
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dalius Ratautas | -- | 2541 | 2024-01-02 14:34:34 | | | |
| 2 | Peter Tang | Meta information modification | 2541 | 2024-01-03 01:50:43 | | |
Video Upload Options
The field of biosensors is filled with reports and designs of various sensors, with the vast majority focusing on glucose sensing. However, in addition to glucose, there are many other important analytes that are worth investigating as well. In particular, L-amino acids appear as important diagnostic markers for a number of conditions. The need to determine L-amino acids from clinical samples has risen. More clinical data appear to demonstrate that abnormal concentrations of L-amino acids are related to various clinical conditions such as inherited metabolic disorders, dyslipidemia, type 2 diabetes, muscle damage, etc.
1. Typical Concentrations of Total L-Amino Acids in Human Blood
2. Overview of L-Amino Acids in Clinical Conditions
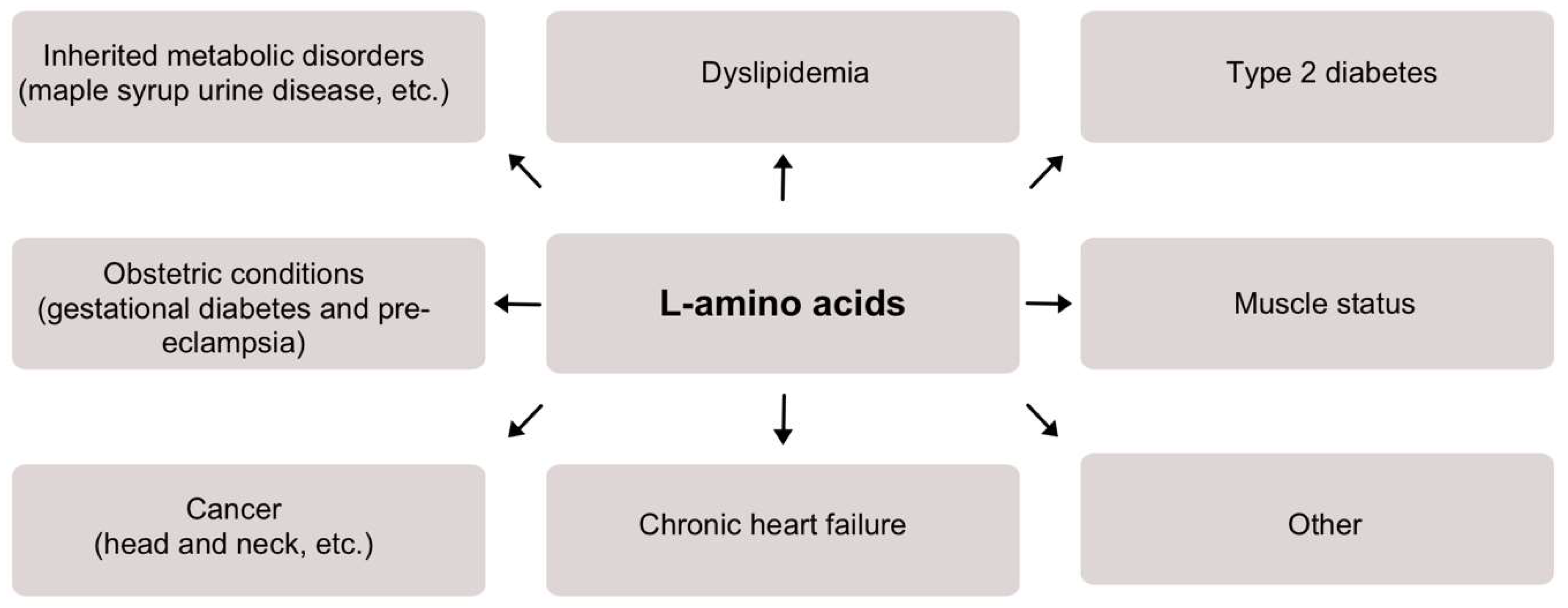
2.1. Inherited Metabolic Disorders
2.2. Dyslipidemia
2.3. Type 2 Diabetes
2.4. Obstetric Conditions
2.5. Muscle Status
2.6. Cancer
2.7. Chronic Heart Failure
2.8. Other
3. Biosensors for Measurement of Total L-Amino Acid Concentration
3.1. General Overview of Enzymatic Biosensors
|
Generation |
Key Advantages |
Key Disadvantages |
|---|---|---|
|
First (electrons are being transferred via native mediator, e.g., H2O2) |
Simple to design Native mediator is constantly being produced by the enzyme, thus cannot be depleted The sensing layer can be separated from the electrode surface The sensing layer can be made using excessive amount of enzyme to thus increase stability |
Depended on the concentration of the dissolved oxygen in a solution Lack of sensitivity High operational potential (e.g., +0.6 V vs. Ag/AgCl) |
|
Second (electrons are being transferred via synthetic mediator, e.g., tetrathiafulvalene) |
Usually do not depend on the concentration of the dissolved oxygen in a solution Have better sensitivities in comparisonto first generation The sensing layer can be made using excessive amount of enzyme to thus increase stability Low operational potential (e.g., +0.2 V vs. Ag/AgCl) |
Complex design Synthetic mediator needs to be constantly added to the solution Desorption of mediator from the surfacecan occur |
|
Third (electrons are being transferred directly) |
Usually do not depend on the concentration of the dissolved oxygen in a solution Highest sensitivities in comparison to the first and second generations Lowest operational potentials (e.g., 0 V vs. Ag/AgCl) No mediator (native or synthetic) is needed for the electrode operation |
Most complex design Can be implemented for a limited number of selective enzymes Low operational stability in comparison to other generations |
