
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daria Molodtsova | -- | 1682 | 2023-12-12 18:32:38 | | | |
| 2 | Peter Tang | Meta information modification | 1682 | 2023-12-13 03:31:31 | | |
Video Upload Options
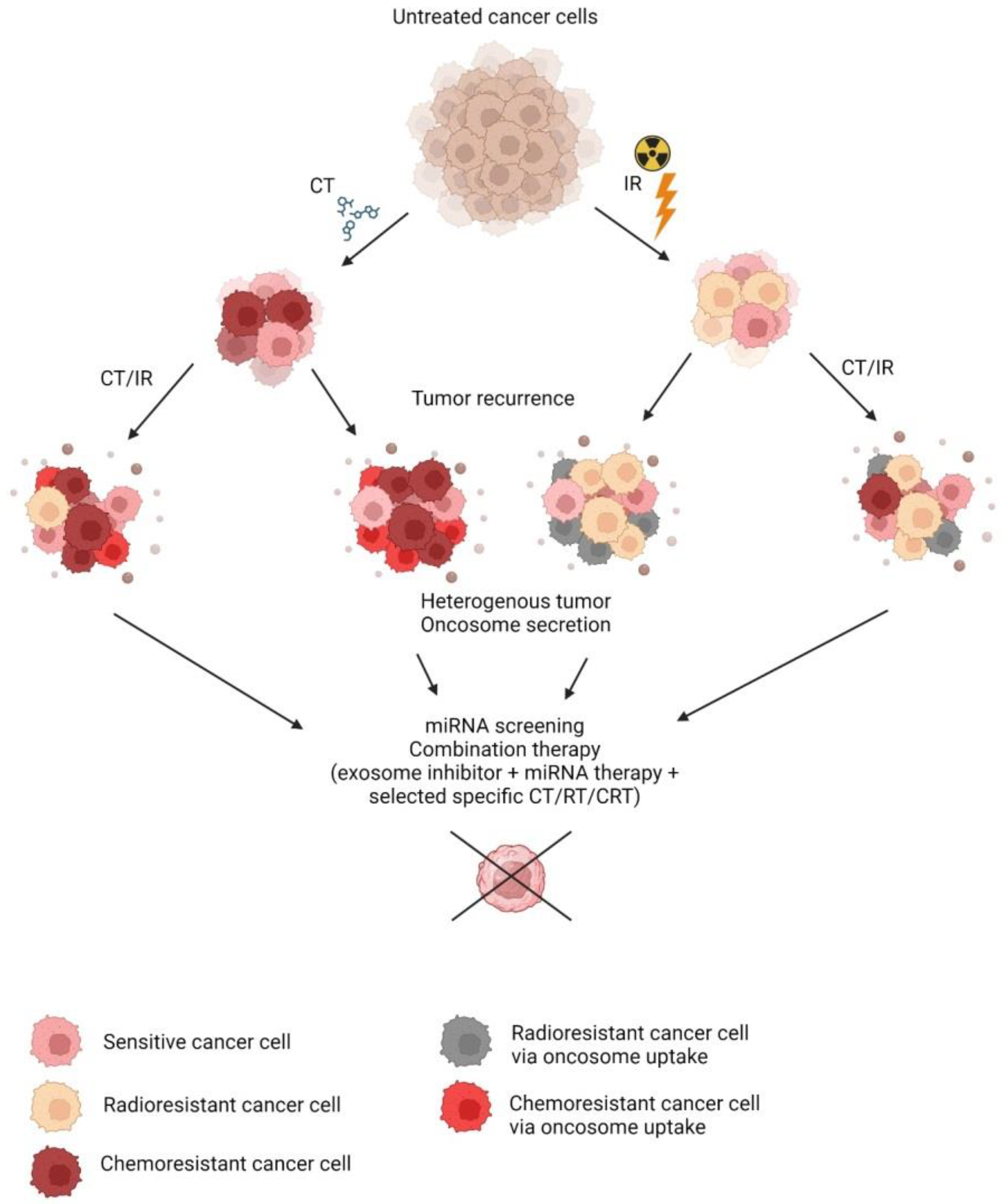
Chemoradiation therapy (CRT) is a commonly indicated treatment in the case of cancer. In combination with surgery or alone, it offers a relief of condition or even a cure for some patients. Resistance to chemo- or radiotherapy is the main obstacle to consistent treatment outcomes in oncology patients.
