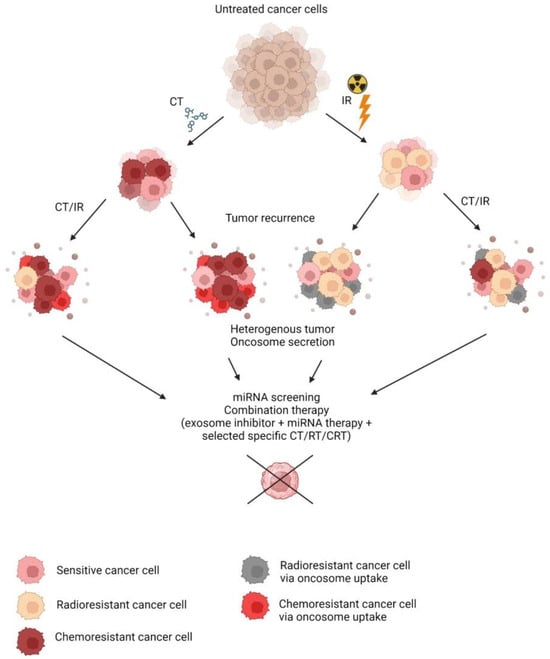
Chemoradiation therapy (CRT) is a commonly indicated treatment in the case of cancer. In combination with surgery or alone, it offers a relief of condition or even a cure for some patients. Resistance to chemo- or radiotherapy is the main obstacle to consistent treatment outcomes in oncology patients.
- radioresistance
- chemoresistance
- cancer cell
- conditioned media
- secretory factors
- oncosomes
- exosomes
- miRNA
1. Introduction
2. Conditioned Media
To reveal the influence of TME on the development of resistance of cancer cells, experiments with conditioned media (CM) were conducted. CM contains various paracrine factors and oncosomes that convey molecular signals and aid in cellular communication. Among the resistance-associated secretory molecules, circular RNAs (circRNA) and cytokines have been identified. Cytokines bind to cellular receptors and initiate a signal cascade, while circRNA can enter by endocytosis. circITGB6 was found to be associated with cisplatin resistance in M2 macrophages, while circATP8B4 facilitates higher viability after ionizing radiation (IR) treatment [1][2][1,2]. In addition to circRNA, interleukin-11 (IL-11), secreted by cancer-associated fibroblasts, previously treated with cisplatin, also induced a significantly higher viability of A549 cells following cisplatin exposure by activating the IL-11R/STAT3 anti-apoptotic signaling pathway [3]. Other studies explored radioresistance induction and showed that CM from previously irradiated cells also showed protective effects in the A549 cells upon additional X-ray exposure, leading to lower apoptotic rates [4]. CM from three types of previously irradiated lung cancer cells facilitated decreased cell death upon irradiation of sensitive cells via increased plasminogen activator inhibitor-1 (PAI-1) that upregulated AKT and ERK1/2 pathways and inhibited caspase-3 activity [5]. In vivo TME contains senescent cells that are formed after anti-cancer therapy that express the senescence-associated secretory phenotype (SASP). Senescent cells are more resistant to exposure due to their dormant state, polyploidy, and apoptosis evasion via senescent cell anti-apoptotic pathways, and they secrete various cytokines such as TGFβ1, TGFβ3, IL1β, IL-6, IL-8, CXCL1, CXCL2, and CXCL5 in addition to miRNA containing oncosomes that can promote tumor progression and confer subsequent therapy resistance [6][7][6,7]. Therapeutic approaches help to ablate senescent cells and were shown to improve treatment outcomes.3. Diagnostic Biomarkers of Resistance
The detection of secretory factors holds potential as a diagnostic biomarker for tumor cell resistance status to predict a therapy response. miRNAs are a promising tool in biomarker development, as they are highly stable molecules in circulation [8][30]. Minimally invasive diagnostic approaches have been made using plasma levels of some miRNAs. miR-208a holds potential as a serum biomarker of NSCLC radioresistance, while other detected differentially expressed miRNAs await further investigation [9][39]. miR-29a-3p and miR-150-5p from blood were also found to be reflective of NSCLC radioresistance [10][36]. Eleven serum miRNAs were predictive of NSCLC patients’ resistance to RT [11][106]. Various circulatory exosome-shuttled miRNAs were also predictive of chemotherapy resistance status, as presented in a review [12][107]. Candidate secretory miRNAs involved with radio- and chemoresistance of NSCLC as an example are presented in Table 1, and they can be a starting point in the development of a minimally invasive diagnostic panel. Other diagnostic approaches are based on liquid and tumor biopsies, which can include miRNA from isolated oncosomes or other secretory factors, as well as intercellular miRNA analysis. Candidate intercellular miRNAs predictive of chemo- or radioresistance status have been proposed in numerous studies and are summarized in reviews [13][14][108,109].|
