1. Introduction
Metallurgical slags resulting from zinc and lead production processes are characterised by a diversity of technical parameters, chemical and mineral composition, which in turn depend on the type of feedstock used, i.e., zinc–lead concentrates (type of bed), additives used in the processing (e.g., fluxes), the technological process applied and its course.
Currently, two methods are used to obtain zinc and lead, the pyrometallurgical method and the hydrometallurgical method.
2. Processes for the Recovery of Metals from Zn-Pb Metallurgical Slags
Metallurgical slags that contain metals at concentrations of several per cent can serve as a source of metals. The mineral and chemical composition of metallurgical slags determines their processing method. Due to the variety of forms of metal occurrence in slags, it is extremely difficult to identify the optimal technological process for their processing. Metals from slags (so-called secondary metals) generated in the pyrometallurgical process to obtain Zn-Pb are typically recovered using pyrometallurgical and hydrometallurgical methods.
2.1. Pyrometallurgical Methods
Pyrometallurgical processes, sometimes referred to as thermal metallurgy, are based on the processing of slags at high temperatures in various furnace types, including shaft, rotary, electric, and muffle furnaces
[1][2][3][4].
2.1.1. Fuming Process
One widely used method of pyrometallurgical processing of metallurgical waste is fuming, which involves recovering zinc and lead from liquid slag blown with air and coal dust or natural gas to provide a reducing atmosphere. The metals contained in the slag are reduced and evaporated and then re-oxidised
[3][4][5][6][7][8].
This process follows these summary chemical equations
[3][4]:
These reactions take place at a temperature of around 1500 °C. Fuming is usually carried out in cyclone furnaces or converters.
Spent gases containing a mixture of metal oxides are cooled, and the dust is retained in bag filters. The resulting dust, with a content of 60–75% Zn and 15–25% Pb, is then processed either pyrometallurgically (ISA furnaces) or hydrometallurgically (electrolysis). The waste material of the process is slag with a Zn content of 1.5–2.5% and a Pb content of ~0.2%, which is then granulated or cast into slabs used for the manufacture of construction aggregates
[3][4].
The second most common pyrometallurgical method involves remelting lead metallurgical slags in Isasmelt and Kaldo furnaces. These furnaces are used to process both primary and secondary raw materials, such as zinc electrolysis slurries, zinc-bearing slags, EAF dust, and various metallurgical waste types. Fuel and process gases are fed into the furnace via a lance directly below the surface of the liquid slag, which provides highly turbulent conditions favourable for the mass and heat transfer processes.
The design of the Isasmelt furnace and the use of a steel lance allow the melting, oxidation and reduction processes to be performed. The remelting process is carried out at a temperature of 1150–1250 °C
[3][4][9] (
Figure 1).
Figure 1. Scheme of Isasmelt technology
[10].
To increase the efficiency, this operation is split between two furnaces. In the first furnace, a continuous melting process is performed, with oxidation conducted by air injected through a lance. The lead obtained in the process is tapped out of the furnace via a siphon, while the lead-bearing slag is transferred intermittently to the second furnace, in which the lead is reduced with coal, and, after the lead is drained, slag fuming is performed. The end product of the process is dust containing 46–58% Zn, 18–30% Pb, and 0.1–2.7% Cd in addition to waste slag
[10][11][12][13][14][15].
2.1.3. Kaldo Process
Kaldo furnaces do not perform charge sintering as a separate step in the process. Instead, the secondary materials together with lead sulphide concentrate (dried to a moisture content of <1% and a particle size of <2 mm) are fed directly into the furnace and then melted at 1400 °C and oxidised. The Kaldo furnace is a tilting rotary vertical converter equipped with a system of three concentric lances: the inner lance is used to deliver the charge, the middle lance delivers the fuel, and the outer lance delivers air and oxygen
[3][4][9].
2.1.4. Electric Furnace Process
Another metal recovery approach, particularly to copper recovery from the slags of nonferrous metallurgy, is the traditional method of reducing liquid slags in an electric furnace using reducing and sulphurising agents such as coal, carbide, pyrite, and pyrrhotite in addition to reducing gases. The recovery rates of copper and other metals using this method are below 90%, and the disadvantages of this method include its significant energy consumption, long process duration, and the need for reducing agents
[16].