1. Introduction
2. Conditioned Media
3. Diagnostic Biomarkers of Resistance
|
Type of Resistance |
miRNA |
|---|---|
|
Tyrosine kinase inhibitors |
mir-BART14, mir-1469, mir-16-1, mir-196, mir-4791, mir-4796, mir-548aq, mir-72, mir-H19, mir-138-2, mir-153, mir-585, mir-4803, mir-744, mir-769 [15] mir-184, mir-3913 [16] mir-658, mir-564 [17] mir-1468, mir-23 [18] mir-136 [19] mir-214 [20] mir-210 [21] mir-615 [22] |
|
Cisplatin |
mir-20a [23] mir-193a [24] mir-524 [25] mir-4443 [26] mir-1246 [27] mir-425 [28] mir-103a [29] mir-1273a [30] mir-100 [31] |
|
IR |
mir-196a [32] mir-208a [9] mir-29a, mir-150 [10] |
3.1. Therapeutic Approaches
|
miRNA |
Number of References |
Type of Resistance |
|---|---|---|
|
mir-21 |
13 |
5FU [50] Cisplatin and paclitaxel [51] Cisplatin and docetaxel [52] Cisplatin, docetaxel, and IR [53] |
|
mir-145 |
10 |
IR [54] Docetaxel [60] Pemetrexed [61] Paclitaxel [62] Cisplatin and pemetrexed [63] |
|
mir-200c |
6 |
Paclitaxel [68] Vincristine, cisplatin, and MDR [69] |
|
mir-17 |
6 |
|
|
mir-34a |
5 |
|
|
mir-326 |
5 |
Matrine [84] Gefitinib [85] |
|
mir-200a |
5 |
|
|
mir-200b |
5 |
References
- Li, H.; Luo, F.; Jiang, X.; Zhang, W.; Xiang, T.; Pan, Q.; Cai, L.; Zhao, J.; Weng, D.; Li, Y.; et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J. ImmunoTherapy Cancer 2022, 10, e004029.
- Zhao, M.; Xu, J.; Zhong, S.; Liu, Y.; Xiao, H.; Geng, L.; Liu, H. Expression profiles and potential functions of circular RNAs in extracellular vesicles isolated from radioresistant glioma cells. Oncol. Rep. 2019, 41, 1893–1900.
- Tao, L.; Huang, G.; Wang, R.; Pan, Y.; He, Z.; Chu, X.; Song, H.; Chen, L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci. Rep. 2016, 6, 38408.
- Yoshino, H.; Nawamaki, M.; Murakami, K.; Kashiwakura, I. Effects of irradiated cell conditioned medium on the response of human lung cancer cells to anticancer treatment in vitro. World Acad. Sci. J. 2019, 1, 92–97.
- Kang, J.; Kim, W.; Kwon, T.; Youn, H.; Kim, J.S.; Youn, B. Plasminogen activator inhibitor-1 enhances radioresistance and aggressiveness of non-small cell lung cancer cells. Oncotarget 2016, 7, 23961–23974.
- Chambers, C.R.; Ritchie, S.; Pereira, B.A.; Timpson, P. Overcoming the senescence-associated secretory phenotype (SASP): A complex mechanism of resistance in the treatment of cancer. Mol. Oncol. 2021, 15, 3242–3255.
- Hoare, M.; Ito, Y.; Kang, T.-W.; Weekes, M.P.; Matheson, N.J.; Patten, D.A.; Shetty, S.; Parry, A.J.; Menon, S.; Salama, R.; et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016, 18, 979–992.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Tang, Y.; Cui, Y.; Li, Z.; Jiao, Z.; Zhang, Y.; He, Y.; Chen, G.; Zhou, Q.; Wang, W.; Zhou, X.; et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 7.
- Dinh, T.-K.T.; Fendler, W.; Chałubińska-Fendler, J.; Acharya, S.S.; O’Leary, C.; Deraska, P.V.; D’Andrea, A.D.; Chowdhury, D.; Kozono, D. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat. Oncol. 2016, 11, 61.
- Sun, Y.; Hawkins, P.G.; Bi, N.; Dess, R.T.; Tewari, M.; Hearn, J.W.D.; Hayman, J.A.; Kalemkerian, G.P.; Lawrence, T.S.; Ten Haken, R.K.; et al. Serum MicroRNA Signature Predicts Response to High-Dose Radiation Therapy in Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 107–114.
