
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jordi B. Torrelles | -- | 2205 | 2023-12-12 00:10:40 | | | |
| 2 | Lindsay Dong | Meta information modification | 2205 | 2023-12-18 01:33:18 | | |
Video Upload Options
The presence of lipoarabinomannan (LAM) in the Mycobacterium tuberculosis (Mtb) cell envelope was first reported close to 100 years ago. Since then, numerous studies have been dedicated to the isolation, purification, structural definition, and elucidation of the biological properties of Mtb LAM. The significance of LAM remains high to this date, mainly due to its distinct immunological properties in conjunction with its role as a biomarker for diagnostic tests due to its identification in urine, and thus can serve as a point-of-care diagnostic test for tuberculosis (TB). LAM has been thoroughly studied and massive amounts of information on this intriguing molecule are now available.
1. Introduction
2. Defining the Basic Structure of LAM
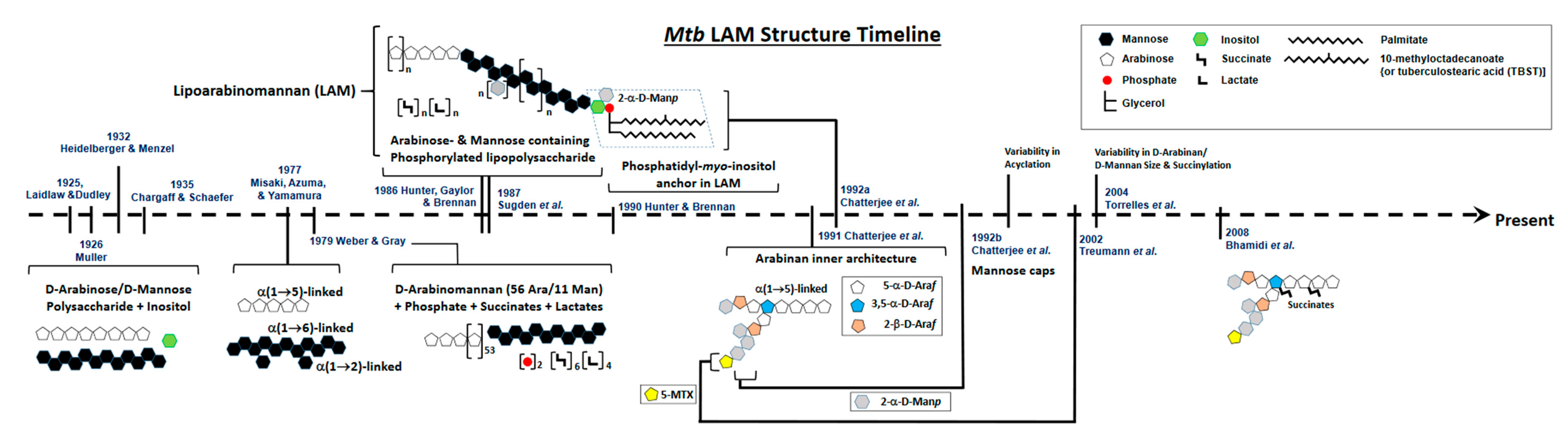
3. Evolution of LAM Structural Studies across Species
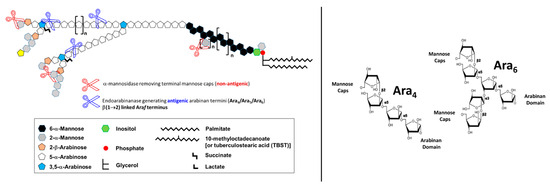
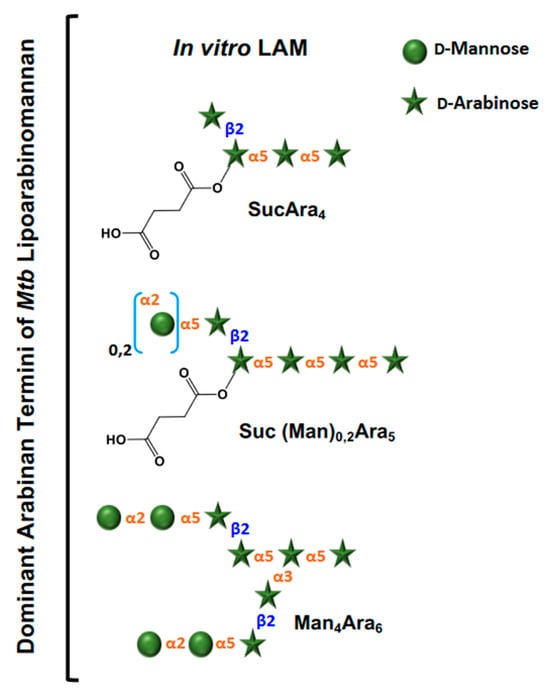
Essentially, LAM throughout contains four structural domains: a mannosylated phosphatidyl myo-inositol (PI) anchor, a D-mannan core, a D-arabinan domain, and different capping motifs that contribute to species and strain diversity [28][29]. The mannan core consists of a chain of α-(1 → 6)-linked mannopyranose (Manp) residues, some of which are modified by the addition of α-(1 → 2)-linked Manp motifs, usually, but not always, as a single residue. An arabinan, composed of solely D-arabinofuranose (Araf) residues, is attached to the non-reducing end of the mannan core [30]. Capping motifs can be added at specific positions contributing to intra- and inter-species structural variability [27]. Fast-growing mycobacterial species predominately produce AraLAM (uncapped LAM) or PILAM (phosphoinositol capped, as defined for M. smegmatis) [31]. Slow-growing mycobacteria like Mtb and M. leprae produce LAM with α-(1 → 2)-linked Manp capping residues, giving a molecule referred to as ManLAM [8][32]. Within the Mtb complex group, variations regarding primarily the degree of terminal mannose capping present in ManLAM can range between 40–70% [24][25][33][34][35]. Some fast-growing and/or non-pathogenic mycobacteria also produce ManLAM; however, these differ in the Man content of the capping motifs. In addition to Manp capping, ManLAM from strains of the Mtb complex group also contains a unique residue –MTX on the terminal Manp caps [36][37][38][39].