2.1.5. Thermal Electrolysis
Another method of copper recovery from Zn-Pb metallurgical slags involves subjecting the molten slag to thermal electrolysis, which is carried out collectively (i.e., in the same furnace) or selectively in several units, where metallic alloys of different compositions are obtained by applying different current–voltage conditions
[3][4][17]. Copper recovery in this approach is carried out by fitting graphite electrodes that supply a direct current. The flow of current through the liquid slag results in electrolytic separation of the metals dissolved within it, in addition to the separation of metallic precipitates by electrocapillary movement in the electric field. The physical and chemical properties of the slag are adjusted, depending on its composition, by introducing suitable additives, such as calcium fluoride, sodium chloride, or sodium carbonate
[3][4][17]. This process yields Cu (>90%) and other accompanying metals, e.g., Pb, Zn, and Ni, in the form of an alloy that can be further processed
[3][4][17].
2.1.6. Black Sea Copper Works Process
An innovative process for recovering copper from slag has been developed at the Black Sea Copper Works (Turkey), in which the slag is cooled in air for 24 h and then crushed and ground until 80% is a fraction finer than 0.1 mm; this fraction is subsequently floated to produce a flotation concentrate for remelting.
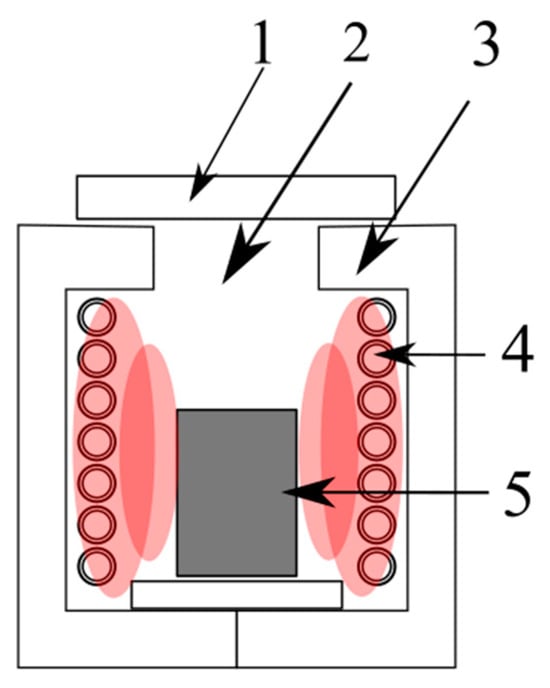
Various other methods have been developed for metal recovery from slags; however, most of them have only been tested on a laboratory scale. An example of an attempt to recover metals from Zn-Pb metallurgical slags at a laboratory scale involves processing a refining slag in a resistance pit furnace at 1250 °C (
Figure 2) with a pre-oxidation roasting step conducted by blowing 50 dm
3/h of air through the slag at 800 °C to allow the following reactions to proceed:
It is mainly these reactions that take place in the workspace of the furnace:
Figure 2. Scheme of the soaking furnace: (1) furnace cover, (2) heating chamber, (3) furnace casing, (4) heating elements made of Kanthal wire, (5) crucible
[18].
In this process, the furnace charge consists of refining slags, coal (reducing agent), and limestone CaCO
3 (flux). During the processing of the slags, sample weight loss was observed, which primarily occurred due to zinc evaporation caused by the reduction process. This zinc then is oxidised and accumulates on the filter in the form of ZnO (
Figure 3a)
[18]. In addition to the zinc oxidation reaction, the observed change in the sample weight in this method may result from various other factors, including the reduction of lead oxide, the combustion of coal and sulphur, or the addition of limestone or iron. The lead yield in this process is higher than 75%. It is difficult to determine the efficiency of zinc recovery from slags because during their processing, Zn is oxidised, and the resulting ZnO accumulates not only on the filter but also in the furnace chamber (
Figure 3b)
[18]. This ZnO would require further leaching with a sulphuric (VI) acid solution to obtain Zn.
Figure 3. Zinc oxide depositing (
a) on the filter and (
b) in the furnace chamber
[18].
2.2. Hydrometallurgical Methods
2.2.1. Chemical Leaching
Hydrometallurgical methods of metal recovery from waste include leaching, solution purification, metal separation, and isolation of pure metals at temperatures below 100 °C.
The leaching process, which forms the basis of hydrometallurgical processes, can be performed using acid solutions (such as sulphuric or hydrochloric acid), ammonia solutions, or alkaline hydroxide solutions. The choice of the leaching medium depends on the chemical form in which the metal is present in the waste
[3][4][19][20][21][22][23][24]. Most studies to date have investigated the leaching of Zn-Pb metallurgical waste using sulphuric (VI) acid solutions, with the choice of leaching conditions dependent, among other factors, on the mineral and chemical composition of the metallurgical waste, as the solubility of its constituent phases in sulphuric (VI) acid solutions varies significantly
[3][4][19][20][21][22][23][24]. Depending on the form of zinc present, the zinc leaching process occurs according to the equations exemplified below
[3][4]:
The rates of the above reactions are determined by several factors, the most important of which are the mineral composition, material grain size, and sulphuric (VI) acid concentration. Zinc silicate (ZnSiO3) solubilises at a much lower rate than the oxide form (ZnO), while the efficiency of the leaching process following Reactions (17) and (19) increases significantly with increasing temperature. Other slag components, i.e., Cu, Ni, Cd, Pb, etc., react similarly to sulphuric (VI) acid.