- Mostafazadeh, M.; Samadi, N.; Kahroba, H.; Baradaran, B.; Haiaty, S.; Nouri, M. Potential roles and prognostic significance of exosomes in cancer drug resistance. Cell Biosci. 2021, 11, 1.
- Ahmad, P.; Sana, J.; Slavik, M.; Slampa, P.; Smilek, P.; Slaby, O. MicroRNAs Involvement in Radioresistance of Head and Neck Cancer. Dis. Markers 2017, 2017, 8245345.
- Long, L.; Zhang, X.; Bai, J.; Li, Y.; Wang, X.; Zhou, Y. Tissue-specific and exosomal miRNAs in lung cancer radiotherapy: From regulatory mechanisms to clinical implications. Cancer Manag. Res. 2019, 11, 4413–4424.
- Sorokin, M.; Kholodenko, R.; Grekhova, A.; Suntsova, M.; Pustovalova, M.; Vorobyeva, N.; Kholodenko, I.; Malakhova, G.; Garazha, A.; Nedoluzhko, A.; et al. Acquired resistance to tyrosine kinase inhibitors may be linked with the decreased sensitivity to X-ray irradiation. Oncotarget 2017, 9, 5111–5124.
- Li, X.; Chen, C.; Wang, Z.; Liu, J.; Sun, W.; Shen, K.; Lv, Y.; Zhu, S.; Zhan, P.; Lv, T.; et al. Elevated exosome-derived miRNAs predict osimertinib resistance in non-small cell lung cancer. Cancer Cell Int. 2021, 21, 428.
- Azuma, Y.; Yokobori, T.; Mogi, A.; Yajima, T.; Kosaka, T.; Iijima, M.; Shimizu, K.; Shirabe, K.; Kuwano, H. Cancer exosomal microRNAs from gefitinib-resistant lung cancer cells cause therapeutic resistance in gefitinib-sensitive cells. Surg. Today 2020, 50, 1099–1106.
- Janpipatkul, K.; Trachu, N.; Watcharenwong, P.; Panvongsa, W.; Worakitchanon, W.; Metheetrairut, C.; Oranratnachai, S.; Reungwetwattana, T.; Chairoungdua, A. Exosomal microRNAs as potential biomarkers for osimertinib resistance of non-small cell lung cancer patients. Cancer Biomark. 2021, 31, 281–294.
- Gu, G.; Hu, C.; Hui, K.; Zhang, H.; Chen, T.; Zhang, X.; Jiang, X. Exosomal miR-136-5p Derived from Anlotinib-Resistant NSCLC Cells Confers Anlotinib Resistance in Non-Small Cell Lung Cancer Through Targeting PPP2R2A. Int. J. Nanomed. 2021, 16, 6329–6343.
- Zhang, Y.; Li, M.; Hu, C. Exosomal transfer of miR-214 mediates gefitinib resistance in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018, 507, 457–464.
- Hisakane, K.; Seike, M.; Sugano, T.; Yoshikawa, A.; Matsuda, K.; Takano, N.; Takahashi, S.; Noro, R.; Gemma, A. Exosome-derived miR-210 involved in resistance to osimertinib and epithelial–mesenchymal transition in EGFR mutant non-small cell lung cancer cells. Thorac. Cancer 2021, 12, 1690–1698.
- Pan, R.; Zhou, H. Exosomal Transfer of lncRNA H19 Promotes Erlotinib Resistance in Non-Small Cell Lung Cancer via miR-615-3p/ATG7 Axis. Cancer Manag. Res. 2020, 12, 4283–4297.
- Shi, L.; Zhu, W.; Huang, Y.; Zhuo, L.; Wang, S.; Chen, S.; Zhang, B.; Ke, B. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e989.
- Wu, H.; Mu, X.; Liu, L.; Wu, H.; Hu, X.; Chen, L.; Liu, J.; Mu, Y.; Yuan, F.; Liu, W.; et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020, 11, 801.
- Xie, H.; Yao, J.; Wang, Y.; Ni, B. Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 2022, 29, 1257–1271.
- Song, Z.; Jia, G.; Ma, P.; Cang, S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021, 276, 119399.
- Liu, Z.; Zhao, W.; Yang, R. MiR-1246 is responsible for lung cancer cells-derived exosomes-mediated promoting effects on lung cancer stemness via targeting TRIM17. Environ. Toxicol. 2022, 37, 2651–2659.