4. Biological Properties of Mtb LAM
5. LAM as a Diagnosis Biomarker for TB Disease
References
- Brennan, P.J.; Nikaido, H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63.
- Dahl, J.L. Electron microscopy analysis of Mycobacterium tuberculosis cell division. FEMS Microbiol. Lett. 2004, 240, 15–20.
- Chiaradia, L.; Lefebvre, C.; Parra, J.; Marcoux, J.; Burlet-Schiltz, O.; Etienne, G.; Tropis, M.; Daffé, M. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 2017, 7, 12807.
- Daffé, M.; Marrakchi, H. Unraveling the Structure of the Mycobacterial Envelope. Microbiol. Spectr. 2019, 7.
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope—A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59.
- Gaylord, H.; Brennan, P.J. Leprosy and the leprosy bacillus: Recent developments in characterization of antigens and immunology of the disease. Annu. Rev. Microbiol. 1987, 41, 645–675.
- McNeil, M.R.; Brennan, P.J. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res. Microbiol. 1991, 142, 451–463.
- Chatterjee, D.; Khoo, K.-H. Mycobacterial lipoarabinomannan: An extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 1998, 8, 113–120.
- Kang, P.B.; Azad, A.K.; Torrelles, J.B.; Kaufman, T.M.; Beharka, A.; Tibesar, E.; DesJardin, L.E.; Schlesinger, L.S. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 2005, 202, 987–999.
- Hett, E.C.; Rubin, E.J. Bacterial growth and cell division: A mycobacterial perspective. Microbiol. Mol. Biol. Rev. 2008, 72, 126–156.
- Torrelles, J.B. Broadening our view about the role of Mycobacterium tuberculosis cell envelope components during infection: A battle for survival. In Understanding Tuberculosis—Analyzing the Origin of Mycobacterium tuberculosis Pathogenicity; Cardona, P.J., Ed.; Intech: Rijeka, Croatia, 2012; pp. 1–46.
- Shi, L.; Torrelles, J.B.; Chatterjee, D. Lipoglycans of Mycobacterium tuberculosis: Isolation, purification, and characterization. In Mycobacteria Protocols, 2nd ed.; Parish, T., Brown, A.C., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 23–45.
- Besra, G.S.; Morehouse, C.B.; Rittner, C.M.; Waechter, C.J.; Brennan, P.J. Biosynthesis of mycobacterial lipoarabinomannan. J. Biol. Chem. 1997, 272, 18460–18466.
