Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cyril Fersing | -- | 1896 | 2024-01-02 09:27:19 | | | |
| 2 | Jessie Wu | Meta information modification | 1896 | 2024-01-03 03:12:09 | | | | |
| 3 | Jessie Wu | Meta information modification | 1896 | 2024-01-05 07:40:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Souche, C.; Fouillet, J.; Rubira, L.; Donzé, C.; Deshayes, E.; Fersing, C. Chemistry and Radiochemistry of DOTAZOL. Encyclopedia. Available online: https://encyclopedia.pub/entry/53313 (accessed on 07 February 2026).
Souche C, Fouillet J, Rubira L, Donzé C, Deshayes E, Fersing C. Chemistry and Radiochemistry of DOTAZOL. Encyclopedia. Available at: https://encyclopedia.pub/entry/53313. Accessed February 07, 2026.
Souche, Céleste, Juliette Fouillet, Léa Rubira, Charlotte Donzé, Emmanuel Deshayes, Cyril Fersing. "Chemistry and Radiochemistry of DOTAZOL" Encyclopedia, https://encyclopedia.pub/entry/53313 (accessed February 07, 2026).
Souche, C., Fouillet, J., Rubira, L., Donzé, C., Deshayes, E., & Fersing, C. (2024, January 02). Chemistry and Radiochemistry of DOTAZOL. In Encyclopedia. https://encyclopedia.pub/entry/53313
Souche, Céleste, et al. "Chemistry and Radiochemistry of DOTAZOL." Encyclopedia. Web. 02 January, 2024.
Copy Citation
Bisphosphonates are therapeutic agents that have been used for almost five decades in the treatment of various bone diseases, such as osteoporosis, Paget disease and prevention of osseous complications in cancer patients. In nuclear medicine, simple bisphosphonates such as 99mTc-radiolabelled oxidronate and medronate remain first-line bone scintigraphic imaging agents for both oncology and non-oncology indications. In addition, bifunctional bisphosphonates such as DOTAZOL can be tagged with radiometals for theranostic applications.
DOTAZOL
bisphosphonates
theranostics
nuclear medicine
Radiochemistry
1. Introduction
DOTAZOL is based on the structure of third-generation hydroxylated amino-bisphosphonate zoledronate, functionalized at position four of the imidazole ring by an ethyl-monoamide-DOTA moiety [1].
2. Synthesis of DOTAZOL
The first synthesis of DOTAZOL was reported by Meckel et al. in 2017, who presented this macrocyclic bisphosphonate as a promising potential theranostic tool in the management of skeletal metastases [2]. The first step of the synthesis sequence (Scheme 1) consisted of the functionalization of ω-N-acetylhistamine with an acetic arm on the imidazole nitrogen. The use of benzyl bromoacetate with cesium carbonate in DMF formed the desired compound in the average yield via a N-alkylation reaction, followed by a deprotection step catalyzed by palladium on carbon in methanol under hydrogen atmosphere. The hydroxybisphosphonate core was then obtained in a modest yield by reacting a mixture of phosphorus trichloride and phosphorus acid (probably to increase reaction yield compared to PCl3 alone [3]) in methanesulfonic acid with the latter 1H-imidazole-1-acetic acid derivative. Interestingly, cleavage of the acetamide to form the primary amine was achieved during the same step, based on the strong acidic condition after the hydrolysis of phosphorus trichloride. Finally, functionalization with the chelating moiety was achieved through the nucleophilic substitution of activated DOTA-NHS-ester in water in the presence of sodium carbonate [4].
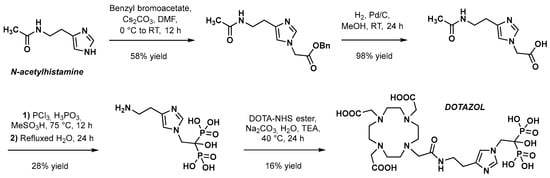
Scheme 1. Original synthesis sequence of DOTAZOL as described by Meckel et al. [2].
