Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Nowińska | -- | 2773 | 2023-12-30 06:59:54 | | | |
| 2 | Mona Zou | Meta information modification | 2773 | 2024-01-03 09:21:13 | | | | |
| 3 | Mona Zou | Meta information modification | 2773 | 2024-01-03 09:25:08 | | | | |
| 4 | Mona Zou | Meta information modification | 2773 | 2024-01-03 09:25:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nowińska, K.; Adamczyk, Z. Zn-Pb Metallurgical Slags as Source of Metal Recovery. Encyclopedia. Available online: https://encyclopedia.pub/entry/53283 (accessed on 07 February 2026).
Nowińska K, Adamczyk Z. Zn-Pb Metallurgical Slags as Source of Metal Recovery. Encyclopedia. Available at: https://encyclopedia.pub/entry/53283. Accessed February 07, 2026.
Nowińska, Katarzyna, Zdzisław Adamczyk. "Zn-Pb Metallurgical Slags as Source of Metal Recovery" Encyclopedia, https://encyclopedia.pub/entry/53283 (accessed February 07, 2026).
Nowińska, K., & Adamczyk, Z. (2023, December 30). Zn-Pb Metallurgical Slags as Source of Metal Recovery. In Encyclopedia. https://encyclopedia.pub/entry/53283
Nowińska, Katarzyna and Zdzisław Adamczyk. "Zn-Pb Metallurgical Slags as Source of Metal Recovery." Encyclopedia. Web. 30 December, 2023.
Copy Citation
The mineralogical and chemical characteristics of zinc and lead smelting slags are presented, with particular reference to the slags formed during the simultaneous production of Zn and Pb by the Imperial Smelting Process. These slags, because of the presence of many metals in their composition, mainly in the form of crystalline phases, are a valuable source for their extraction. Slags from Zn-Pb metallurgy are processed on an industrial scale using pyrometallurgical and hydrometallurgical methods, alongside which a number of experiments conducted to recover metals as efficiently as possible, including bioleaching experiments.
metallurgy
zinc
lead
slag
metal recovery
1. Introduction
Metallurgical slags resulting from zinc and lead production processes are characterised by a diversity of technical parameters, chemical and mineral composition, which in turn depend on the type of feedstock used, i.e., zinc–lead concentrates (type of bed), additives used in the processing (e.g., fluxes), the technological process applied and its course.
Currently, two methods are used to obtain zinc and lead, the pyrometallurgical method and the hydrometallurgical method.
2. Processes for the Recovery of Metals from Zn-Pb Metallurgical Slags
Metallurgical slags that contain metals at concentrations of several per cent can serve as a source of metals. The mineral and chemical composition of metallurgical slags determines their processing method. Due to the variety of forms of metal occurrence in slags, it is extremely difficult to identify the optimal technological process for their processing. Metals from slags (so-called secondary metals) generated in the pyrometallurgical process to obtain Zn-Pb are typically recovered using pyrometallurgical and hydrometallurgical methods.
2.1. Pyrometallurgical Methods
Pyrometallurgical processes, sometimes referred to as thermal metallurgy, are based on the processing of slags at high temperatures in various furnace types, including shaft, rotary, electric, and muffle furnaces [1][2][3][4].
2.1.1. Fuming Process
One widely used method of pyrometallurgical processing of metallurgical waste is fuming, which involves recovering zinc and lead from liquid slag blown with air and coal dust or natural gas to provide a reducing atmosphere. The metals contained in the slag are reduced and evaporated and then re-oxidised [3][4][5][6][7][8].
This process follows these summary chemical equations [3][4]:
(ZnO)slag + CO = Zn(g) + CO2
(Cd)slag + CO = Cd(g) + CO2
(PbO)slag + CO = Pb(g) + CO2
(Fe2O3)slag + CO = 2(FeO)slag + CO2
C + CO2 = 2CO
These reactions take place at a temperature of around 1500 °C. Fuming is usually carried out in cyclone furnaces or converters.