- Kaur, D.; Guerin, M.E.; Skovierova, H.; Brennan, P.J.; Jackson, M. Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv. Appl. Microbiol. 2009, 69, 23–78.
- Laidlaw, P.P.; Dudley, H.W. A specific pre-cipitating substance from tubercle bacilli. Br. J. Exp. Pathol. 1925, 6, 197–201.
- Heidelberger, M.; Menzel, A.E. Specific and non-specific cell polysaccharides of the humantype of tubercle bacillus, H37. Proc. Soc. Exp. Biol. Med. 1932, 29, 631–633.
- Mueller, J.H. A chemical study of the specific elements of tuberculin: II. The preparation of resi¬due antigen from old tuberculin. J. Exp. Med. 1926, 43, 9–12.
- Menzel, A.E.; Heidelberger, M. Specific and non-specific cell polysaccharides of an avian strain of tubercle bacillus. J. Biol. Chem. 1939, 127, 221–236.
- Chargaff, E.; Schaefer, W. A specific polysaccharide from the Bacillus Calmette-Guerin (BCG). J. Biol. Chem. 1935, 112, 393–405.
- Misaki, A.; Azuma, I.; Yamamura, Y. Structural and immunochemical studies on D-arabino-D-mannans and D-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J. Biochem. 1977, 82, 1759–1770.
- Weber, P.L.; Gray, G.R. Structural and immunochemical characterization of the acidic arabinomannan of Mycobacterium smegmatis. Carbohydr. Res. 1979, 74, 259–278.
- Hunter, S.W.; Gaylord, H.; Brennan, P.J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 1986, 261, 12345–12351.
- Sugden, E.A.; Samagh, B.S.; Bundle, D.R.; Duncan, J.R. Lipoarbinomannan and Lipid-free arabinomannan antigens of Mycobacterium paratuberculosis. Infect. Immun. 1987, 55, 762–770.
- Chatterjee, D.; Bozic, C.M.; McNeil, M.; Brennan, P.J. Structural features of the arabinan component of the lipoarabinomannan of Mycobacterium tuberculosis. J. Biol. Chem. 1991, 266, 9652–9660.
- Chatterjee, D.; Hunter, S.W.; McNeil, M.; Brennan, P.J. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphophatidylinositols. J. Biol. Chem. 1992, 267, 6228–6233.
- Amin, A.G.; De, P.; Spencer, J.S.; Brennan, P.J.; Daum, J.; Andre, B.G.; Joe, M.; Bai, Y.; Laurentius, L.; Porter, M.D.; et al. Detection of lipoarabinomannan in urine and serum of HIV-positive and HIV-negative TB suspects using an improved capture-enzyme linked immuno absorbent assay and gas chromatography/mass spectrometry. Tuberculosis 2018, 111, 178–187.
- De, P.; Amin, A.G.; Flores, D.; Simpson, A.; Dobos, K.; Chatterjee, D. Structural implications of lipoarabinomannan glycans from global clinical isolates in diagnosis of Mycobacterium tuberculosis infection. J. Biol. Chem. 2021, 297, 101265.
- Turner, J.; Torrelles, J.B. Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76, fty026.
- Nigou, J.; Gilleron, M.; Puzo, G. Lipoarabinomannans: From structure to biosynthesis. Biochimie 2003, 85, 153–166.
- Angala, S.; Li, W.; Boot, C.M.; Jackson, M.; McNeil, M.R. Secondary Extended Mannan Side Chains and Attachment of the Arabinan in Mycobacterial Lipoarabinomannan. Commun. Chem. 2020, 3, 101.
- Palcekova, Z.; Angala, S.K.; Belardinelli, J.M.; Eskandarian, H.A.; Joe, M.; Brunton, R.; Rithner, C.; Jones, V.; Nigou, J.; Lowary, T.L.; et al. Disruption of the SucT acyltransferase in Mycobacterium smegmatis abrogates succinylation of cell envelope polysaccharides. J. Biol. Chem. 2019, 294, 10325–10335.