- Kilic, S.; Lezaja, A.; Gatti, M.; Bianco, E.; Michelena, J.; Imhof, R.; Altmeyer, M. Phase separation of 53 BP 1 determines liquid-like behavior of DNA repair compartments. EMBO J. 2019, 38, e101379.
- Wang, H.; Huang, H.; Wang, L.; Liu, Y.; Wang, M.; Zhao, S.; Lu, G.; Kang, X. Cancer-associated fibroblasts secreted miR-103a-3p suppresses apoptosis and promotes cisplatin resistance in non-small cell lung cancer. Aging 2021, 13, 14456–14468.
- Zhao, X.; Li, M.; Dai, X.; Yang, Y.; Peng, Y.; Xu, C.; Dai, N.; Wang, D. Downregulation of exosomal miR-1273a increases cisplatin resistance of non-small cell lung cancer by upregulating the expression of syndecan binding protein. Oncol. Rep. 2020, 44, 2165–2173.
- Qin, X.; Yu, S.; Zhou, L.; Shi, M.; Hu, Y.; Xu, X.; Shen, B.; Liu, S.; Yan, D.; Feng, J. Cisplatin-resistant lung cancer cell–derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100–5p-dependent manner. Int. J. Nanomed. 2017, 12, 3721–3733.
- Yao, F.; Shi, W.; Fang, F.; Lv, M.Y.; Xu, M.; Wu, S.Y.; Huang, C.L. Exosomal miR-196a-5p enhances radioresistance in lung cancer cells by downregulating NFKBIA. Kaohsiung J. Med. Sci. 2023, 39, 554–564.
- Cui, C.; Zhong, B.; Fan, R.; Cui, Q. HMDD v4.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 2023, gkad717.
- Hydbring, P.; Badalian-Very, G. Clinical applications of microRNAs. F1000Research 2013, 2, 136.
- Mrowczynski, O.D.; Madhankumar, A.B.; Sundstrom, J.M.; Zhao, Y.; Kawasawa, Y.I.; Slagle-Webb, B.; Mau, C.; Payne, R.A.; Rizk, E.B.; Zacharia, B.E.; et al. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget 2018, 9, 36083–36101.
- Tang, C.-H.; Sento, S.; Sasabe, E.; Yamamoto, T. Application of a Persistent Heparin Treatment Inhibits the Malignant Potential of Oral Squamous Carcinoma Cells Induced by Tumor Cell-Derived Exosomes. PLoS ONE 2016, 11, e0148454.
- Lebedeva, I.V.; Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; et al. Exosome Release and Low pH Belong to a Framework of Resistance of Human Melanoma Cells to Cisplatin. PLoS ONE 2014, 9, e88193.
- Eliopoulos, N.; Francois, M.r.; Boivin, M.-N.l.; Martineau, D.; Galipeau, J. Neo-Organoid of Marrow Mesenchymal Stromal Cells Secreting Interleukin-12 for Breast Cancer Therapy. Cancer Res. 2008, 68, 4810–4818.
- Huang, T.; Song, X.; Xu, D.; Tiek, D.; Goenka, A.; Wu, B.; Sastry, N.; Hu, B.; Cheng, S.-Y. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics 2020, 10, 8721–8743.
- Schulz, A.; Meyer, F.; Dubrovska, A.; Borgmann, K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers 2019, 11, 862.
- Zhang, C.-C.; Li, Y.; Feng, X.-Z.; Li, D.-B. Circular RNA circ_0001287 inhibits the proliferation, metastasis, and radiosensitivity of non-small cell lung cancer cells by sponging microRNA miR-21 and up-regulating phosphatase and tensin homolog expression. Bioengineered 2021, 12, 414–425.
- Zhang, J.; Zhang, C.; Hu, L.; He, Y.; Shi, Z.; Tang, S.; Chen, Y. Abnormal Expression of miR-21 and miR-95 in Cancer Stem-Like Cells is Associated with Radioresistance of Lung Cancer. Cancer Investig. 2015, 33, 165–171.
- Sun, Y.; Liu, W.; Zhao, Q.; Zhang, R.; Wang, J.; Pan, P.; Shang, H.; Liu, C.; Wang, C. Down-Regulating the Expression of miRNA-21 Inhibits the Glucose Metabolism of A549/DDP Cells and Promotes Cell Death Through the PI3K/AKT/mTOR/HIF-1α Pathway. Front. Oncol. 2021, 11, 653596.