3.2. Overview of Biosensors for L-Amino Acid Detection
Table 2 summarizes most significant biosensors for L-amino acid detection.
Table 2. A comparative table of most significant biosensors for L-amino acid detection (majority for total L-amino acids, some for specific).
|
Biosensor |
Enzyme, Nanomaterials Used |
Analysis Method |
Analytical Parameters (as Reported) |
Real Sample |
|---|---|---|---|---|
|
Nanjo and Guilbault [43] |
Enzyme: L-amino acid oxidase (Crotalus adamanteus) Nanomaterials: N/A |
Chronoamperometry |
Sensitivity: N/A LOD: 10−6–10−5 M Linear range: N/A Stability: stable for at least 4 months |
N/A |
|
Varadi et al. [44] |
Enzyme: L-amino acid oxidase (Crotalus adamanteus), D-amino acid oxidase (porcine kidney) Nanomaterials: N/A |
Flow-through amperometry |
Sensitivity: N/A LOD: N/A Linear range: 0.1–3 mM Stability: 900–1000 measurements |
Brewing process samples (ginger and brown beer) |
|
Sarkar et al. [45] |
Enzyme: L-amino acid oxidase (Crotalus adamanteus), D-amino acid oxidase (porcine kidney) Nanomaterials: N/A |
Chronoamperometry |
Sensitivity: N/A LOD: 0.15–0.47 mM Linear range: 0.47–2.5 mM Stability: 40% activity loss after 56 days |
Milk, fruit juice, urine |
|
Lata and Pundir [46] |
Enzyme: L-amino acid oxidase (goat kidney) Nanomaterials: MWCNT |
Linear square voltammetry |
Sensitivity: N/A LOD: 0.5 µM Linear range: 0.5 µM–100 mM Stability: 70% left after 140 days |
Fruit juices, alcoholic beverages |
|
Dominguez et al. [47] |
Enzyme: L-amino acid oxidase (Gratelis Adamate) *, D-amino acid oxidase (porcine kidney) Nanomaterials: N/A |
Chronoamperometry |
Sensitivity: 10–480 µA M−1 LOD: 1.1–160 µM Linear range: 10−6–10−4 M Stability: around 10 days with no need to regenerate the electrode surface |
Grapes |
|
Odewunmi et al. [48] |
Enzyme: enzyme-free Nanomaterials: AgO nanoparticles |
Chronoamperometry |
Sensitivity: 4230 µA mM−1 cm−2 LOD: 0.42 µM Linear range: 60–500 µM Stability: N/A |
Human serum |
|
Garcia-Carmona et al. [49] |
Enzyme: enzyme-free Nanomaterials: MWCNT |
Differential pulse voltammograms |
Sensitivity: 0.012 µA µM−1 LOD: 8 µM Linear range: 25–750 µM Stability: N/A |
Human serum |
|
Hua et al. [50] |
Enzyme: enzyme-free Nanomaterials: tetrahedral copper metal–organic frameworks |
Linear sweep voltammetry |
Sensitivity: N/A LOD: 25 nM Linear range: N/A Stability: no significant change after 6-month storage |
Human blood |
|
Miškinis et al. [51] |
Enzyme: L-amino acid oxidase (Crotalus adamanteus) Nanomaterials: gold nanoparticles |
Chronocoulometry |
Sensitivity: 0.73 µC/µM LOD: 5.5 µM Linear range: 5.5–100 µM Stability: 50% of the initial activity after 10 days of storage |
Human serum and blood |
* As reported in the research.