- Torrelles, J.B.; Khoo, K.H.; Sieling, P.A.; Modlin, R.L.; Zhang, N.; Marques, A.M.; Treumann, A.; Rithner, C.D.; Brennan, P.J.; Chatterjee, D. Truncated Structural Variants of Lipoarabinomannan in Mycobacterium leprae and an Ethambutol-resistant Strain of Mycobacterium tuberculosis. J. Biol. Chem. 2004, 279, 41227–41239.
- Chatterjee, D.; Lowell, K.; Rivoire, B.; McNeil, M.R.; Brennan, P.J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J. Biol. Chem. 1992, 267, 6234–6239.
- Chatterjee, D.; Khoo, K.H.; McNeil, M.R.; Dell, A.; Morris, H.R.; Brennan, P.J. Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology 1993, 3, 497–506.
- Khoo, K.-H.; Tang, J.-B.; Chatterjee, D. Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J. Biol. Chem. 2001, 276, 3863–3871.
- De, P.; Shi, L.; Boot, C.; Ordway, D.; McNeil, M.; Chatterjee, D. Comparative Structural Study of Terminal Ends of Lipoarabinomannan from Mice Infected Lung Tissues and Urine of a Tuberculosis Positive Patient. ACS Infect. Dis. 2020, 6, 291–301.
- Treumann, A.; Xidong, F.; McDonnell, L.; Derrick, P.J.; Ashcroft, A.E.; Chatterjee, D.; Homans, S.W. 5-Methylthiopentose: A new substituent on lipoarabinomannan in Mycobacterium tuberculosis. J. Mol. Biol. 2002, 316, 89–100.
- Turnbull, W.B.; Shimizu, K.H.; Chatterjee, D.; Homans, S.W.; Treumann, A. Identification of the 5-methylthiopentosyl substituent in Mycobacterium tuberculosis lipoarabinomannan. Angew. Chem. Int. Ed. Engl. 2004, 43, 3918–3922.
- Angala, S.K.; McNeil, M.R.; Shi, L.; Joe, M.; Pham, H.; Zuberogoitia, S.; Nigou, J.; Boot, C.M.; Lowary, T.L.; Gilleron, M.; et al. Biosynthesis of the Methylthioxylose Capping Motif of Lipoarabinomannan in Mycobacterium tuberculosis. ACS Chem. Biol. 2017, 12, 682–691.
- Torrelles, J.B.; Sieling, P.A.; Zhang, N.; Keen, M.A.; McNeil, M.R.; Belisle, J.T.; Modlin, R.L.; Brennan, P.J.; Chatterjee, D. Isolation of a distinct Mycobacterium tuberculosis mannose-capped lipoarabinomannan isoform responsible for recognition by CD1b-restricted T cells. Glycobiology 2012, 22, 1118–1127.
- Venisse, A.; Berjeaud, J.M.; Chaurand, P.; Gilleron, M.; Puzo, G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J. Biol. Chem. 1993, 268, 12401–12411.
- Sada, E.; Brennan, P.J.; Herrera, T.; Torres, M. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 1990, 28, 2587–2590.
- Cho, S.N.; Shin, J.S.; Kim, J.D.; Chong, Y. Production of monoclonal antibodies to lipoarabinomannan-B and use in the detection of mycobacterial antigens in sputum. Yonsei Med. J. 1990, 31, 333–338.
- Sibley, L.D.; Adams, L.B.; Krahenbuhl, J.L. Inhibition of interferon-gamma-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin. Exp. Immunol. 1990, 80, 141–148.
- Sibley, L.D.; Hunter, S.W.; Brennan, P.J.; Krahenbuhl, J.L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect. Immun. 1988, 56, 1232–1236.
- Kaplan, G.; Gandhi, R.R.; Weinstein, D.E.; Levis, W.R.; Patarroyo, M.E.; Brennan, P.J.; Cohn, Z.A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J. Immunol. 1987, 138, 3028–3034.
- Chan, J.; Fan, X.; Hunter, S.W.; Brennan, P.J.; Bloom, B.R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 1991, 59, 1755–1761.
- Moreno, C.; Mehlert, A.; Lamb, J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin. Exp. Immunol. 1988, 74, 206–210.
- Moreno, C.; Taverne, J.; Mehlert, A.; Bate, C.A.W.; Brealey, R.J.; Meager, A.; Rook, G.A.W.; Playfair, J.H.L. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumor necrosis factor from human and murine macrophages. Clin. Exp. Immunol. 1989, 76, 240–245.
