The drug formulation process often involves combining inactive ingredients and additional substances with APIs to produce drug products with specific characteristics. Improving this process to achieve an optimal drug formulation can involve various objectives such as increasing efficacy, extending the duration of therapeutic effects, reducing adverse effects, prolonging the shelf life of active ingredients, and enhancing compatibility with patient intake patterns
[1]. APIs can be formulated using different material combinations, including neutral boosters such as polymers, lipids, surfactants, and other active ingredients, depending on the desired delivery method and specific application requirements. Such formulations are made possible by utilizing various types of delivery systems, including different kinds of microparticles (MPs), nanoparticles (NPs), and complex multi-component systems
[2][3][4][5][2,3,4,5]. Typical practices involving these delivery mechanisms are often evolving, resulting in the development of drug products in various forms, such as solids, liquids, or non-oral administration methods
[38][50].
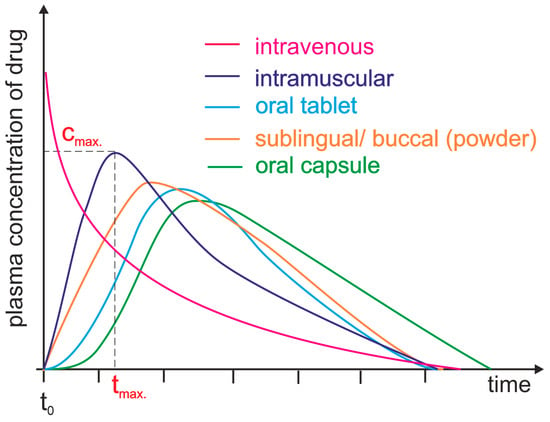
Drug forms such as tablets, capsules, granules, powders, suspensions, solutions, emulsions, inserts, ointments, inserts, aerosols, patches, and transdermal systems affect the drug’s dissolution rate and absorption
[39][51]. Excipients, such as binders, solvents, and stabilizers, can affect a drug’s bioavailability through interactions with the drug or changes in its solubility
[40][52]. Drug release techniques can control the active ingredient’s release rate, which affects its availability and action
[37][48]. Acetylsalicylic acid, commonly known as aspirin, is available in various administration forms. It can be taken orally as tablets in enteral, enteric-coated, effervescent, and controlled-release forms.
Patient factors refer to individual patient characteristics that can affect the bioavailability of a drug
[41][55]. These include age, gender, genotype, health status, and diet. The drug absorption, metabolism, and elimination processes may differ depending on age. Children and the elderly may exhibit differences in metabolic enzymes, renal function, and blood flow, which can affect the bioavailability of drugs.
The protein known as P-glycoprotein (P-gp) plays a critical role in creating barriers within cells, particularly in the endothelial cells of the blood vessels. Its primary role is to prevent the entry of various substances, including drugs, into neural tissue by removing them from endothelial cells and returning them into the bloodstream. P-gp is a multidrug transporter that can recognize many compounds with different chemical structures and molecular weights (ranging from 330 to 4000 Da)
[42][61]. It can transport hydrophobic and inert substances, as well as cations, but it cannot transport anions. The log
p value of approximately 2.2 for DTG
[43][62] indicates that it is only partially subject to bioaccumulation due to its moderate hydrophobicity
[44][63]. P-gp is a protein crucial in transporting substances into and out of cells. Dolutegravir (DTG) is a substance for which P-gp is particularly important. Studies have shown that when DTG enters endothelial cells from the blood, it is pumped back into the bloodstream due to P-gp activity. However, disruption of the blood–brain barrier caused by HIV can lead to the dysfunction of P-gp, making it easier for drugs like DTG to penetrate brain tissues. This can result in higher concentrations of DTG in the brain, leading to unwanted side effects such as insomnia and headaches
[45][64]. It is important to note that P-gp is present in tissues with a secretory function, such as the small intestine, liver, and kidney. If there is a pathological dysfunction of the P-gp protein, it can result in increased symptoms of dysfunction in these tissues. Recent studies reveal that P-gp triggers the production of effector T cells after viral infection.
34. Methods for Assessing Drug Bioavailability
Three main categories of methods are used to assess drug bioavailability—in vitro methods, in vivo methods, and new techniques and tools.
