
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alicja Nowaczyk | -- | 3887 | 2024-01-02 22:06:49 | | | |
| 2 | Lindsay Dong | Meta information modification | 3887 | 2024-01-03 02:52:57 | | |
Video Upload Options
Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. In addition to biochemical activity, bioavailability also plays a critical role in achieving the desired therapeutic effects. This may seem obvious, but it is worth noting that a drug can only produce the expected effect if the proper level of concentration can be achieved at the desired point in a patient’s body. Given the differences between patients, drug dosages, and administration forms, understanding and controlling bioavailability has become a priority in pharmacology.
1. Introduction
2. Bioavailability of Drugs: Basic Concepts and Controlling Factors
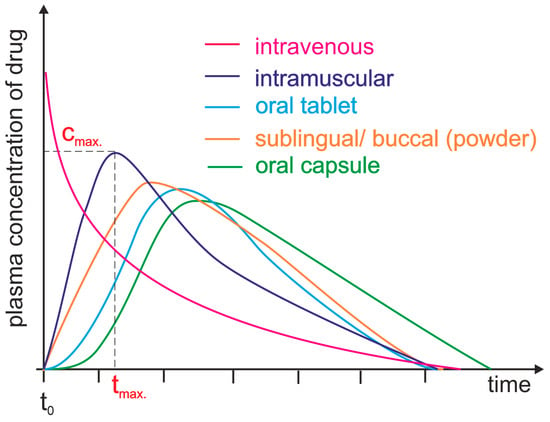
3. Methods for Assessing Drug Bioavailability
4. Drugs with Poorly Described Bioavailability
5. Conclusions
References
- Herkenne, C.; Alberti, I.; Naik, A.; Kalia, Y.N.; Mathy, F.X.; Preat, V.; Guy, R.H. In vivo methods for the assessment of topical drug bioavailability. Pharm. Res. 2008, 25, 87–103.
- Olivares-Morales, A.; Hatley, O.J.; Turner, D.; Galetin, A.; Aarons, L.; Rostami-Hodjegan, A. The use of ROC analysis for the qualitative prediction of human oral bioavailability from animal data. Pharm. Res. 2014, 31, 720–730.
- Caldwell, J.; Gardner, I.; Swales, N. An introduction to drug disposition: The basic principles of absorption, distribution, metabolism, and excretion. Toxicol. Pathol. 1995, 23, 102–114.
- Martinez, M.N.; Amidon, G.L. A mechanistic approach to understanding the factors affecting drug absorption: A review of fundamentals. J. Clin. Pharmacol. 2002, 42, 620–643.
- Doogue, M.P.; Polasek, T.M. The ABCD of clinical pharmacokinetics. Ther. Adv. Drug Saf. 2013, 4, 5–7.
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673.
- Hatton, G.B.; Madla, C.M.; Rabbie, S.C.; Basit, A.W. Gut reaction: Impact of systemic diseases on gastrointestinal physiology and drug absorption. Drug Discov. Today 2019, 24, 417–427.
- Wagner, J.G. History of pharmacokinetics. Pharmacol. Therapeut. 1981, 12, 537–562.
- Lin, L.; Wong, H. Predicting oral drug absorption: Mini review on physiologically-based pharmacokinetic models. Pharmaceutics 2017, 9, 41.
- Wang, Y.; Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int. J. Nanomed. 2020, 15, 6295–6310.
- Alston, A.B.; Digigow, R.; Fluhmann, B.; Wacker, M.G. Putting square pegs in round holes: Why traditional pharmacokinetic principles cannot universally be applied to iron-carbohydrate complexes. Eur. J. Pharm. Biopharm. 2023, 188, 6–14.
- Hedaya, M.A. Basic Pharmacokinetics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012.
- Wang, F.; Yang, G.; Zhou, Y.; Song, H.; Xiong, L.; Wang, L.; Shen, X. Pharmacokinetics of niazirin from Moringa oleifera Lam in rats by UPLC-MS/MS: Absolute bioavailability and dose proportionality. eFood 2022, 3, e39.