Spent gases containing a mixture of metal oxides are cooled, and the dust is retained in bag filters. The resulting dust, with a content of 60–75% Zn and 15–25% Pb, is then processed either pyrometallurgically (ISA furnaces) or hydrometallurgically (electrolysis). The waste material of the process is slag with a Zn content of 1.5–2.5% and a Pb content of ~0.2%, which is then granulated or cast into slabs used for the manufacture of construction aggregates [3][4].
2.1.2. Isasmelt Process
The second most common pyrometallurgical method involves remelting lead metallurgical slags in Isasmelt and Kaldo furnaces. These furnaces are used to process both primary and secondary raw materials, such as zinc electrolysis slurries, zinc-bearing slags, EAF dust, and various metallurgical waste types. Fuel and process gases are fed into the furnace via a lance directly below the surface of the liquid slag, which provides highly turbulent conditions favourable for the mass and heat transfer processes.
The design of the Isasmelt furnace and the use of a steel lance allow the melting, oxidation and reduction processes to be performed. The remelting process is carried out at a temperature of 1150–1250 °C [3][4][9] (Figure 1).

Figure 1. Scheme of Isasmelt technology [10].
To increase the efficiency, this operation is split between two furnaces. In the first furnace, a continuous melting process is performed, with oxidation conducted by air injected through a lance. The lead obtained in the process is tapped out of the furnace via a siphon, while the lead-bearing slag is transferred intermittently to the second furnace, in which the lead is reduced with coal, and, after the lead is drained, slag fuming is performed. The end product of the process is dust containing 46–58% Zn, 18–30% Pb, and 0.1–2.7% Cd in addition to waste slag [10][11][12][13][14][15].
2.1.3. Kaldo Process
Kaldo furnaces do not perform charge sintering as a separate step in the process. Instead, the secondary materials together with lead sulphide concentrate (dried to a moisture content of <1% and a particle size of <2 mm) are fed directly into the furnace and then melted at 1400 °C and oxidised. The Kaldo furnace is a tilting rotary vertical converter equipped with a system of three concentric lances: the inner lance is used to deliver the charge, the middle lance delivers the fuel, and the outer lance delivers air and oxygen [3][4][9].
2.1.4. Electric Furnace Process
Another metal recovery approach, particularly to copper recovery from the slags of nonferrous metallurgy, is the traditional method of reducing liquid slags in an electric furnace using reducing and sulphurising agents such as coal, carbide, pyrite, and pyrrhotite in addition to reducing gases. The recovery rates of copper and other metals using this method are below 90%, and the disadvantages of this method include its significant energy consumption, long process duration, and the need for reducing agents [16].
2.1.5. Thermal Electrolysis
Another method of copper recovery from Zn-Pb metallurgical slags involves subjecting the molten slag to thermal electrolysis, which is carried out collectively (i.e., in the same furnace) or selectively in several units, where metallic alloys of different compositions are obtained by applying different current–voltage conditions [3][4][17]. Copper recovery in this approach is carried out by fitting graphite electrodes that supply a direct current. The flow of current through the liquid slag results in electrolytic separation of the metals dissolved within it, in addition to the separation of metallic precipitates by electrocapillary movement in the electric field. The physical and chemical properties of the slag are adjusted, depending on its composition, by introducing suitable additives, such as calcium fluoride, sodium chloride, or sodium carbonate [3][4][17]. This process yields Cu (>90%) and other accompanying metals, e.g., Pb, Zn, and Ni, in the form of an alloy that can be further processed [3][4][17].
2.1.6. Black Sea Copper Works Process
An innovative process for recovering copper from slag has been developed at the Black Sea Copper Works (Turkey), in which the slag is cooled in air for 24 h and then crushed and ground until 80% is a fraction finer than 0.1 mm; this fraction is subsequently floated to produce a flotation concentrate for remelting.