In vitro methods are laboratory-based and involve studying drug bioavailability under controlled conditions outside the living organism. These methods enable researchers to study drug absorption, metabolism, and transport processes and to evaluate the impact of physicochemical factors on drug bioavailability
[46][68]. There are different in vitro methods used for drug testing. One such method is drug solubility testing, in which the solubility of a drug is measured in various environments, including the use of buffer solutions with different pH levels. This test helps determine how well a drug dissolves and is absorbed in the digestive environment
[47][69].
Cell cultures are another example in which human or animal cells are used to simulate processes such as drug absorption, metabolism, and transport. These cultures can also be used to examine the effects of digestive enzyme activity or transporters on bioavailability
[48][72]. Narrow liver microsomes containing microsomal enzymes accurately represent the liver’s metabolic activity, with various applications. Studying drug metabolism using hepatic microsomes allows for the estimation of how a drug may be metabolized before it is eliminated from the system
[49][73].
Through in vivo techniques, the bioavailability of a drug in a living system can be investigated. These methods consider the entire bioavailability process, including drug interactions, metabolism, elimination, and patient response. In vivo methods involve administering drugs to patients or animals and then analyzing samples of blood, urine, or other body fluids to determine the concentration of the drug over time
[50][74].
With the advancements in science and technology, new methods and tools for evaluating drug bioavailability are being developed. Medical imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), are some of the techniques that are currently being developed. These techniques enable the monitoring and tracking of drug distribution in the body, allowing for the observation of the drug’s path post-administration and the assessment of its concentration in various tissues and organs
[51][79]. Other advanced techniques include pharmacogenetic studies, which deal with the impact of genetic differences on drug responses. They allow for the determination of the effect of genetic polymorphism in metabolic enzymes and transporters on drug bioavailability in different individuals
[52][80]. Pharmacokinetic modeling, or computer simulation, has become a valuable medical tool that uses mathematical models and algorithms to predict drug performance in living organisms, considering factors like dosage, absorption, transport rates, and enzyme concentrations, thereby optimizing treatment efficacy
[53][81].
45. Drugs with Poorly Described Bioavailability
Drugs with poorly described bioavailability are those for which there is limited information regarding the ADME process in the body. There may be various reasons for the lack of detailed data on drug bioavailability
[16][21]. In this context, four distinct groups of drugs can be identified: (1) drugs with complex metabolism and elimination, (2) drugs with limited solubility, (3) drugs with specific absorption, and (4) other cases. Drugs with complex metabolism and elimination undergo intricate chemical metabolism and removal processes, influenced by factors such as interactions with other drugs, differences in gene-type metabolism, diseases, and the overall health of the patient
[23][34].
Warfarin is a drug used for preventing and treating thrombosis. However, its metabolism is quite complex. It is mainly metabolized in the liver by cytochrome P450 enzymes through a series of steps involving hydroxylation, reduction, and conjugation. After metabolism, the drug is eliminated from the body as metabolites through the kidneys. However, the activity of cytochrome P450 enzymes varies widely due to genetic differences and interactions with other drugs and food.
Carbamazepine is a medication used to treat epilepsy, trigeminal neuralgia, and bipolar affective disorder. Certain enzymes, particularly CYP3A4 and CYP2C9 isoenzymes, metabolize the drug in the liver. Its metabolites are excreted in both urine and feces. Carbamazepine’s metabolism and bioavailability can be significantly affected by interactions with other drugs that inhibit or induce the cytochrome enzymes
[54][55][84,85].
Digoxin is a medication used to treat heart failure and certain cardiac arrhythmias. Although it is primarily metabolized in the liver, it is eliminated from the body mainly through renal excretion. The metabolism of digoxin is complex, with glucuronidation being the primary metabolic pathway. However, the therapeutic window for digoxin is narrow, which means that even small changes in bioavailability and elimination can cause toxicity or a lack of effectiveness
[56][57][86,87].