- Gao, W.; Lu, X.; Liu, L.; Xu, J.; Feng, D.; Shu, Y. MiRNA-21. Cancer Biol. Ther. 2014, 13, 330–340.
- Dong, Z.; Ren, L.I.; Lin, L.I.; Li, J.; Huang, Y.; Li, J. Effect of microRNA-21 on multidrug resistance reversal in A549/DDP human lung cancer cells. Mol. Med. Rep. 2015, 11, 682–690.
- Li, B.; Ren, S.; Li, X.; Wang, Y.; Garfield, D.; Zhou, S.; Chen, X.; Su, C.; Chen, M.; Kuang, P.; et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer 2014, 83, 146–153.
- Wang, S.; Su, X.; Bai, H.; Zhao, J.; Duan, J.; An, T.; Zhuo, M.; Wang, Z.; Wu, M.; Li, Z.; et al. Identification of plasma microRNA profiles for primary resistance to EGFR-TKIs in advanced non-small cell lung cancer (NSCLC) patients with EGFR activating mutation. J. Hematol. Oncol. 2015, 8, 127.
- Huang, W.-C.; Yadav, V.K.; Cheng, W.-H.; Wang, C.-H.; Hsieh, M.-S.; Huang, T.-Y.; Lin, S.-F.; Yeh, C.-T.; Kuo, K.-T. The MEK/ERK/miR-21 Signaling Is Critical in Osimertinib Resistance in EGFR-Mutant Non-Small Cell Lung Cancer Cells. Cancers 2021, 13, 6005.
- Lee, J.W.; Shen, H.; Zhu, F.; Liu, J.; Xu, T.; Pei, D.; Wang, R.; Qian, Y.; Li, Q.; Wang, L.; et al. Alteration in Mir-21/PTEN Expression Modulates Gefitinib Resistance in Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e103305.
- Ding, S.; Zheng, Y.; Xu, Y.; Zhao, X.; Zhong, C. MiR-21/PTEN signaling modulates the chemo-sensitivity to 5-fluorouracil in human lung adenocarcinoma A549 cells. Int. J. Clin. Exp. Pathol. 2019, 12, 2339–2352.
- Su, C.; Cheng, X.; Li, Y.; Han, Y.; Song, X.; Yu, D.; Cao, X.; Liu, Z. MiR-21 improves invasion and migration of drug-resistant lung adenocarcinoma cancer cell and transformation of EMT through targetingHBP1. Cancer Med. 2018, 7, 2485–2503.
- Zhang, Y.-Q.; Chen, R.-L.; Shang, L.-Q.; Yang, S.-M. Nicotine-induced miR-21-3p promotes chemoresistance in lung cancer by negatively regulating FOXO3a. Oncol. Lett. 2022, 24, 260.
- Liu, Z.-L.; Wang, H.; Liu, J.; Wang, Z.-X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell. Biochem. 2012, 372, 35–45.
- Li, H.; Zhao, S.; Chen, X.; Feng, G.; Chen, Z.; Fan, S. MiR-145 modulates the radiosensitivity of non-small cell lung cancer cells by suppression of TMOD3. Carcinogenesis 2022, 43, 288–296.
- Zhang, H.; Luo, Y.; Xu, W.; Li, K.; Liao, C. Silencing long intergenic non-coding RNA 00707 enhances cisplatin sensitivity in cisplatin-resistant non-small-cell lung cancer cells by sponging miR-145. Oncol. Lett. 2019, 18, 6261–6268.
- Bar, J.; Gorn-Hondermann, I.; Moretto, P.; Perkins, T.J.; Niknejad, N.; Stewart, D.J.; Goss, G.D.; Dimitroulakos, J. miR Profiling Identifies Cyclin-Dependent Kinase 6 Downregulation as a Potential Mechanism of Acquired Cisplatin Resistance in Non–Small-Cell Lung Carcinoma. Clin. Lung Cancer 2015, 16, e121–e129.
- Chang, Y.-F.; Lim, K.-H.; Chiang, Y.-W.; Sie, Z.-L.; Chang, J.; Ho, A.-S.; Cheng, C.-C. STAT3 induces G9a to exacerbate HER3 expression for the survival of epidermal growth factor receptor-tyrosine kinase inhibitors in lung cancers. BMC Cancer 2019, 19, 959.
- Yu, C.; Li, B.; Wang, J.; Zhang, Z.; Li, S.; Lei, S.; Wang, Q. miR-145-5p Modulates Gefitinib Resistance by Targeting NRAS and MEST in Non-Small Cell Lung Cancer. Ann. Clin. Lab. Sci. 2021, 51, 625–637.