Minor components present in the zinc-bearing material can be classified into two groups
[3][4]:
- –
-
Those passing into solution during the leaching process, including Cu, Cd, Fe, Mg, and Ni;
- –
-
Those forming insoluble salts, including PbSO4, CaSO4∙2H2O, BaSO4.
The next step in the zinc recovery process is the purification of the zinc sulphate (VI) solution, which is then electrolysed. In practice, chemical purification of the electrolyte may be carried out continuously or in several stages. The average composition of the crude ZnSO
4 solution is 135 mg/dm
3 Zn, 470 mg/dm
3 Cu, 168 mg/dm
3 Cd, 18 mg/dm
3 Fe, 0.9 mg/dm
3 As, 0.11 mg/dm
3 Sb, and 12.7 mg/dm
3 Co
[3].
Purification of the zinc sulphate solution is conducted using various methods, which are selected according to the type of contaminant to be removed. Some impurities (As, Sb, Fe) are removed during neutral leaching, as the salts of these elements hydrolyse under these conditions and are adsorbed onto the Fe(OH)3 surface. Hydrolytic purification involves selecting suitable process conditions (solution pH, temperature, etc.) to ensure that the usually sparingly soluble hydroxides of metals that contaminate the solution are formed.
Depending on the conditions of the hydrolytic electrolyte purification process, iron precipitates from the solution in the form of goethite (FeOOH), hematite (Fe
2O
3), or jarosite (MFe
3(SO
4)
2(OH)
6, where M = NH
4+, K
+, Na
+, or 0.5Pb
2+). The preferred precipitate type in hydrometallurgical processing is jarosite. The obtained jarosite is characterised by highly favourable filtration properties, which significantly reduce operating costs for the process. In addition, during jarosite precipitation, it is also possible to remove other impurities from the electrolyte, e.g., arsenic
[3][4]. To remove copper, cadmium, nickel, and cobalt from the zinc sulphate (VI) solution, the cementation method (internal electrolysis) is applied, which involves precipitating the more noble metal from the electrolyte with zinc dust.
Ammonia solutions are another group of agents commonly used for metal leaching in hydrometallurgical processes. A Spanish company, CENIM, and a Portuguese company, LENTI, developed a CENIM–LENTI technology to process sulphide concentrates and zinc-bearing waste using ammonium salts. The main waste-leaching reagent in this process is ammonium chloride. Zinc in the leach residue is present as ZnO-Fe
2O
3, which is then extracted with a solution of di-2-ethylhexylphosphoric acid (D2EHPA) and isolated using electrolysis
[3][4][9][25][26].
A study using acetic acid as the leaching agent for Pb from slags was performed by Forte et al.
[27]. In their study, the leaching process involved dissolving metallic Pb in concentrated acetic acid and then precipitating PbSO
4 by adding H
2SO
4 to the solution. However, the major disadvantage of this method is that only lead present in metallic form is recovered.
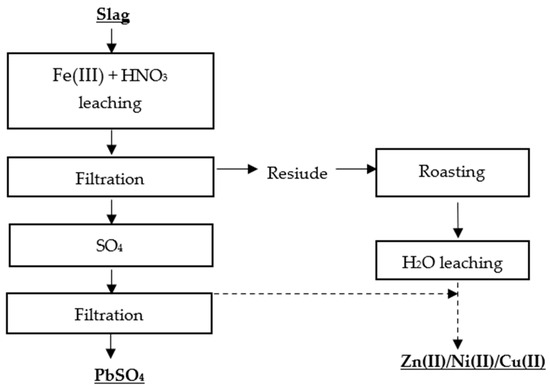
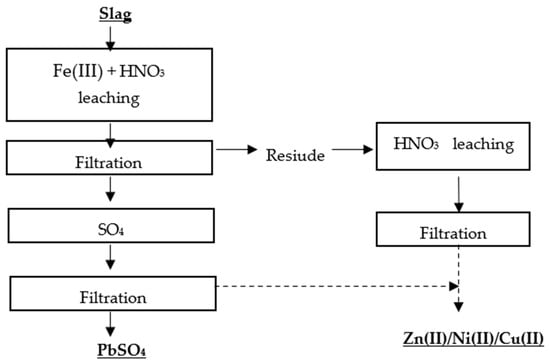
Kim et al.