Various other methods have been developed for metal recovery from slags; however, most of them have only been tested on a laboratory scale. An example of an attempt to recover metals from Zn-Pb metallurgical slags at a laboratory scale involves processing a refining slag in a resistance pit furnace at 1250 °C (Figure 2) with a pre-oxidation roasting step conducted by blowing 50 dm3/h of air through the slag at 800 °C to allow the following reactions to proceed:
ZnS + 1.5O2 → ZnO + SO2
PbS + 1.5O2 → PbO + SO2
It is mainly these reactions that take place in the workspace of the furnace:
2ZnO + C → Zng + CO2
ZnO + CO → Zng + CO2
2PbO + C → Pb + CO2
PbO + CO → Pb + CO2
CaCO3 → CaO + CO2
2Zng + O2 → 2ZnO
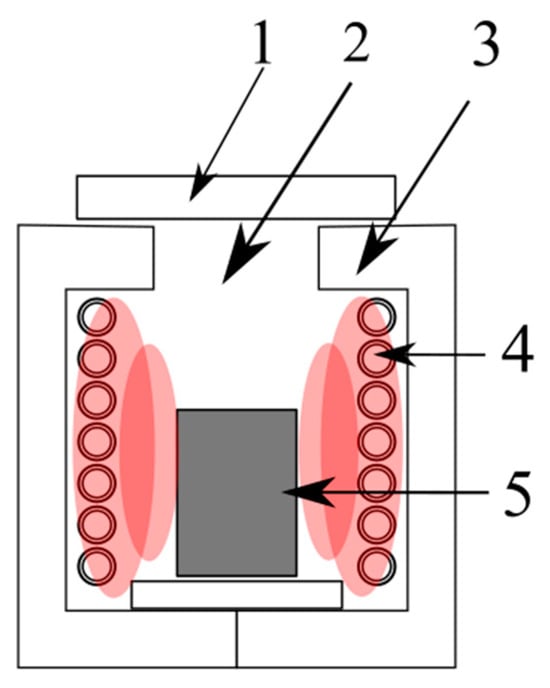
Figure 2. Scheme of the soaking furnace: (1) furnace cover, (2) heating chamber, (3) furnace casing, (4) heating elements made of Kanthal wire, (5) crucible [18].
In this process, the furnace charge consists of refining slags, coal (reducing agent), and limestone CaCO3 (flux). During the processing of the slags, sample weight loss was observed, which primarily occurred due to zinc evaporation caused by the reduction process. This zinc then is oxidised and accumulates on the filter in the form of ZnO (Figure 3a) [18]. In addition to the zinc oxidation reaction, the observed change in the sample weight in this method may result from various other factors, including the reduction of lead oxide, the combustion of coal and sulphur, or the addition of limestone or iron. The lead yield in this process is higher than 75%. It is difficult to determine the efficiency of zinc recovery from slags because during their processing, Zn is oxidised, and the resulting ZnO accumulates not only on the filter but also in the furnace chamber (Figure 3b) [18]. This ZnO would require further leaching with a sulphuric (VI) acid solution to obtain Zn.

Figure 3. Zinc oxide depositing (a) on the filter and (b) in the furnace chamber [18].
2.2. Hydrometallurgical Methods
2.2.1. Chemical Leaching
Hydrometallurgical methods of metal recovery from waste include leaching, solution purification, metal separation, and isolation of pure metals at temperatures below 100 °C.