Type of Resistance |
miRNA |
|---|---|
|
Tyrosine kinase inhibitors |
mir-BART14, mir-1469, mir-16-1, mir-196, mir-4791, mir-4796, mir-548aq, mir-72, mir-H19, mir-138-2, mir-153, mir-585, mir-4803, mir-744, mir-769 [15][41] |
|
Cisplatin |
|
|
IR |
3.1. Therapeutic Approaches
Since the resistance-mediating effects of oncosomes were characterized, the inhibition of their secretion, biogenesis, or uptake was attempted in combination with anticancer therapy that led to tumor sensitization and higher antineoplastic efficiency in multiple in vitro studies, as summarized in a review [12][107]. The administration of exosome inhibitors heparin and simvastatin can help alleviate the detrimental effects of the oncosome injection derived from resistant cells in mice [35][36][23,111]. Alkylation of TME also reduces oncosome release, and intraperitoneal injections of proton pump inhibitors in combination with chemotherapy in mice led to decreased plasma exosome levels; however, no differences in tumor weight were noted due to selected time intervals [37][112]. Upon the discovery of secretory regulatory RNA factors conveying chemo- and radioresistance, approaches were made to up- or downregulate them in vivo. Injections of exosomes containing mir-214 antagomir sensitized lung tumors in mice, pre-treated with oncosomes from gefitinib-resistant PC9 cells [20][46]. As an example, antisense oligonucleotide targeting allowed for the knockout of circITGB6 in vivo with intraperitoneal injections, and in combination with cisplatin treatment, it led to significantly lower ovarian circITGB6-transfected tumor size and increased survival in mice compared to cisplatin alone [1]. Knockdown of IL-11 in mice with lung cancer also led to better effects of cisplatin treatment [3]. Inhibition of a paracrine factor PAI-1 in mice with lung cancer by oral administration of tiplaxtitnin successfully sensitized tumors to radiation therapy and led to a significantly decreased tumor volume [5]. The animal studies discussed above provide a valuable model for the in vivo investigation of therapeutic opportunities and successful outcomes proceeding further to clinical trials. Preliminary investigations were carried out on cell cultures, while 3D cultures were also used to simulate tumor formation in vitro. Recent neo-organoid developments are a very promising treatment based on cell integration into a 3D scaffold with the following implantation into the body. Neo-organoid implantation with Matrigel-imbedded MSCs overexpressing IL-12 led to significantly better results, as compared to non-genetically modified MSCs with 67% of mice with breast cancer xenografts being completely tumor free 55 days after treatment [38][113]. Such approaches pave the way to abrogate the subset of resistance-acquiring cancer tumor cells via the acquisition of secreted factors. However, the question remains open as to how kill the resistant and CSCs that are also present as a subset of a heterogenous tumor in this model. Today, the major directions in CSC-targeted therapy research include immunotherapy, inhibition of key signaling pathways, inhibition of DNA repair, and awakening quiescent CSCs [39][40][114,115]. Studies of differentially expressed intracellular miRNAs between resistant and sensitive cancer cells point out the miRNA control of cancer cells’ response to IR or CT, and its direct manipulation can be used to sensitize the tumor prior to therapy. Table 2 presents a summary of the candidate deregulated miRNA of resistant NSCLC cells as an example of possible therapeutic targets.|
miRNA |
Number of References |
Type of Resistance |
|---|---|---|
|
mir-21 |
13 |
Cisplatin [43][44][45][118,119,120] EGFR-TKI [46][47][48][49][121,122,123,124] Cisplatin and paclitaxel [51][126] |
|
mir-145 |
10 |
|
|
mir-200c |
6 |
|
|
mir-17 |
6 |
|
|
mir-34a |
5 |
|
|
mir-326 |
5 |
|
|
mir-200a |
5 |
|
|
mir-200b |
5 |