- Currie, G.M. Pharmacology, part 2: Introduction to pharmacokinetics. J. Nucl. Med. Technol. 2018, 46, 221–230.
- Tuntland, T.; Ethell, B.; Kosaka, T.; Blasco, F.; Zang, R.X.; Jain, M.; Hoffmaster, K. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front. Pharmacol. 2014, 5, 174.
- Wei, M.; Zhang, X.; Pan, X.; Wang, B.; Ji, C.; Qi, Y.; Zhang, J.Z. HobPre: Accurate prediction of human oral bioavailability for small molecules. J. Cheminform. 2022, 14, 1–10.
- Rowland, M.; Tozer, T.N. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011.
- Kalaimathi, K.; Shine, K.; Gandhi, G.R.; Vijayakumar, S.; Ayyanar, M.; Amalraj, S.; Jose, J. Cyanobacterial metabolites as novel potential suppressors of breast cancer: A comparative in silico pharmacological assessment. Intell. Pharm. 2023, 1, 133–144.
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Müllertz, A. Impact of gastrointestinal physiology on drug absorption in special populations—An UNGAP review. Eur. J. Pharm. Sci. 2020, 147, 105280.
- Belayneh, A.; Molla, F. The effect of coffee on pharmacokinetic properties of drugs: A review. Biomed. Res. Int. 2020, 2020, 7909703.
- Sochacka, J.; Lipska, I. Rola α1-kwaśnej glikoproteiny surowicy krwi ludzkiej w procesie wiązania leków, sytuacja w Polsce i na świecie. Farmacja Polska 2014, 70, 55–62.
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer metabolism: Phenotype, signaling and therapeutic targets. Cells 2020, 9, 2308.
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Bi, H. Current trends in drug metabolism and pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144.
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411.
- Settimo, L.; Bellman, K.; Knegtel, R.M. Comparison of the accuracy of experimental and predicted pKa values of basic and acidic compounds. Pharm. Res. 2014, 31, 1082–1095.
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of physiological pH and chemical pKa under the umbrella of physiologically based pharmacokinetic modeling of drug absorption, distribution, metabolism, excretion, and toxicity. Expert Opin. Drug Met. 2021, 17, 1103–1124.
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Augustijns, P. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812.
- Wong, K.H.; Riaz, M.K.; Xie, Y.; Zhang, X.; Liu, Q.; Chen, H.; Yang, Z. Review of current strategies for delivering Alzheimer’s disease drugs across the blood-brain barrier. Int. J. Mol. Sci. 2019, 20, 381.
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122.
- Kratzer, I.; Ek, J.; Stolp, H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183430.
- Uchida, Y.; Goto, R.; Usui, T.; Tachikawa, M.; Terasaki, T. Blood-Arachnoid Barrier as a Dynamic Physiological and Pharmacological Interface between Cerebrospinal Fluid and Blood. In Drug Delivery to the Brain: Physiological Concepts, Methodologies and Approaches; Springer International Publishing: Cham, Germany, 2022; pp. 93–121.
- Kiecker, C. The origins of the circumventricular organs. J. Anat. 2018, 232, 540–553.
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliver. Rev. 2020, 165, 1–14.
- Villaseñor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular transport and regulation of transcytosis across the blood–brain barrier. Cell. Mol. Life Sci. 2019, 76, 1081–1092.
- Yazdani, S.; Jaldin-Fincati, J.R.; Pereira, R.V.; Klip, A. Endothelial cell barriers: Transport of molecules between blood and tissues. Traffic 2019, 20, 390–403.
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 1–24.
- Sánchez-Félix, M.; Burke, M.; Chen, H.H.; Patterson, C.; Mittal, S. Predicting bioavailability of monoclonal antibodies after subcutaneous administration: Open innovation challenge. Adv. Drug Deliver. Rev. 2020, 167, 66–77.
- Bannigan, P.; Aldeghi, M.; Bao, Z.; Häse, F.; Aspuru-Guzik, A.; Allen, C. Machine learning directed drug formulation development. Adv. Drug Deliver. Rev. 2021, 175, 113806.