The leaching process, which forms the basis of hydrometallurgical processes, can be performed using acid solutions (such as sulphuric or hydrochloric acid), ammonia solutions, or alkaline hydroxide solutions. The choice of the leaching medium depends on the chemical form in which the metal is present in the waste [3][4][19][20][21][22][23][24]. Most studies to date have investigated the leaching of Zn-Pb metallurgical waste using sulphuric (VI) acid solutions, with the choice of leaching conditions dependent, among other factors, on the mineral and chemical composition of the metallurgical waste, as the solubility of its constituent phases in sulphuric (VI) acid solutions varies significantly [3][4][19][20][21][22][23][24]. Depending on the form of zinc present, the zinc leaching process occurs according to the equations exemplified below [3][4]:
ZnO + H2SO4 = ZnSO4 + H2O
ZnFe2O4 + 4H2SO4 = ZnSO4 + Fe2(SO4)3 + 4H2O
Fe3O4 + 4H2SO4 = FeSO4 + Fe2(SO4)3 + 4H2O
Ca[Zn(OH)3]2 · H2O + 3H2SO4 = CaSO4 + 2ZnSO4 + 8H2O
ZnSiO3 + H2SO4 = ZnSO4 + SiO2 + H2O
The rates of the above reactions are determined by several factors, the most important of which are the mineral composition, material grain size, and sulphuric (VI) acid concentration. Zinc silicate (ZnSiO3) solubilises at a much lower rate than the oxide form (ZnO), while the efficiency of the leaching process following Reactions (17) and (19) increases significantly with increasing temperature. Other slag components, i.e., Cu, Ni, Cd, Pb, etc., react similarly to sulphuric (VI) acid.
- –
-
Those passing into solution during the leaching process, including Cu, Cd, Fe, Mg, and Ni;
- –
-
Those forming insoluble salts, including PbSO4, CaSO4∙2H2O, BaSO4.
The next step in the zinc recovery process is the purification of the zinc sulphate (VI) solution, which is then electrolysed. In practice, chemical purification of the electrolyte may be carried out continuously or in several stages. The average composition of the crude ZnSO4 solution is 135 mg/dm3 Zn, 470 mg/dm3 Cu, 168 mg/dm3 Cd, 18 mg/dm3 Fe, 0.9 mg/dm3 As, 0.11 mg/dm3 Sb, and 12.7 mg/dm3 Co [3].
Purification of the zinc sulphate solution is conducted using various methods, which are selected according to the type of contaminant to be removed. Some impurities (As, Sb, Fe) are removed during neutral leaching, as the salts of these elements hydrolyse under these conditions and are adsorbed onto the Fe(OH)3 surface. Hydrolytic purification involves selecting suitable process conditions (solution pH, temperature, etc.) to ensure that the usually sparingly soluble hydroxides of metals that contaminate the solution are formed.
Depending on the conditions of the hydrolytic electrolyte purification process, iron precipitates from the solution in the form of goethite (FeOOH), hematite (Fe2O3), or jarosite (MFe3(SO4)2(OH)6, where M = NH4+, K+, Na+, or 0.5Pb2+). The preferred precipitate type in hydrometallurgical processing is jarosite. The obtained jarosite is characterised by highly favourable filtration properties, which significantly reduce operating costs for the process. In addition, during jarosite precipitation, it is also possible to remove other impurities from the electrolyte, e.g., arsenic [3][4]. To remove copper, cadmium, nickel, and cobalt from the zinc sulphate (VI) solution, the cementation method (internal electrolysis) is applied, which involves precipitating the more noble metal from the electrolyte with zinc dust.
Ammonia solutions are another group of agents commonly used for metal leaching in hydrometallurgical processes. A Spanish company, CENIM, and a Portuguese company, LENTI, developed a CENIM–LENTI technology to process sulphide concentrates and zinc-bearing waste using ammonium salts. The main waste-leaching reagent in this process is ammonium chloride. Zinc in the leach residue is present as ZnO-Fe2O3, which is then extracted with a solution of di-2-ethylhexylphosphoric acid (D2EHPA) and isolated using electrolysis [3][4][9][25][26].
PbCl2 (s) + 2Cl− (aq) → PbCl42− (aq)
A study using acetic acid as the leaching agent for Pb from slags was performed by Forte et al. [27]. In their study, the leaching process involved dissolving metallic Pb in concentrated acetic acid and then precipitating PbSO4 by adding H2SO4 to the solution. However, the major disadvantage of this method is that only lead present in metallic form is recovered.