References
- Stein, W.H.; Moore, S. The free amino acids of human blood plasma. J. Biol. Chem. 1954, 211, 915–926.
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312.
- Aliu, E.; Kanungo, S.; Arnold, G.L. Amino acid disorders. Ann. Transl. Med. 2018, 6, 471.
- Nunes, V.; Niinikoski, H. Lysinuric Protein Intolerance. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, M.G., Pagon, R.A., Wallace, S.E., Bean, J.H.L., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993.
- Blackburn, P.R.; Gass, J.M.; e Vairo, F.P.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple syrup urine disease: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 57–66.
- Kim, M.J.; Park, S.; Yang, H.J.; Shin, P.K.; Hur, H.J.; Park, S.J.; Lee, K.H.; Hong, M.; Kim, J.H.; Choi, S.W.; et al. Alleviation of Dyslipidemia via a Traditional Balanced Korean Diet Represented by a Low Glycemic and Low Cholesterol Diet in Obese Women in a Randomized Controlled Trial. Nutrients 2022, 14, 235.
- Elshorbagy, A.K.; Samocha-Bonet, D.; Jernerén, F.; Turner, C.; Refsum, H.; Heilbronn, L.K. Food Overconsumption in Healthy Adults Triggers Early and Sustained Increases in Serum Branched-Chain Amino Acids and Changes in Cysteine Linked to Fat Gain. J. Nutr. 2018, 148, 1073–1080.
- Mikkola, T.M.; Salonen, M.K.; Kajantie, E.; Kautiainen, H.; Eriksson, J.G. Associations of Fat and Lean Body Mass with Circulating Amino Acids in Older Men and Women. J. Gerontol. Ser. A 2020, 75, 885–891.
- Bi, X.; Tey, S.L.; Loo, Y.T.; Henry, C.J. Central adiposity-induced plasma-free amino acid alterations are associated with increased insulin resistance in healthy Singaporean adults. Eur. J. Clin. Nutr. 2017, 71, 1080–1087.
- Wiklund, P.; Zhang, X.; Tan, X.; Keinänen-Kiukaanniemi, S.; Alen, M.; Cheng, S. Serum Amino Acid Profiles in Childhood Predict Triglyceride Level in Adulthood: A 7-Year Longitudinal Study in Girls. J. Clin. Endocrinol. Metab. 2016, 101, 2047–2055.
- Mook-Kanamori, D.O.; Römisch-Margl, W.; Kastenmüller, G.; Prehn, C.; Petersen, A.K.; Illig, T.; Gieger, C.; Wang-Sattler, R.; Meisinger, C.; Peters, A.; et al. Increased amino acids levels and the risk of developing of hypertriglyceridemia in a 7-year follow-up. J. Endocrinol. Investig. 2014, 37, 369–374.
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Mechanism of Amino Acid-Induced Skeletal Muscle Insulin Resistance in Humans. Diabetes 2002, 51, 599–605.
- Adams, S.H. Emerging Perspectives on Essential Amino Acid Metabolism in Obesity and the Insulin-Resistant State. Adv. Nutr. 2011, 2, 445–456.
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453.
- Tillin, T.; Hughes, A.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.; Godsland, I.F.; Eastwood, S.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall and Brent REvisited) Study. Diabetologia 2015, 58, 968–979.
- Karusheva, Y.; Koessler, T.; Strassburger, K.; Markgraf, D.; Mastrototaro, L.; Jelenik, T.; Simon, M.-C.; Pesta, D.; Zaharia, O.-P.; Bódis, K.; et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2019, 110, 1098–1107.