[28] proposed two methods for the selective recovery of lead, copper, nickel, and zinc from lead slag. The first metal recovery method was based on a two-stage leaching process, in which the first stage involved leaching the metals with Fe(III) + HNO
3, and the second stage involved roasting the residue and leaching it with water (
Figure 4 and
Figure 5).
Figure 4. Flowsheet of two-stage leaching of Pb, Zn, Ni, Cu
[28].
Figure 5. Flowsheet of single-stage leaching of Pb, Zn, Ni, Cu without roasting
[28].
Attempts have also been made to process hydrometallurgical waste using sodium hydroxide. The main problem with this method is the presence of sparingly soluble zinc compounds in the waste, e.g., ferrites.
Leaching of zinc from metallurgical waste has been conducted using NaOH solution under various conditions. This process was most commonly performed at temperatures of 25 and 90 °C, using NaOH solutions of 2–6 mol/dm
3 concentration for 4 h (traditional method). Pressure leaching in an autoclave using a 6 mol/dm
3 NaOH solution at 120–200 °C for 4 h was also investigated, as was the leaching process after pretreating the waste in a microwave oven (1 kW, 2.45 GHz). The best results were obtained with the traditional method, in which the zinc recovery reached 74%. In addition to iron oxides, the residual solid phase after leaching contained insoluble zinc ferrites
[29][30][31].
2.2.2. Bioleaching
Another group of methods used to recover metals from metallurgical slags involves bioleaching, a process in which microorganisms are used to convert solid, insoluble metals and their compounds to water-soluble forms
[32][33]. Most microorganisms capable of biohydrometallurgical processes belong to the group of chemolithotrophs, which use carbon dioxide as their source of cellular carbon. For their energy source, they can use reduced sulphur and iron compounds or oxidation reactions of elemental sulphur, sulphides, or thiosulphates.
The microorganisms involved in leaching include not only bacteria (genera:
Acidithiobacillus,
Thiobacillus) but also fungi (including the genera
Penicillium,
Aspergillus,
Fusarium,
Alternaria, and
Candida). In practice, biohydrometallurgical processes are carried out using mixtures of microbial populations that occur naturally in iron- and sulphur-rich environments; bacterial monocultures are not used. The bioleaching process follows two main mechanisms: indirect and direct
[34][35][36][37].
The indirect mechanism involves chemical and bacterial oxidation, with microbial oxidation of Fe
2+ ions derived from minerals used to form Fe
3+ ions, which then participate in the leaching process. Microorganisms are the source of the leaching agent, which chemically oxidises the sulphide minerals. In this model, no physical contact occurs between the bacterial cell and the mineral surface
[34][35][36][37].
In the direct mechanism, the electrons obtained in the bacterial oxidation process are sourced directly from the reduced minerals. In this model, there is physical contact between the bacterial cell and the mineral surface. These reactions are most often associated with the oxidation of pyrite.
Microorganism selection is a key factor when performing bioleaching of metallurgical slags. The indigenous microorganisms present at the site where the slags are deposited have the highest contaminant removal efficiency by adapting to conditions with higher metal content. For this reason,
Acidithiobacillus bacteria are the most commonly used in studies of bioleaching of components from metallurgical slags because environmental bacteria from these genera have been widely reported in slag deposition sites
[34][35][36][37].
Laboratory-scale bioleaching experiments include batch leaching, semi-open flow-through leaching, and continuously stirred tank reactors
[36][37][38][39][40][41]. On a laboratory scale, a method has been developed to process slags from zinc and lead production from former Yugoslavian plants by gravity enrichment of the slags, resulting in a concentrate with a 94.5 wt% Pb content. Waste from the enrichment process containing 38.7 wt% Fe, 32.3 wt% SiO
2, 5.8 wt% Zn, and 3.0 wt% Pb is subjected to bacterial leaching using autotrophic thionic bacteria to recover zinc
[3][4][17].
Bioleaching can be used not only to extract valuable metals but also to remove toxic elements by disrupting the amorphous structure of slags. The efficiency of bioleaching depends on various factors, including the pH, leaching time and temperature, and slag structure
[39][40][41]. However, despite its high efficiency, bioleaching to date has only been performed on a laboratory scale. Due to the long processing times, low yields, and problems with separating metals from the solution associated with this approach, it has not yet been applied at an industrial scale.
This entry is adapted from the peer-reviewed paper 10.3390/ma16237295