Kim et al. [28] proposed two methods for the selective recovery of lead, copper, nickel, and zinc from lead slag. The first metal recovery method was based on a two-stage leaching process, in which the first stage involved leaching the metals with Fe(III) + HNO3, and the second stage involved roasting the residue and leaching it with water (Figure 4 and Figure 5).
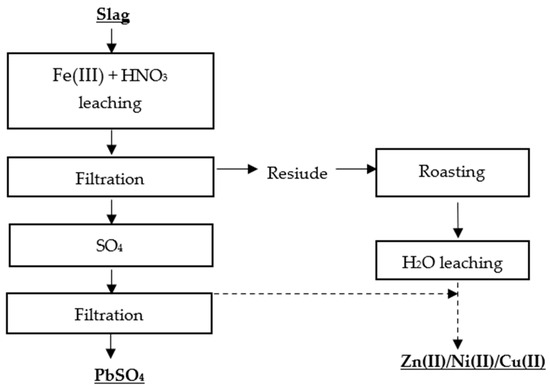
Figure 4. Flowsheet of two-stage leaching of Pb, Zn, Ni, Cu [28].
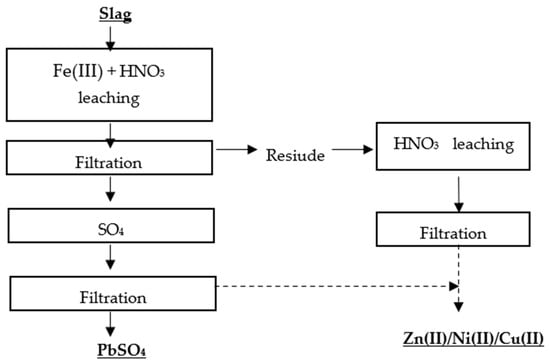
Figure 5. Flowsheet of single-stage leaching of Pb, Zn, Ni, Cu without roasting [28].
Attempts have also been made to process hydrometallurgical waste using sodium hydroxide. The main problem with this method is the presence of sparingly soluble zinc compounds in the waste, e.g., ferrites.
Leaching of zinc from metallurgical waste has been conducted using NaOH solution under various conditions. This process was most commonly performed at temperatures of 25 and 90 °C, using NaOH solutions of 2–6 mol/dm3 concentration for 4 h (traditional method). Pressure leaching in an autoclave using a 6 mol/dm3 NaOH solution at 120–200 °C for 4 h was also investigated, as was the leaching process after pretreating the waste in a microwave oven (1 kW, 2.45 GHz). The best results were obtained with the traditional method, in which the zinc recovery reached 74%. In addition to iron oxides, the residual solid phase after leaching contained insoluble zinc ferrites [29][30][31].
2.2.2. Bioleaching
Another group of methods used to recover metals from metallurgical slags involves bioleaching, a process in which microorganisms are used to convert solid, insoluble metals and their compounds to water-soluble forms [32][33]. Most microorganisms capable of biohydrometallurgical processes belong to the group of chemolithotrophs, which use carbon dioxide as their source of cellular carbon. For their energy source, they can use reduced sulphur and iron compounds or oxidation reactions of elemental sulphur, sulphides, or thiosulphates.
The microorganisms involved in leaching include not only bacteria (genera: Acidithiobacillus, Thiobacillus) but also fungi (including the genera Penicillium, Aspergillus, Fusarium, Alternaria, and Candida). In practice, biohydrometallurgical processes are carried out using mixtures of microbial populations that occur naturally in iron- and sulphur-rich environments; bacterial monocultures are not used. The bioleaching process follows two main mechanisms: indirect and direct [34][35][36][37].