- Wang, Y.; Lian, Y.M.; Ge, C.Y. MiR-145 changes sensitivity of non-small cell lung cancer to gefitinib through targeting ADAM19. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5831–5839.
- Pan, Y.; Chen, J.; Tao, L.; Zhang, K.; Wang, R.; Chu, X.; Chen, L. Long noncoding RNA ROR regulates chemoresistance in docetaxel-resistant lung adenocarcinoma cells via epithelial mesenchymal transition pathway. Oncotarget 2017, 8, 33144–33158.
- Chang, W.-W.; Wang, B.-Y.; Chen, S.-H.; Chien, P.-J.; Sheu, G.-T.; Lin, C.-H. miR-145-5p Targets Sp1 in Non-Small Cell Lung Cancer Cells and Links to BMI1 Induced Pemetrexed Resistance and Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2022, 23, 15352.
- Guan, X.; Guan, Y. miR-145-5p attenuates paclitaxel resistance and suppresses the progression in drug-resistant breast cancer cell lines. Neoplasma 2020, 67, 972–981.
- Zheng, F.; Xu, R. CircPVT1 contributes to chemotherapy resistance of lung adenocarcinoma through miR-145-5p/ABCC1 axis. Biomed. Pharmacother. 2020, 124, 109828.
- Fukuda, K.; Takeuchi, S.; Arai, S.; Katayama, R.; Nanjo, S.; Tanimoto, A.; Nishiyama, A.; Nakagawa, T.; Taniguchi, H.; Suzuki, T.; et al. Epithelial-to-Mesenchymal Transition Is a Mechanism of ALK Inhibitor Resistance in Lung Cancer Independent of ALK Mutation Status. Cancer Res. 2019, 79, 1658–1670.
- Gao, H.-X.; Yan, L.; Li, C.; Zhao, L.-M.; Liu, W. miR-200c regulates crizotinib-resistant ALK-positive lung cancer cells by reversing epithelial-mesenchymal transition via targeting ZEB1. Mol. Med. Rep. 2016, 14, 4135–4143.
- Fukuda, K.; Takeuchi, S.; Arai, S.; Kita, K.; Tanimoto, A.; Nishiyama, A.; Yano, S. Glycogen synthase kinase-3 inhibition overcomes epithelial-mesenchymal transition-associated resistance to osimertinib in EGFR-mutant lung cancer. Cancer Sci. 2020, 111, 2374–2384.
- Zhou, G.; Zhang, F.; Guo, Y.; Huang, J.; Xie, Y.; Yue, S.; Chen, M.; Jiang, H.; Li, M. miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibites cell migration via targeting ZEB1. Biomed. Pharmacother. 2017, 85, 113–119.
- Zhao, Y.-f.; Han, M.-l.; Xiong, Y.-j.; Wang, L.; Fei, Y.; Shen, X.; Zhu, Y.; Liang, Z.-q. A miRNA-200c/cathepsin L feedback loop determines paclitaxel resistance in human lung cancer A549 cells in vitro through regulating epithelial–mesenchymal transition. Acta Pharmacol. Sin. 2017, 39, 1034–1047.
- Zhu, W.; Xu, H.; Zhu, D.; Zhi, H.; Wang, T.; Wang, J.; Jiang, B.; Shu, Y.; Liu, P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother. Pharmacol. 2011, 69, 723–731.
- Zhao, J.; Fu, W.; Liao, H.; Dai, L.; Jiang, Z.; Pan, Y.; Huang, H.; Mo, Y.; Li, S.; Yang, G.; et al. The regulatory and predictive functions of miR-17 and miR-92 families on cisplatin resistance of non-small cell lung cancer. BMC Cancer 2015, 15, 731.
- Fröhlich, H.; Jiang, Z.; Yin, J.; Fu, W.; Mo, Y.; Pan, Y.; Dai, L.; Huang, H.; Li, S.; Zhao, J. miRNA 17 Family Regulates Cisplatin-Resistant and Metastasis by Targeting TGFbetaR2 in NSCLC. PLoS ONE 2014, 9, e94639.
- Chatterjee, A.; Chattopadhyay, D.; Chakrabarti, G. miR-16 targets Bcl-2 in paclitaxel-resistant lung cancer cells and overexpression of miR-16 along with miR-17 causes unprecedented sensitivity by simultaneously modulating autophagy and apoptosis. Cell. Signal. 2015, 27, 189–203.