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615.
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; De Angelis, M.H.; Peters, A.; et al. Identification of Serum Metabolites Associated with Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2013, 62, 639–648.
- Nevalainen, J.; Sairanen, M.; Appelblom, H.; Gissler, M.; Timonen, S.; Ryynänen, M. First-Trimester Maternal Serum Amino Acids and Acylcarnitines Are Significant Predictors of Gestational Diabetes. Rev. Diabet. Stud. 2016, 13, 236–245.
- Evans, R.W.; Powers, R.W.; Ness, R.B.; Cropcho, L.J.; Daftary, A.R.; Harger, G.F.; Vergona, R.; Finegold, D.N. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction 2003, 125, 785–790.
- Lehmann, M.; Huonker, M.; Dimeo, F.; Heinz, N.; Gastmann, U.; Treis, N.; Steinacker, J.M.; Keul, J.; Kajewski, R.; Häussinger, D. Serum Amino Acid Concentrations in Nine Athletes Before and After the 1993 Colmar Ultra Triathlon. Int. J. Sports Med. 1995, 16, 155–159.
- Coombes, J.S.; McNaughton, L.R. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sports Med. Phys. Fit. 2000, 40, 240–246.
- Yoshikawa, N.; Yamamoto, M.; Kuribara-Souta, A.; Uehara, M.; Yamazaki, H.; Tanaka, H. Amino Acid Profile in 18 Patients with Rheumatic Diseases Treated with Glucocorticoids and BCAAs. J. Nutr. Sci. Vitaminol. 2021, 67, 180–188.
- Pavlickova Aimova, P.; Hronek, M.; Zadak, Z. The Importance and Dosage of Amino Acids in Nutritional Support of Various Pathological Conditions in ICU Patients; Biomedical Papers of the Medical Faculty of the Palacky University: Olomouc, Czech Republic, 2014; Volume 158, pp. 346–355.
- Vicka, V.; Vickiene, A.; Miskinyte, S.; Bartuseviciene, I.; Lisauskiene, I.; Serpytis, M.; Ringaitiene, D.; Sipylaite, J. Role of Fat-Free Mass Index on Amino Acid Loss during CRRT in Critically Ill Patients. Medicina 2023, 59, 389.
- Kitagawa, M.; Haji, S.; Amagai, T. High Serum Essential Amino Acids as a Predictor of Skeletal Muscle Depletion in Patients with Cachexia and Advanced Gastrointestinal Cancers. Nutr. Clin. Pract. 2017, 32, 645–651.
- Cadoni, G.; Giraldi, L.; Chiarla, C.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Settimi, S.; Galli, J.; Paludetti, G.; Arzani, D.; et al. Prognostic Role of Serum Amino Acids in Head and Neck Cancer. Dis. Markers 2020, 2020, 2291759.
- Kang, J.-S. Dietary restriction of amino acids for Cancer therapy. Nutr. Metab. 2020, 17, 20.
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma Amino Acid Profiling Identifies Specific Amino Acid Associations with Cardiovascular Function in Patients with Systolic Heart Failure. PLoS ONE 2015, 10, e0117325.
- Tsuji, S.; Koyama, S.; Taniguchi, R.; Fujiwara, T.; Fujiwara, H.; Sato, Y. Nutritional status of outpatients with chronic stable heart failure based on serum amino acid concentration. J. Cardiol. 2018, 72, 458–465.
- Puskarich, M.A.; McHugh, C.; Flott, T.L.; Karnovsky, A.; Jones, A.E.; Stringer, K.A. Serum Levels of Branched Chain Amino Acids Predict Duration of Cardiovascular Organ Failure in Septic Shock. Shock 2021, 56, 65–72.
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic Changes in Amino Acid Concentration Profiles in Patients with Sepsis. PLoS ONE 2015, 10, e0121933.
- Mizuno, K.; Tanaka, M.; Nozaki, S.; Yamaguti, K.; Mizuma, H.; Sasabe, T.; Sugino, T.; Shirai, T.; Kataoka, Y.; Kajimoto, Y.; et al. Mental fatigue-induced decrease in levels of several plasma amino acids. J. Neural Transm. 2007, 114, 555–561.