The indirect mechanism involves chemical and bacterial oxidation, with microbial oxidation of Fe2+ ions derived from minerals used to form Fe3+ ions, which then participate in the leaching process. Microorganisms are the source of the leaching agent, which chemically oxidises the sulphide minerals. In this model, no physical contact occurs between the bacterial cell and the mineral surface [34][35][36][37].
MeS + Fe2(SO4)3MeSO4 + FeSO4 + S
S + 3O2 + 2H2O → 2H2SO4
In the direct mechanism, the electrons obtained in the bacterial oxidation process are sourced directly from the reduced minerals. In this model, there is physical contact between the bacterial cell and the mineral surface. These reactions are most often associated with the oxidation of pyrite.
4FeS2 + 15O2 + 2H2O → 2Fe2(SO4)3 + 2H2SO4
MeS + 2O2 → MeSO4
Microorganism selection is a key factor when performing bioleaching of metallurgical slags. The indigenous microorganisms present at the site where the slags are deposited have the highest contaminant removal efficiency by adapting to conditions with higher metal content. For this reason, Acidithiobacillus bacteria are the most commonly used in studies of bioleaching of components from metallurgical slags because environmental bacteria from these genera have been widely reported in slag deposition sites [34][35][36][37].
Laboratory-scale bioleaching experiments include batch leaching, semi-open flow-through leaching, and continuously stirred tank reactors [36][37][38][39][40][41]. On a laboratory scale, a method has been developed to process slags from zinc and lead production from former Yugoslavian plants by gravity enrichment of the slags, resulting in a concentrate with a 94.5 wt% Pb content. Waste from the enrichment process containing 38.7 wt% Fe, 32.3 wt% SiO2, 5.8 wt% Zn, and 3.0 wt% Pb is subjected to bacterial leaching using autotrophic thionic bacteria to recover zinc [3][4][17].
Bioleaching can be used not only to extract valuable metals but also to remove toxic elements by disrupting the amorphous structure of slags. The efficiency of bioleaching depends on various factors, including the pH, leaching time and temperature, and slag structure [39][40][41]. However, despite its high efficiency, bioleaching to date has only been performed on a laboratory scale. Due to the long processing times, low yields, and problems with separating metals from the solution associated with this approach, it has not yet been applied at an industrial scale.
References
- Bernasowski, M.; Klimczyk, A.; Stachura, R. Overview of Zinc Production in Imperial Smelting Process. In Proceedings of the Conference Iron and Steelmaking, Horní Bečva, Czech Republic, 4–6 October 2017.
- Potysz, A.; Van Hullebusch, E.D. Secondary metal recovery from Slags. In Metallurgical Slags, 1st ed.; Piatak, N., Ettler, V., Eds.; Royal Society of Chemistry: London, UK, 2021.
- Jarosiński, A. Innowacyjne i Proekologiczne Metody Przeróbki Materiałów Cynkonośnych; Wydawnictwo IGSMiE PAN: Kraków, Poland, 2012.
- Ulewicz, M. Procesy Odzysku i Recyklingu Metali Nieżelaznych i Stali; Wydawnictwo Politechniki Częstochowskiej: Częstochowa, Poland, 2015.
- Reddy, R.G.; Prabhu, V.L.; Mantha, D. Zinc fuming from lead blast furnace slag. High Temp. Mater. Process. 2002, 21, 377–386.
- Jak, E.; Hayes, P. Phase equilibria and thermodynamics of zinc fuming slags. Can. Metall. Q. 2002, 41, 163–174.
- Verscheure, K.; Van Camp, M.; Blanpain, B.; Wollants, P.; Hayes, P.; Jak, E. Continuous fuming of zinc bearing residues: Part I. Model Development. Metall. Mater. Trans. B 2007, 38B, 13–20.
- Verscheure, K.; Van Camp, M.; Blanpain, B.; Wollants, P.; Hayes, P.; Jak, E. Continuous fuming of zinc bearing residues: Part II. The submerged plasma zinc fuming process. Metall. Mater. Trans. 2007, 38, 21–33.