- Mari, B.; Chatterjee, A.; Chattopadhyay, D.; Chakrabarti, G. miR-17-5p Downregulation Contributes to Paclitaxel Resistance of Lung Cancer Cells through Altering Beclin1 Expression. PLoS ONE 2014, 9, e95716.
- Yin, J.; Hu, W.; Pan, L.; Fu, W.; Dai, L.; Jiang, Z.; Zhang, F.; Zhao, J. let-7 and miR-17 promote self-renewal and drive gefitinib resistance in non-small cell lung cancer. Oncol. Rep. 2019, 42, 495–508.
- Gong, J.; He, L.; Ma, J.; Zhang, J.; Wang, L.; Wang, J. The relationship between miR-17-5p, miR-92a, and let-7b expression with non-small cell lung cancer targeted drug resistance. J. BUON 2017, 22, 454–461.
- Yu, G.; Zhong, N.; Chen, G.; Huang, B.; Wu, S. Downregulation of PEBP4, a target of miR-34a, sensitizes drug-resistant lung cancer cells. Tumor Biol. 2014, 35, 10341–10349.
- Luo, S.; Shen, M.; Chen, H.; Li, W.; Chen, C. Long non-coding RNA TP73-AS1 accelerates the progression and cisplatin resistance of non-small cell lung cancer by upregulating the expression of TRIM29 via competitively targeting microRNA-34a-5p. Mol. Med. Rep. 2020, 22, 3822–3832.
- Yang, X.; Sun, Q.; Song, Y.; Li, W.; Falzone, L. circHUWE1 Exerts an Oncogenic Role in Inducing DDP-Resistant NSCLC Progression Depending on the Regulation of miR-34a-5p/TNFAIP8. Int. J. Genom. 2021, 2021, 3997045.
- Zhou, J.-Y.; Chen, X.; Zhao, J.; Bao, Z.; Chen, X.; Zhang, P.; Liu, Z.-F.; Zhou, J.-Y. MicroRNA-34a overcomes HGF-mediated gefitinib resistance in EGFR mutant lung cancer cells partly by targeting MET. Cancer Lett. 2014, 351, 265–271.
- Xiong, R.; Sun, X.x.; Wu, H.r.; Xu, G.w.; Wang, G.x.; Sun, X.h.; Xu, M.q.; Xie, M.r. Mechanism research of miR-34a regulates Axl in non-small-cell lung cancer with gefitinib-acquired resistance. Thorac. Cancer 2019, 11, 156–165.
- Li, J.; Li, S.; Chen, Z.; Wang, J.; Chen, Y.; Xu, Z.; Jin, M.; Yu, W. miR-326 reverses chemoresistance in human lung adenocarcinoma cells by targeting specificity protein 1. Tumor Biol. 2016, 37, 13287–13294.
- Wei, J.; Meng, G.; Wu, J.; Wang, Y.; Zhang, Q.; Dong, T.; Bao, J.; Wang, C.; Zhang, J. MicroRNA-326 impairs chemotherapy resistance in non small cell lung cancer by suppressing histone deacetylase SIRT1-mediated HIF1α and elevating VEGFA. Bioengineered 2022, 13, 5685–5699.
- Wu, Y.; Cheng, K.; Liang, W.; Wang, X. lncRNA RPPH1 promotes non-small cell lung cancer progression through the miR-326/WNT2B axis. Oncol. Lett. 2020, 20, 105.
- Dong, C.; Yang, L.; Zhao, G. Circ-PGAM1 Enhances Matrine Resistance of Non-Small Cell Lung Cancer via the miR-326/CXCR5 Axis. Cancer Biother. Radiopharm. 2022.
- Zheng, Y.; Guo, Z.; Li, Y. Long non-coding RNA prostate cancer-associated transcript 6 inhibited gefitinib sensitivity of non-small cell lung cancer by serving as a competing endogenous RNA of miR-326 to up-regulate interferon-alpha receptor 2. Bioengineered 2022, 13, 3785–3796.
- Tang, W.; Yu, X.; Zeng, R.; Chen, L. LncRNA-ATB Promotes Cisplatin Resistance in Lung Adenocarcinoma Cells by Targeting the miR-200a/β-Catenin Pathway. Cancer Manag. Res. 2020, 12, 2001–2014.
