; Roselli, L.; Tagliatesta, P.; Canini, A.; Gismondi, A. Biostimulants promoting growth of Vicia faba L. seedlings: Inulin coated ZnO nanoparticles. Chem. Biol.Technol. Agric. 2023, 10, 134.
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Shakeel, A. Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019.
- Bansal, V.; Ramanathan, R.; Bhargava, S.K. Fungus-mediated Biological Approaches Towards ‘Green’ Synthesis of Oxide Nanomaterials. Aust. J. Chem. 2011, 64, 279–293.