- Cybulski, A.; Prajsnar, R.; Kulawik, S.; Michalski, R.; Basiura, B. Rozwiązania techniczno-technologiczne w pirometalurgii cynku i ołowiu na Świecie. Rudy Met. 2016, 63, 19–27.
- Bakker, M.L.; Alvear, G.R.F.; Kreuh, M. IsasmeltTM—Making a splash for nickiel. Miner. Eng. 2011, 24, 610–619.
- Arthur, P.; Edwards, J.; Technology, X. IsasmeltTM—A Quiet Revolution. In Proceedings of the European Metallurgical Conference, Hannover, Germany, 16–19 September 2023.
- Errington, W.J.; Fewings, J.H.; Keran, V.P.; Denholm, W.T. The Isasmelt lead smelting process. Trans. Inst. Min. Metall. Sect. C 1987, 96, 1–6.
- Hogg, B.; Prince, M.; Letchford, M.; Burrows, A.; Tokzhigitov, T.; Azekenov, T. Successful Development and Optimisation of Lead ISASMELT™ Furnace Slag Tapping System at Kazzinc Ltd. In Furnace Tapping, 1st ed.; Steenkamp, J.D., Gregurek, D., Reynolds, Q.G., Alvear Flores, G., Joubert, H., Mackey, P.J., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2022; pp. 245–249.
- Shanmuganathan, P.; Lakshmipathiraj, P.; Srikanth, S.; Nachiappan, A.L.; Sumathy, A. Toxicity characterization and long-term stability studies on copper slag from the ISASMELT process. Resour. Conserv. Recycl. 2008, 52, 601–611.
- Zhao, B.; Errington, B.; Yang, G.; Wang, J.; Dong, Y.; Jak, E.; Hayes, P.C. Characterisation of ISASMELT™ slag and lead blast furnace sinters. In Proceedings of the International Symposium on Lead and Zinc Processing, Kyoto, Japan, 17–19 October 2005.
- Derin, B.; Şahin, F.C.; Yűcel, O. The electrical characteristics of copper slags in a 270 kVA DC arc furnace. In Proceedings of the 3rd BMC Conference, Ohrid, North Macedonia, 2–3 July 2003.
- Lia, Y.; Weid, C.; Liud, C.; Jiangd, J.; Wangd, F. Sulfidation roasting of low grade lead–zinc oxide ore with elemental sulfur. Miner. Eng. 2010, 23, 563–566.
- Nowińska, K. Formy Występowania Metali w Żużlach z Hutnictwa Cynku i Ołowiu w Aspekcie Środowiskowym i Możliwości Ich Odzysku; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2022.
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recycl. 2001, 33, 1–22.
- Abdel Basin, S.M.; Rabah, M.A. Hydrometallurgical recovery of metal values from brass melting slag. Hydrometallurgy 1999, 53, 31–44.
- RaO, S.; Liu, Z.; Wang, D.; Cao, H.; Zhu, W.; Zhang, K.; Tao, J. Hydrometallurgical process for recovery of Zn, Pb, Ga and Ge from Zn refinery residues. Trans. Nonferrous Met. Soc. China 2021, 31, 555–564.
- Kul, M.; Topkaya, Y. Recovery of germanium and other valuable metals from zinc plant residue. Hydrometallurgy 2008, 92, 87–94.
- Kurama, H.; Göktepe, F. Recovery of zinc from waste material using hydro metallurgical processes. Environ. Prog. 2004, 22, 161–166.
- Xie, H.; Zhang, L.; Li, H.; Koppala, S.; Yin, S.; Li, S.; Yang, K.; Zhu, F. Efficient recycling of Pb from zinc leaching residues by using the hydrometallurgical method. Mater. Res. Express 2019, 6, 075505.