The overall yield of this synthesis sequence is 2.5%, the most limiting steps being the formation of the bisphosphonate motif (28%) and, most importantly, the anchoring of the DOTA group (16%). Regarding this final step, because unprotected bisphosphonates have poor solubility in organic solvents and dissolve only in aqueous buffers, an amide bond formation is not an appropriate strategy because most active esters tend to hydrolyze in aqueous buffers. Thus, the solution proposed here is to use DOTA-NHS-ester; however, it was reported that this compound led to an increased amount of free DOTA as a hydrolysis product of the NHS ester, resulting in successive, laborious final purification cycles. In 2022, Greifenstein et al. proposed a practical and efficient alternative coupling method between the 12-membered macrocycle and the bisphosphonate moiety. This ligation method, which relies on the pH-sensitive reactivity of diethyl squarate, is progressively gaining prominence in the development of novel radiopharmaceuticals [5]. Although the compound that was ultimately obtained was not DOTAZOL (the squaric acid replacing the imidazole ring), the stability of squaric acid esters in aqueous buffers would provide a much more straightforward coupling reaction method (Scheme 2), with no significant formation of side products.
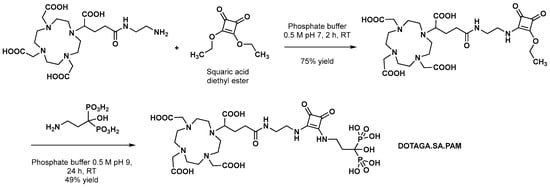
Scheme 2. Synthesis sequence of DOTAGA.SA.PAM, a DOTAZOL-related bisphosphonate, as described by Greifenstein et al. [6].
3. DOTAZOL Radiolabeling with 68Ga or 177Lu
The initial 68Ga radiolabeling of DOTAZOL was performed manually, involving sodium acetate buffer 0.5 M pH 4 and post-processed 68Ga in acetone/HCl and 25 nmol ligand; the mixture was heated at 98 °C for 15 min. After terminal purification on a weak anion exchange cartridge, the radiochemical purity (RCP) of [68Ga]Ga-DOTAZOL was >98% with the RCY between 80 and 95% [2]. The same method was reported for the preparation of 68Ga-labeled DOTAZOL for clinical use [7]. Meisenheimer et al. proposed an alternate 68Ga radiolabeling protocol using ammonium acetate 0.08 M with ethanol as a cosolvent, concentrated 68Ga in NaCl 5 M pH 1, and heated at 95 °C for 10 min [8]. A thorough study of the reaction conditions was carried out simultaneously and showed that, interestingly, the nature of the reaction vial had a significant influence on the overall reaction outcome. Several polyvalent cations present in the glass could interact with the bisphosphonate molecules, leading to their precipitation and/or adsorption on the reaction vessel. This issue has apparently not been reported in the literature for other DOTA-bisphosphonates; however, it should be considered in the context of unsatisfactory radiolabeling results involving the ionic molecules likely to form this type of interaction [9]. The terminal purification step via solid phase extraction (SPE) was also identified as critical in the [68Ga]Ga-DOTAZOL preparation process. The use of a weak anion exchange cartridge to trap the product and then elute it with phosphate buffer saline could be considered [2]; however, the screening of dozens of SPE cartridges did not identify any fully suitable model as thecartridges showed either no retention, the retention of all chemical species, or e thpartial/imperfect retention of one or other species [8]. This parameter therefore requires in-depth study and adaptation to each DOTA-Bisphosphonate used. Indeed, similar issues have been reported with other compounds such as BPAMD, although apparently reliable solutions have been found for this derivative [10][11]. Finally, the automation of the 68Ga radiolabeling of DOTAZOL was reported to be quite challenging (notably due to the influence of the reaction vial and to the SPE purification step) [8], which could complicate its use in humans on a larger scale. Thus, the optimized conditions for the automated preparation of [68Ga]Ga-DOTAZOL have yet to be identified and adapted to the various GMP-compliant synthesis modules available at present [12]. The ideal scenario for successful and lasting integration into routine clinical practice would probably be the industrial development of DOTAZOL, formulated in a single-vial cold kit for 68Ga radiolabeling, which would guarantee the easy preparation of the radiopharmaceutical.