- Yu, X.; Qian-Qian, L.; Cong, Y.; Xiao-Bing, Z.; Hong-Zhu, D. Reduction of essential amino acid levels and sex-specific alterations in serum amino acid concentration profiles in children with autism spectrum disorder. Psychiatry Res. 2021, 297, 113675.
- Tinkov, A.A.; Skalnaya, M.G.; Skalny, A.V. Serum trace element and amino acid profile in children with cerebral palsy. J. Trace Elem. Med. Biol. 2021, 64, 126685.
- Figura, M.; Kuśmierska, K.; Bucior, E.; Szlufik, S.; Koziorowski, D.; Jamrozik, Z.; Janik, P. Serum amino acid profile in patients with Parkinson’s disease. PLoS ONE 2018, 13, e0191670.
- Ooi, P.H.; Gilmour, S.M.; Yap, J.; Mager, D.R. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin. Nutr. ESPEN 2018, 28, 41–51.
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214.
- Duranton, F.; Lundin, U.; Gayrard, N.; Mischak, H.; Aparicio, M.; Mourad, G.; Daurès, J.-P.; Weinberger, K.M.; Argilés, A. Plasma and Urinary Amino Acid Metabolomic Profiling in Patients with Different Levels of Kidney Function. Clin. J. Am. Soc. Nephrol. 2014, 9, 37–45.
- Zhang, Y.; Zhou, Q.; Yang, R.; Hu, C.; Huang, Z.; Zheng, C.; Liang, Q.; Gong, R.; Zhu, X.; Gong, H.; et al. Serum branched-chain amino acids are associated with leukocyte telomere length and frailty based on residents from Guangxi longevity county. Sci. Rep. 2020, 10, 10252.
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014, 25, 558–566.
- Dato, S.; Hoxha, E.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Amino acids and amino acid sensing: Implication for aging and diseases. Biogerontology 2019, 20, 17–31.
- Nanjo, M.; Guilbault, G. Enzyme electrode for l-amino acids and glucose. Anal. Chim. Acta 1974, 73, 367–373.
- Varadi, M.; Adanyi, N.; Szabo, E.E.; Trummer, N. Determination of the ratio of D- and L-amino acids in brewing by an immobilised amino acid oxidase enzyme reactor coupled to amperometric detection. Biosens. Bioelectron. 1999, 14, 335–340.
- Sarkar, P.; Tothill, I.E.; Setford, S.J.; Turner, A.P.F. Screen-printed amperometric biosensors for the rapid measurement of L- and D-amino acids. Analyst 1999, 124, 865–870.
- Lata, S.; Pundir, C.S. L-amino acid biosensor based on L-amino acid oxidase immobilized onto NiHCNFe/c-MWCNT/PPy/GC electrode. Int. J. Biol. Macromol. 2013, 54, 250–257.
- Domínguez, R.; Serra, B.; Reviejo, A.J.; Pingarrón, J.M. Chiral Analysis of Amino Acids Using Electrochemical Composite Bienzyme Biosensors. Anal. Biochem. 2001, 298, 275–282.
- Odewunmi, N.A.; Kawde, A.-N.; Ibrahim, M. In-situ single-step electrochemical AgO modified graphite pencil electrode for trace determination of DL-methionine in human serum sample. Sens. Actuators B Chem. 2019, 281, 765–773.
- García-Carmona, L.; Moreno-Guzmán, M.; Sierra, T.; González, M.C.; Escarpa, A. Filtered carbon nanotubes-based electrodes for rapid sensing and monitoring of L-tyrosine in plasma and whole blood samples. Sens. Actuators B Chem. 2018, 259, 762–767.
- Hua, Y.; Lv, X.; Cai, Y.; Liu, H.; Li, S.; Wan, Y.; Wang, H. Highly selective and reproducible electroanalysis for histidine in blood with turn-on responses at a potential approaching zero using tetrahedral copper metal organic frameworks. Chem. Commun. 2019, 55, 1271–1274.
- Miškinis, J.; Ramonas, E.; Gurevičienė, V.; Razumienė, J.; Dagys, M.; Ratautas, D. Capacitance-Based Biosensor for the Measurement of Total Loss of L-Amino Acids in Human Serum during Hemodialysis. ACS Sens. 2022, 7, 3352–3359.