- Liu, X.; Chen, L.; Wang, T. Overcoming cisplatin resistance of human lung cancer by sinomenine through targeting the miR-200a-3p-GLS axis. J. Chemother. 2022, 35, 357–366.
- Zhen, Q.; Liu, J.; Gao, L.; Liu, J.; Wang, R.; Chu, W.; Zhang, Y.; Tan, G.; Zhao, X.; Lv, B. MicroRNA-200a Targets EGFR and c-Met to Inhibit Migration, Invasion, and Gefitinib Resistance in Non-Small Cell Lung Cancer. Cytogenet. Genome Res. 2015, 146, 1–8.
- Nishijima, N.; Seike, M.; Soeno, C.; Chiba, M.; Miyanaga, A.; Noro, R.; Sugano, T.; Matsumoto, M.; Kubota, K.; Gemma, A. miR-200/ZEB axis regulates sensitivity to nintedanib in non-small cell lung cancer cells. Int. J. Oncol. 2016, 48, 937–944.
- Rastogi, I.; Rajanna, S.; Webb, A.; Chhabra, G.; Foster, B.; Webb, B.; Puri, N. Mechanism of c-Met and EGFR tyrosine kinase inhibitor resistance through epithelial mesenchymal transition in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2016, 477, 937–944.
- Chen, J.; Liu, X.; Xu, Y.; Zhang, K.; Huang, J.; Pan, B.; Chen, D.; Cui, S.; Song, H.; Wang, R.; et al. TFAP2C-Activated MALAT1 Modulates the Chemoresistance of Docetaxel-Resistant Lung Adenocarcinoma Cells. Mol. Ther.-Nucleic Acids 2019, 14, 567–582.
- Jiang, M.; Qi, F.; Zhang, K.; Zhang, X.; Ma, J.; Xia, S.; Chen, L.; Yu, Z.; Chen, J.; Chen, D. MARCKSL1–2 reverses docetaxel-resistance of lung adenocarcinoma cells by recruiting SUZ12 to suppress HDAC1 and elevate miR-200b. Mol. Cancer 2022, 21, 150.
- Pan, B.; Feng, B.; Chen, Y.; Huang, G.; Wang, R.; Chen, L.; Song, H. MiR-200b regulates autophagy associated with chemoresistance in human lung adenocarcinoma. Oncotarget 2015, 6, 32805–32820.
- Chen, D.-Q.; Pan, B.-Z.; Huang, J.-Y.; Zhang, K.; Cui, S.-Y.; De, W.; Wang, R.; Chen, L.-B. HDAC 1/4-mediated silencing of microRNA-200b promotes chemoresistance in human lung adenocarcinoma cells. Oncotarget 2014, 5, 3333–3349.
- Feng, B.; Wang, R.; Song, H.-Z.; Chen, L.-B. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer 2012, 118, 3365–3376.
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637.
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: A first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017, 18, 1386–1396.
- Qi, Y.; Huang, Y.; Pang, L.; Gu, W.; Wang, N.; Hu, J.; Cui, X.; Zhang, J.; Zhao, J.; Liu, C.; et al. Prognostic value of the MicroRNA-29 family in multiple human cancers: A meta-analysis and systematic review. Clin. Exp. Pharmacol. Physiol. 2017, 44, 441–454.
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic advances of miRNAs: A preclinical and clinical update. J. Adv. Res. 2021, 28, 127–138.
- To, K.K.W.; Fong, W.; Tong, C.W.S.; Wu, M.; Yan, W.; Cho, W.C.S. Advances in the discovery of microRNA-based anticancer therapeutics: Latest tools and developments. Expert Opin. Drug Discov. 2019, 15, 63–83.
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179.
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132.
- He, Y.; Zhang, X.; Chang, J.; Kim, H.-N.; Zhang, P.; Wang, Y.; Khan, S.; Liu, X.; Zhang, X.; Lv, D.; et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat. Commun. 2020, 11, 1996.
- Hu, L.; Li, H.; Zi, M.; Li, W.; Liu, J.; Yang, Y.; Zhou, D.; Kong, Q.-P.; Zhang, Y.; He, Y. Why Senescent Cells Are Resistant to Apoptosis: An Insight for Senolytic Development. Front. Cell Dev. Biol. 2022, 10, 822816.
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2022, 290, 1362–1383.
- Short, S.; Fielder, E.; Miwa, S.; von Zglinicki, T. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine 2019, 41, 683–692.