- Limpa, J.L.; Figueiredo, J.M.; Amer, S.; Luis, A. The CENIM-LNETI process: A new process for the hydrometallurgical treatment of complex sulphides in ammonium chloride solutions. Hydrometallurgy 1997, 28, 149–161.
- Velásquez-Yévenes, L.; Ram, R. The aqueous chemistry of the copper-ammonia system and its implications for the sustainable recovery of copper. Clean. Eng. Technol. 2022, 9, 100515.
- Forte, F.; Horckmans, L.; Broos, K.; Kim, E.; Kukurugya, F.; Binnemans, K. Closed-loop solvometallurgical process for recovery of lead from iron-rich secondary lead smelter residues. RSC Adv. 2017, 7, 49999–50005.
- Kim, E.; Horckmans, L.; Spooren, J.; Vrancken, K.C.; Quaghebeur, M.; Broos, K. Hydrometallurgy, Selective leaching of Pb, Cu, Ni and Zn from secondary lead smelting residues. Hydrometallurgy 2017, 169, 372–381.
- Li, Y.; Liu, Z.; Li, Q.; Zhao, Z.; Liu, Z.; Zeng, L. Removal of arsenic from Waelz zinc oxide using a mixed NaOH–Na2S leach. Hydrometallurgy 2011, 108, 165–170.
- Piatak, N.M. Environmental Characteristics and Utilization Potential of Metallurgical Slag. In Environmental Geochemistry, 2nd ed.; De Vivo, B., Belkin, H.E., Lima, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018.
- Radzymińska-Lenarcik, E.; Sulewski, M.; Urbaniak, W. Wydobywanie metali z odpadów hydrometalurgicznej przeróbki rud cynkowych. Przemysł Chem. 2017, 96, 1000–1004.
- Mikoda, B.; Kucha, H.; Potysz, A.; Kmiecik, E. Metallurgical slags from Cu production and Pb recovery in Poland—Their environmental stability and resource potential. Appl. Geochem. 2018, 98, 459–472.
- Mikoda, B.; Kucha, H.; Potysz, A.; Kmiecik, E. Bacterial leaching of critical metal values from Polish copper metallurgical slags using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445.
- Pandey, S.C.; Pande, V.; Sati, D.; Samant, M. (Eds.) Bioremediation of heavy metals by soil-dwelling microbes: An environment survival approach. In Advanced Microbial Techniques in Agriculture, Environment, and Health Management, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2023.
- Tezyapar Kara, I.; Kremser, K.; Wagland, S.T.; Coulon, F. Bioleaching metal-bearing wastes and by-products for resource recovery: A review. Environ. Chem. Lett. 2023, 21, 3329–3350.
- Klimiuk, E.; Łebkowska, M. Biotechnologia w Ochronie Środowiska; PWN: Warszawa, Poland, 2008.
- Błaszczyk, M.K. Mikroorganizmy w Ochronie Środowiska; PWN: Warszawa, Poland, 2009.
- Tuovinen, O.H.; Särkijärvi, S.; Peuraniemi, E.; Junnikkala, S.; Puhakka, J.A.; Kaksonen, A.H. Acid Leaching of Cu and Zn from a Smelter Slag with a Bacterial Consortium. Adv. Mater. Res. 2015, 1130, 660–663.
- Blažek, V.; Závada, J.; Bouchal, T.; Lébr, J.; Fečko, P. Ługowanie miedzi i cyny z odpadów elektronicznych za pomocą bakterii Acidithiobacillus ferrooxidans. Inżynieria Miner. 2012, 13, 1–7.
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J. Perspectives regarding the use of metallurgical slags as secondary metal resources—A review of bioleaching approaches. J. Environ. Manag. 2018, 219, 138–152.
- Potysz, A.; Pędziwiatr, A.; Hedwig, S.; Lenz, M. Bioleaching and toxicity of metallurgical wastes. J. Environ. Chem. Eng. 2020, 8, 104450.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
4 times
(View History)
Update Date:
03 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