- Barnes, P.F.; Chatterjee, D.; Brennan, P.J.; Rea, T.H.; Modlin, R.L. Tumor necrosis factor production in patients with leprosy. Infect. Immun. 1992, 60, 1441–1446.
- Chatterjee, D.; Roberts, A.D.; Lowell, K.; Brennan, P.J.; Orme, I.M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect. Immun. 1992, 60, 1249–1253.
- Adams, L.B.; Fukutomi, Y.; Krahenbuhl, J.L. Regulation of murine macrophage effector functions by lipoarabinomannan from mycobacterial strains with different degrees of virulence. Infect. Immun. 1993, 61, 4173–4181.
- Barnes, P.F.; Chatterjee, D.; Abrams, J.S.; Lu, S.; Wang, E.; Yamamura, M.; Brennan, P.J.; Modlin, R.L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan: Relationship to chemical structure. J. Immunol. 1992, 149, 541–547.
- Zhang, Y.; Rom, W.N. Regulation of the interleukin-1b (IL-1b) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol. Cell Biol. 1993, 13, 3831–3837.
- Maeda, N.; Nigou, J.; Herrmann, J.L.; Jackson, M.; Amara, A.; Lagrange, P.H.; Puzo, G.; Gicquel, B.; Neyrolles, O. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 2003, 278, 5513–5516.
- Schlesinger, L.S.; Hull, S.R.; Kaufman, T.M. Binding of the Terminal Mannosyl Units of Lipoarabinomannan from a Virulent Strain of Mycobacterium Tuberculosis to Human Macrophages. J. Immunol. 1994, 152, 4070–4079.
- Venisse, A.; Fournié, J.-J.; Puzo, G. Mannosylated lipoarabinomannan interacts with phagocytes. Eur. J. Biochem. 1995, 231, 440–447.
- Fratti, R.A.; Chua, J.; Vergne, I.; Deretic, V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 2003, 100, 5437–5442.
- Schlesinger, L.S.; Kaufman, T.M.; Iyer, S.; Hull, S.R.; Marchiando, L.K. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J. Immunol. 1996, 157, 4568–4575.
- Hunter, S.W.; Brennan, P.J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J. Biol. Chem. 1990, 265, 9272–9279.
- Kaur, D.; Obregon-Henao, A.; Pham, H.; Chatterjee, D.; Brennan, P.J.; Jackson, M. Lipoarabinomannan of Mycobacterium: Mannose capping by a multifunctional terminal mannosyltransferase. Proc. Natl. Acad. Sci. USA 2008, 105, 17973–17977.
- Alderwick, L.J.; Birch, H.L.; Mishra, A.K.; Eggeling, L.; Besra, G.S. Structure, function and biosynthesis of the Mycobacterium tuberculosis cell wall: Arabinogalactan and lipoarabinomannan assembly with a view to discovering new drug targets. Biochem. Soc. Trans. 2007, 35, 1325–1328.
- Chatterjee, D.; Brennan, P.J. Glycosylated components of the mycobacterial cell wall;structure and function. In Microbial Glycobiology: Structures, Relevance and Applications, 1st ed.; Holst, O., Brennan, P.J., Itzstein, V.M., Eds.; Academic Press: Oxford, UK, 2009; pp. 147–167.
- Briken, V.; Porcelli, S.A.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403.
- Rivoire, B.; Ranchoff, B.; Chatterjee, D.; Gaylord, H.; Tsang, A.; Kolk, A.H.J.; Aspinall, G.O.; Brennan, P.J. Generation of monoclonal antibodies to the specific sugar epitopes of Mycobacterium avium complex serovars. Infect. Immun. 1989, 57, 3147–3158.
- Ahmad, R.; Xie, L.; Pyle, M.; Suarez, M.F.; Broger, T.; Steinberg, D.; Ame, S.M.; Lucero, M.G.; Szucs, M.J.; MacMullan, M.; et al. A rapid triage test for active pulmonary tuberculosis in adult patients with persistent cough. Sci. Transl. Med. 2019, 11, eaaw8287.