The original 177Lu radiolabeling protocol was in line with the usual conditions for this type of reaction [13], involving sodium acetate buffer 0.25 M, [177Lu]LuCl3 (1 GBq) in 0.04 M HCl and only 20 nmol (~14 µg) DOTAZOL, which were heated at 98 °C for 30 min [2]. In comparison, the 177Lu radiolabeling of DOTA-IBA employed the exact same reaction conditions, with a heating time of 15 min. The quantitative complexation yields were reached with this method, exempting it from a final purification step. Khawar et al. later described a modified radiolabeling protocol replacing sodium acetate with ascorbate buffer 1.3 M plus gentisic acid 0.3 M as an anti-radiolysis compound [14]. Although the 177Lu activity used was higher (~6 GBq was added to the reaction medium and heated at 95 °C for 30 min), excellent RCP and RCY values were reached (≥98% and ≥95%, respectively); the radiopharmaceutical thus prepared was subsequently administered to humans. This protocol was also applied by both Yadav et al. [15] and Kreppel et al. [16], who gave details of the quantities of the vector used for 177Lu radiolabeling (60 µg), which was logically higher than that used for 68Ga radiolabeling. Regardless of the radiolabeling conditions, the pH of the reaction medium should be carefully controlled to be around 5, which is ideal for lutetium-177 complexation [13]. It is worth mentioning that these preparations were carried out manually, again raising the issue of the need for pharmacotechnical works aimed at developing efficient and reliable automated synthesis routes in accordance with GMP guidelines. Additionally, although all reported clinical uses of [177Lu]Lu-DOTAZOL mention in-house preparation, the industrialized production of this radiopharmaceutical would be a further step toward wider clinical applications.
4. Quality Controls of Radiolabeled DOTAZOL: A Critical Step
As well as several parameters involved in the radiolabeling of DOTAZOL, the quality control methods for determining th RCP of this 68Ga/177Lu-labeled bisphosphonate proved to be critical points in the preparation process of such investigational radiopharmaceuticals.
The initial radio-TLC conditions for controlling the RCP of [68Ga]Ga-DOTAZOL used an acetone/acetylacetone/HCl mixture (10/10/1) as the mobile phase and silica 60 F254 TLC plates as the stationary phase [2]. With this eluent composition, the unbound 68Ga is chelated by acetylacetone to form gallium(III) tris(acetylacetonate), migrating to the solvent front. Colloidal gallium-68 should also convert to ionic [68Ga]Ga3+ due to its extremely low pH, facilitating its subsequent chelation by acetylacetone and its migration to the solvent front. Notably, slightly adapted analytical conditions (e.g., using acetonitrile instead of acetone) have been reported for other 68Ga-labeled bifunctional bisphosphonates [17]. To guarantee the reliability of the results obtained by this analysis and to verify that part of the free gallium identified in radio-TLC does not come from the dissociation of the DOTA-complex [18][19], a threeplate TLC system was later proposed in order to more formally identify all the chemical species potentially present [8]. Table 1 summarizes the migration profiles expected under the different analytical conditions. Interestingly, these conditions were also used to control the RCP of the [68Ga]Ga-DOTAZOL used in humans, but only partially, with the single acetylacetone/acetone (1:1) system [7].
Table 1. Radio-TLC conditions proposed by Meisenheimer et al. for [68Ga]Ga-DOTAZOL analysis [8]. The green-colored cells indicate the chemical species identified in each condition, potentially by cross-referencing with other radio-TLC analyses.
| Mobile Phase | Rf [68Ga]Ga-DOTAZOL | Rf [68Ga]Ga3+ | Rf 68Ga-Colloids |
|---|---|---|---|
| TBAP 60 mM/MeOH (9:1) | 0.7–0.8 | 0.1–0.3 | 0.1–0.2 |
| Citrate buffer pH 4 | 0–0.1 | 0.7–1 | 0.1–0.2 |
| Acetylacetone/acetone (1:1) | 0–0.1 | 0.7–0.8 | 0–0.1/0.5–0.9 |
TBAP: tetrabutylammonium phosphate.
Radio-TLC analytical conditions for [177Lu]Lu-DOTAZOL tended to be much more straightforward and relied on iTLC plates with the citrate buffer 0.1 M pH 4 [2][15], although a three-plate protocol similar to the one for [68Ga]Ga-DOTAZOL was proposed [14]. Overall, in view of the difficulty of discriminating by TLC between two highly hydrophilic chemical entities such as free 68Ga3+/177Lu3+ and radiolabeled bisphosphonates, particular attention should be paid to the rigorous validation of the RCP control conditions for each of these different radiopharmaceuticals. In addition, special consideration should be given to the redaction of the experimental protocols provided in scientific publications to ensure maximum reproducibility of such analytical processes.