- Tanguay, M.; Girard, J.; Scarsi, C.; Mautone, G.; Larouche, R. Pharmacokinetics and comparative bioavailability of a levothyroxine sodium oral solution and soft capsule. Clin. Pharm. Dug Dev. 2019, 8, 521–528.
- Shariare, M.H.; Altamimi, M.A.; Marzan, A.L.; Tabassum, R.; Jahan, B.; Reza, H.M.; Kazi, M. In vitro dissolution and bioavailability study of furosemide nanosuspension prepared using design of experiment (DoE). Saudi Pharm. J. 2019, 27, 96–105.
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The promise of nanotechnology in personalized medicine. J. Pers. Med. 2022, 12, 673.
- Leopoldo, M.; Nardulli, P.; Contino, M.; Leonetti, F.; Luurtsema, G.; Colabufo, N.A. An updated patent review on P-glycoprotein inhibitors (2011–2018). Expert Opin. Ther. Pat. 2019, 29, 455–461.
- Australian Product Information Tivicay (dolutegravir) Film-Coated Tablets and TIVICAY PD (dolutegravir) Dispersible Tablets. Available online: https://www.tga.gov.au/sites/default/files/2022-08/auspar-tivicay-tivicay-pd-220705-pi.pdf (accessed on 10 October 2023).
- Shultz, M.D. Two decades under the influence of the rule of five and the changing properties of approved oral drugs: Miniperspective. J. Med. Chem. 2018, 62, 1701–1714.
- U.S. FDA Approves GlaxoSmithKline’s HIV Drug Tivicay. Available online: https://www.reuters.com/article/us-glaxosmithkline-hivdrug-idUSBRE97B0WU20130812 (accessed on 5 October 2023).
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605.
- Ren, S.; Liu, M.; Hong, C.; Li, G.; Sun, J.; Wang, J.; Xie, Y. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystals. Acta Pharm. Sin. B 2019, 9, 59–73.
- Santbergen, M.J.; Van der Zande, M.; Gerssen, A.; Bouwmeester, H.; Nielen, M.W. Dynamic in vitro intestinal barrier model coupled to chip-based liquid chromatography mass spectrometry for oral bioavailability studies. Anal. Bioanal. Chem. 2020, 412, 1111–1122.
- Attwa, M.W.; AlRabiah, H.; Mostafa, G.A.; Kadi, A.A. Development of an LC-MS/MS method for quantification of sapitinib in human liver microsomes: In silico and in vitro metabolic stability evaluation. Molecules 2023, 28, 2322.
- Shinha, K.; Nihei, W.; Ono, T.; Nakazato, R.; Kimura, H. A pharmacokinetic–pharmacodynamic model based on multi-organ-on-a-chip for drug–drug interaction studies. Biomicrofluidics 2020, 14, 044108.
- Perez-Medina, C.; Teunissen, A.J.; Kluza, E.; Mulder, W.J.; Van der Meel, R. Nuclear imaging approaches facilitating nanomedicine translation. Adv. Drug Deliver. Rev. 2020, 154, 123–141.
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643.
- Dałek, P.; Drabik, D.; Wołczańska, H.; Foryś, A.; Jagas, M.; Jędruchniewicz, N.; Langner, M. Bioavailability by design—Vitamin D3 liposomal delivery vehicles. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102552.
- Li, H.; Zhang, M.; Xiong, L.; Feng, W.; Williams, R.O., III. Bioavailability improvement of carbamazepine via oral administration of modified-release amorphous solid dispersions in rats. Pharmaceutics 2020, 12, 11–1023.
- Fuhr, L.M.; Marok, F.Z.; Hanke, N.; Selzer, D.; Lehr, T. Pharmacokinetics of the CYP3A4 and CYP2B6 inducer carbamazepine and its drug–drug interaction potential: A physiologically based pharmacokinetic modeling approach. Pharmaceutics 2021, 13, 270.