- Choudhary, A.; Patel, D.; Honnen, W.; Lai, Z.; Prattipati, R.S.; Zheng, R.B.; Hsueh, Y.C.; Gennaro, M.L.; Lardizabal, A.; Restrepo, B.I.; et al. Characterization of the Antigenic Heterogeneity of Lipoarabinomannan, the Major Surface Glycolipid of Mycobacterium tuberculosis, and Complexity of Antibody Specificities toward this Antigen. J. Immunol. 2018, 200, 3053–3066.
- Corrigan, D.T.; Ishida, E.; Chatterjee, D.; Lowary, T.L.; Achkar, J.M. Monoclonal antibodies to lipoarabinomannan/arabinomannan—Characteristics and implications for tuberculosis research and diagnostics. Trends Microbiol. 2023, 31, 22–35.
- Lawn, S.D.; Gupta-Wright, A. Detection of lipoarabinomannan (LAM) in urine is indicative of disseminated TB with renal involvement in patients living with HIV and advanced immunodeficiency: Evidence and implications. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 180–185.
- Gupta-Wright, A.; Peters, J.A.; Flach, C.; Lawn, S.D. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: A systematic review and meta-analysis. BMC Med. 2016, 14, 53.
- Shah, M.; Hanrahan, C.; Wang, Z.Y.; Dendukuri, N.; Lawn, S.D.; Denkinger, C.M.; Steingart, K.R. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst. Rev. 2016, 5, CD011420.
- Amin, A.G.; De, P.; Graham, B.; Calderon, R.I.; Franke, M.F.; Chatterjee, D. Urine lipoarabinomannan in HIV uninfected, smear negative, symptomatic TB patients: Effective sample pretreatment for a sensitive immunoassay and mass spectrometry. Sci. Rep. 2021, 11, 2922.
- Amin, A.G.; De, P.; Graham, B.; Jensen, B.L.; Moreau, E.; Chatterjee, D. Overcome low levels of detection limit and choice of antibody affects detection of lipoarabinomannan in pediatric tuberculosis. PLoS ONE 2022, 17, e0275838.
- Sigal, G.B.; Pinter, A.; Lowary, T.L.; Kawasaki, M.; Li, A.; Mathew, A.; Tsionsky, M.; Zheng, R.B.; Plisova, T.; Shen, K.; et al. A Novel Sensitive Immunoassay Targeting the 5-Methylthio-d-Xylofuranose-Lipoarabinomannan Epitope Meets the WHO’s Performance Target for Tuberculosis Diagnosis. J. Clin. Microbiol. 2018, 56, e01338-18.
- Zhang, A.; Jumbe, E.; Krysiak, R.; Sidiki, S.; Kelley, H.V.; Chemey, E.K.; Kamba, C.; Mwapasa, V.; Garcia, J.I.; Norris, A.; et al. Low-cost diagnostic test for susceptible and drug-resistant tuberculosis in rural Malawi. Afr. J. Lab. Med. 2018, 7, 690.
- Lawn, S.D.; Kerkhoff, A.D.; Nicol, M.P.; Meintjes, G. Underestimation of the true specificity of the urine lipoarabinomannan (LAM) point-of-care diagnostic assay for HIV-associated tuberculosis. J. Acquir. Immune Defic. Syndr. 2015, 69, e144.
- Paris, L.; Magni, R.; Zaidi, F.; Araujo, R.; Saini, N.; Harpole, M.; Coronel, J.; Kirwan, D.E.; Steinberg, H.; Gilman, R.H.; et al. Urine lipoarabinomannan glycan in HIV-negative patients with pulmonary tuberculosis correlates with disease severity. Sci. Transl. Med. 2017, 9, eaal2807.
- Nabeemeeah, F.; Sabet, R.; Moloantoa, T.; Waja, Z.; Pretorius, Z.; Majoro, K.; Letutu-Xaba, M.; Vilaplana, C.; Nigou, J.; Martinson, N. Exhaled breath specimens subjected to point-of-care lipoarabinomannan testing. Int. J. Tuberc. Lung Dis. 2023, 27, 703–705.
- Mosquera-Restrepo, S.F.; Zuberogoïtia, S.; Gouxette, L.; Layre, E.; Gilleron, M.; Stella, A.; Rengel, D.; Burlet-Schiltz, O.; Caro, A.C.; Garcia, L.; et al. A Mycobacterium tuberculosis fingerprint in human breath allows tuberculosis detection. Nat. Commun. 2022, 13, 7751.