The HPLC methods for the RCP determination of radiolabeled DOTA-bisphosphonates are considered difficult to implement [6]. For some derivatives, the use of an anion-exchange stationary phase is preferred in order to properly discriminate free radiometal from radiolabeled bisphosphonate [10][11]. Nevertheless, in order to take advantage of the reverse-phase radio-HPLC configuration usually employed for radiopharmaceutical quality controls, several protocols relying on reverse-phase columns were proposed. The original HPLC analysis method of [68Ga]Ga-DOTAZOL used a water/acetonitrile gradient and required sample incubation in 0.25 M desferoxamine for 1 min to complex free 68Ga3+ and discriminate it from the radiolabeled bisphosphonate [2]. However, these conditions made [68Ga]Ga-DOTAZOL pass through the column without retention and were therefore unsuitable for the formal identification of the radiolabeling product. An alternative method using an isocratic mixture of tetrabutylammonium phosphate 59 mM and methanol (9:1) as the mobile phase allowed the slight retention of the [68Ga]Ga-DOTAZOL complex on the reverse stationary phase [8]. This method was also used for quality control of the 68Ga-labeled DOTAZOL evaluated in humans [14]. Finally, an HPLC analysis method for [177Lu]Lu-DOTAZOL that is transposable to other phosphonate-containing radiopharmaceuticals was developed by Eryilmaz et al., based on a disodium hydrogen phosphate mobile phase adjusted at pH 1.5–1.8 with orthophosphoric acid and containing N,N-dimethyltetradecylamine [20]. This latter reagent can form ion pairs with phosphonate groups and increase the retention of bisphosphonates via the lipophilic moiety of the alkylamine. In summary, similar to radio-TLC methods, particular attention should be given to the validation of a reliable and efficient radio-HPLC method for the RCP determination of radiolabeled bisphosphonates to guarantee the accurate analysis of the radiopharmaceutical preparation and to avoid either false positive or false negative results.
5. Conclusion
Second-generation bifunctional bisphosphonates such as DOTAZOL offer promising prospects for the diagnosis and treatment - at present, as a last-line, palliative approach - of skeletal lesions in a variety of cancer diseases. Functionalized by a DOTA chelating group with versatile features, DOTAZOL even offers compatibility with the theranostic triplet 68Ga/177Lu/225Ac, similarly to somatostatin analogs [21].
References
- Ballinger, J.R. 68Ga-DOTA-Zoledronate. In PET Radiopharmaceuticals; Springer International Publishing: Cham, Switzerland, 2022; pp. 62–63. ISBN 978-3-031-10270-7.
- Meckel, M.; Bergmann, R.; Miederer, M.; Roesch, F. Bone Targeting Compounds for Radiotherapy and Imaging: *Me(III)-DOTA Conjugates of Bisphosphonic Acid, Pamidronic Acid and Zoledronic Acid. EJNMMI Radiopharm. Chem. 2017, 1, 14.
- Grun, A.; Kovacs, R.; Nagy, D.I.; Garadnay, S.; Greiner, I.; Keglevich, G. Efficient Synthesis of Benzidronate Applying of Phosphorus Trichloride and Phosphorous Acid. Lett. Drug. Des. Discov. 2015, 12, 78–84.
- Ogawa, K.; Kawashima, H.; Shiba, K.; Washiyama, K.; Yoshimoto, M.; Kiyono, Y.; Ueda, M.; Mori, H.; Saji, H. Development of DOTA-Conjugated Bisphosphonate for Treatment of Painful Bone Metastases. Nucl. Med. Biol. 2009, 36, 129–135.
- Grus, T.; Lahnif, H.; Klasen, B.; Moon, E.-S.; Greifenstein, L.; Roesch, F. Squaric Acid-Based Radiopharmaceuticals for Tumor Imaging and Therapy. Bioconjug. Chem. 2021, 32, 1223–1231.
- Greifenstein, L.; Engelbogen, N.; Máthé, D.; Grus, T.; Rösch, F.; Bergmann, R. Squaric Acid Bisphposphonates for Theranostics of Bone Metastasis—The Easy DOTA-Zoledronate. Front. Nucl. Med. 2022, 2, 870910.