- Hsin, C.H.; Stoffel, M.S.; Gazzaz, M.; Schaeffeler, E.; Schwab, M.; Fuhr, U.; Taubert, M. Combinations of common SNPs of the transporter gene ABCB1 influence apparent bioavailability, but not renal elimination of oral digoxin. Sci. Rep. 2020, 10, 12457.
- Ibrahim, N.A.M. An up-to-date review of digoxin toxicity and its management. Int. J. Res. Pharm. Pharm. Sci. 2019, 4, 59–64.
- Pawar, S.R.; Barhate, S.D. Solubility enhancement (Solid Dispersions) novel boon to increase bioavailability. J. Drug Deliv. Ther. 2019, 9, 583–590.
- Kareem, S.H.K.A. Quality by Design Approach for Bioavailability Enhancement of Some Hydrophobic Drugs. Available online: https://shodhgangotri.inflibnet.ac.in/bitstream/20.500.14146/13393/1/final%20synopsis%20corrected.pdf (accessed on 5 October 2023).
- Pireddu, R.; Schlich, M.; Marceddu, S.; Valenti, D.; Pini, E.; Fadda, A.M.; Sinico, C. Nanosuspensions and microneedles roller as a combined approach to enhance diclofenac topical bioavailability. Pharmaceutics 2020, 12, 1140.
- Sardana, K.; Mathachan, S.R. Super bioavailable itraconazole and its place and relevance in recalcitrant dermatophytosis: Revisiting skin levels of itraconazole and minimum inhibitory concentration data. Indian Dermatol. Online J. 2021, 12, 1.
- Bardelmeijer, H.A.; Beijnen, J.H.; Brouwer, K.R.; Rosing, H.; Nooijen, W.J.; Schellens, J.H.; van Tellingen, O. Increased oral bioavailability of paclitaxel by GF120918 in mice through selective modulation of P-glycoprotein. Clin. Cancer Res. 2000, 6, 4416–4421.
- Virili, C.; Brusca, N.; Capriello, S.; Centanni, M. Levothyroxine therapy in gastric malabsorptive disorders. Front. Endocrinol. 2021, 11, 621616.
- Oglah, M.K.; Bashir, M.K.; Mustafa, Y.F. Hypericin and its analogues: A review of their biological activities. Turk. J. Field Crops 2021, 26, 259–269.
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, toxicity, bioavailability, and formulation of magnolol: An update. Front. Pharmacol. 2021, 12, 632767.
- O’Laughlin, K.; Tobolowsky, F.A.; Elmor, R.; Overton, R.; O’Connor, S.M.; Damon, I.K.; Veillard, M. Clinical use of tecovirimat (Tpoxx) for treatment of monkeypox under an investigational new drug protocol—United States, May–August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1190.
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685.
- Deore, A.B.; Dhumane, J.R.; Wagh, R.; Sonawane, R. The stages of drug discovery and development process. Asian J. Pharm. Res. Dev. 2019, 7, 62–67.
- Trucillo, P. Drug carriers: Classification, administration, release profiles, and industrial approach. Processes 2021, 9, 470.
- Landersdorfer, C.B.; Gwee, A.; Nation, R.L. Clinical pharmacological considerations in an early intravenous to oral antibiotic switch: Are barriers real or simply perceived? Clin. Microbiol. Infec. 2023, 29, 1120–1125.
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Corsetti, M. The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59.
- Drenth-van Maanen, A.C.; Wilting, I.; Jansen, P.A. Prescribing medicines to older people—How to consider the impact of ageing on human organ and body functions. Brit. J. Clin. Pharmacol. 2020, 86, 1921–1930.
- Baillie, T.A.; Cayen, M.N.; Fouda, H.; Gerson, R.J.; Green, J.D.; Grossman, S.J.; Shipley, L.A. Drug metabolites in safety testing. Toxicol. Appl. Pharm. 2002, 182, 188–196.
- May, M.; Schindler, C.; Engeli, S. Modern pharmacological treatment of obese patients. Ther. Adv. Endocrinol. Metab. 2020, 11, 1–19.