Drugs with low water solubility show difficulty dissolving in body fluids, which affects their bioavailability and therapeutic effectiveness due to the hydrophobicity of the drug or the formation of complexes with other substances
[58][59][88,89]. Analgesics like diclofenac possess limited solubility in water, which adversely affects their absorption from the gastrointestinal tract and therefore, their bioavailability. Various strategies can be employed to increase the solubility and improve the absorption of these drugs. These may include modifying the formulation to obtain a more soluble form or using specific carriers that facilitate drug delivery to the site of action
[60][90]. Certain antifungal medications, including itraconazole, exhibit a restricted capacity to dissolve in water, reducing their absorption in the gastrointestinal tract. To overcome this issue, solubility-enhancing substances are administered, or suitable formulations are developed to enhance the effectiveness and bioavailability of these drugs
[61][91]. Paclitaxel is a common anticancer drug used to treat various malignancies in humans. However, it shows limited solubility in water, which makes it difficult to administer and absorb. To overcome this challenge, unique formulations enhance its solubility and delivery to the site of action
[62][92].
Drugs with specific absorption are those whose absorption in the gastrointestinal tract depends on specific mechanisms or conditions. These drugs may be subject to interactions with other substances, pH changes, the presence of transporters, or specific absorption processes. One example of such a drug is levothyroxine, a synthetic hormone used to treat hypothyroidism. Its absorption depends on the presence of iodine in the gut. Iodine ions are essential for forming the active thyroid hormone (thyroxine—T4). Consequently, patients taking levothyroxine must take it on an empty stomach and avoid substances such as calcium, iron, or fiber that may affect the absorption of iodine and the drug itself
[63][95].
Apart from to the examples outlined above, there are numerous other instances in which drug bioavailability is poorly defined or understood. Herbal treatments, anticancer medications, and novel medications are some examples of these. The bioavailability of certain plant-based drugs, such as herbal dietary supplements, can be inadequately described due to the complexity of their active ingredients, which can vary in their chemical form
[64][65][99,100]. Hypericin is a natural compound in the St. John’s wort (Hypericum perforatum) plant. It is used for various health conditions, including treating depression and fighting against different viruses. However, the bioavailability of hypericin is not well understood because it is a complex chemical compound that can undergo several transformations in the body. Different forms of hypericin may include various pharmacokinetic properties, as well as bioavailability
[64][99].
Data on bioavailability may be limited for new drugs that have not undergone comprehensive clinical trials. The absorption, distribution, metabolism, and elimination of a drug are all evaluated in basic pharmacokinetic studies. Nevertheless, data regarding bioavailability are scarce, particularly in large-scale clinical trials with a diverse patient group
[16][21]. An example of a new drug with limited information regarding its bioavailability is tecovirimat (Tpoxx), an antiviral drug that has demonstrated efficacy in animal studies and has been approved by the Food and Drug Administration for the treatment of smallpox, a severe and life-threatening infection caused by the Variola virus of the Orthopoxvirus genus. It belongs to a group of drugs known as orthopoxvirus-specific antivirals. Tecovirimat is an investigational drug and is not currently approved for routine use. It is used in emergencies as part of preparatory measures against smallpox outbreaks
[66][103].
56. Conclusions
Bioavailability data for many active compounds is sparse, despite substantial pharmacological study. These extensive pharmacokinetic studies are required for a broad list of drugs
[67][119]. However, such studies are expensive and complicated; thus, few are performed, and therefore, few can be added to the bioavailability dataset
[68][106]. Moreover, the pharmacokinetic characteristics of individuals vary greatly. Age, gender, genetics, health, and other parameters affect drug absorption and transport. However, a number of results obtained from such studies can be utilized to build generalized models showing the action of APIs in the human body
[19][24].
It was established that drug bioavailability depends on administration
[69][121]. Intravenous medications enter the bloodstream directly, while oral pharmaceuticals must pass through the digestive system and may be destroyed or absorbed incorrectly. Many bioavailability details remain undiscovered, despite broad studies for varied dosing techniques
[70][122]. Since not all drug-food interactions are known, the need remains for further thorough studies of this aspect
[71][123].
In regards to drug therapy safety, bioavailability studies determine doses to reduce dangerous blood active component concentrations, identify medication interaction risk factors, and improve safety
[72][124]. Individualized treatment based on genetics, health, age, and drug interactions is possible because these studies disclose internal factors impacting drug absorption, distribution, and metabolism
[73][125].
Medication bioavailability research is essential for treating rare and complex diseases. Understanding the active ingredient absorption derived from tablets, capsules, injections, and patches improves drug development. Understanding bioavailability improves drug use and health care by optimizing prescription design, treating rare disorders, and discovering new formulations
[74][126].