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Preliminary Results of Biodistribution and Dosimetric Analysis of Ga-DOTAZOL: A New Zoledronate-Based Bisphosphonate for PET/CT Diagnosis of Bone Diseases. Ann. Nucl. Med. 2019, 33, 404–413.
- Meisenheimer, M.; Kürpig, S.; Essler, M.; Eppard, E. DOTA-ZOL: A Promising Tool in Diagnosis and Palliative Therapy of Bone Metastasis-Challenges and Critical Points in Implementation into Clinical Routine. Molecules 2020, 25, 2988.
- Pillai, S.A.; Chobisa, D.; Urimi, D.; Ravindra, N. Pharmaceutical Glass Interactions: A Review of Possibilities. J. Pharm. Sci. 2016, 8, 103–111.
- Fellner, M.; Biesalski, B.; Bausbacher, N.; Kubícek, V.; Hermann, P.; Rösch, F.; Thews, O. 68Ga-BPAMD: PET-Imaging of Bone Metastases with a Generator Based Positron Emitter. Nucl. Med. Biol. 2012, 39, 993–999.
- Meckel, M.; Fellner, M.; Thieme, N.; Bergmann, R.; Kubicek, V.; Rösch, F. In Vivo Comparison of DOTA Based 68Ga-Labelled Bisphosphonates for Bone Imaging in Non-Tumour Models. Nucl. Med. Biol. 2013, 40, 823–830.
- Eppard, E.; Meisenheimer, M.; Fuente, A.D.L.; Kurpig, S.; Essler, M.; Roesch, F. Radiolabelling of DOTAMZOL with 68Ga and 44Sc for Clinical Application. EJEA 2016, 47, OC34.
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. (Russ) Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Chem. Rev. 2015, 115, 2934–2974.
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Biodistribution and Post-Therapy Dosimetric Analysis of Lu-DOTAZOL in Patients with Osteoblastic Metastases: First Results. EJNMMI Res. 2019, 9, 102.
- Yadav, M.P.; Ballal, S.; Meckel, M.; Roesch, F.; Bal, C. Lu-DOTA-ZOL Bone Pain Palliation in Patients with Skeletal Metastases from Various Cancers: Efficacy and Safety Results. EJNMMI Res. 2020, 10, 130.
- Kreppel, B.; Gaertner, F.C.; Ahmadzadehfar, H.; Khawar, A.; Roesch, F.; Kürpig, S.; Meisenheimer, M.; Essler, M.; Bundschuh, R.A. Lu-DOTA-Zoledronate Therapy—First Application in a Patient with Primary Osseous Metastatic Bronchial Carcinoma. Nuklearmedizin 2020, 59, 281–283.
- Ndlovu, H.; Lawal, I.O.; Popoola, G.O.; Brits, B.; Mokoala, K.M.G.; Maserumule, L.C.; Hlongwa, K.N.; Mahapane, J.; Davis, C.; Sathekge, M.M. Ga-NODAGAZOL Uptake in Atherosclerotic Plaques Correlates with the Cardiovascular Risk Profile of Patients. Ann. Nucl. Med. 2022, 36, 684–692.
- Clarke, E.T.; Martell, A.E. Stabilities of Trivalent Metal Ion Complexes of the Tetraacetate Derivatives of 12-, 13- and 14-Membered Tetraazamacrocycles. Inorganica Chim. Acta. 1991, 190, 37–46.
- Kubíček, V.; Havlíčková, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, É.; Lukeš, I. Gallium(III) Complexes of DOTA and DOTA−Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969.
- Eryilmaz, K.; Bakar, H.E.; Kilbas, B. Novel Developed HPLC Analyses of Ga/Lu-EDTMP and Ga/Lu-DOTA-Zoledronate. J. Label Comp. Radiopharm. 2022, 65, 178–186.
- Rubira, L.; Deshayes, E.; Santoro, L.; Kotzki, P.O.; Fersing, C. 225Ac-Labeled Somatostatin Analogs in the Management of Neuroendocrine Tumors: From Radiochemistry to Clinic. Pharmaceutics 2023, 15, 1051.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
873
Revisions:
3 times
(View History)
Update Date:
05 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




