
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guillaume Grzych | -- | 2422 | 2023-12-11 13:13:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 2422 | 2023-12-13 09:38:28 | | |
Video Upload Options
The recreational use of nitrous oxide (N2O), also called laughing gas, has increased significantly in recent years. In 2022, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) recognized it as one of the most prevalent psychoactive substances used in Europe. Chronic nitrous oxide (N2O) exposure can lead to various clinical manifestations. The most frequent symptoms are neurological (sensitive or motor disorders), but there are also other manifestations like psychiatric manifestations or cardiovascular disorders (thrombosis events). N2O also affects various neurotransmitter systems, leading to its anesthetic, analgesic, anxiolytic and antidepressant properties. N2O is very challenging to measure in biological matrices. Thus, in cases of N2O intoxication, indirect biomarkers such as vitamin B12, plasma homocysteine and plasma MMA should be explored for diagnosis and assessment.
1. Introduction
2. Clinical Manifestations
2.1. Brief History of the First Reported Manifestations of Chronic N2O Exposure
2.2. Common Symptoms and Signs
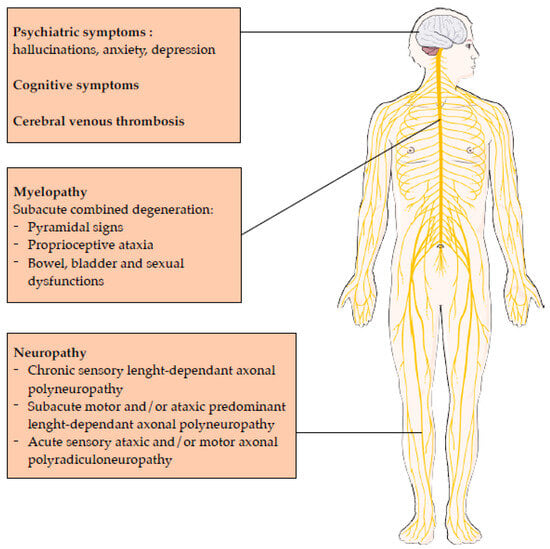
2.3. Central Nervous System Involvement
2.4. Peripheral Nervous System Involvement
-
More motor and sensory nerve injury in the lower limbs compared to the upper limbs,
-
More motor nerve injury than sensory nerve injury in the lower limbs,
-
More demyelinating features in the sensory and motor nerves of the upper limbs, with a marked motor predominance.
2.5. Prognosis of Central and Neurological Nervous System Involvement
3. Pharmacological Effects
3.1. Dependence Producing Potential of Nitrous Oxide
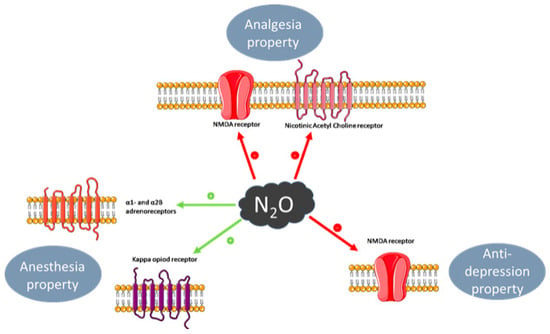
3.2. Anaesthesia
3.3. Analgesia
3.4. Anxiolytic Effect
3.5. Anti-depressant Effect
4. Laboratory Medicine
4.1. Direct N2O Measurement
4.2. Impact on Metabolism
4.2.1. Cobalamin and One Carbon Metabolism
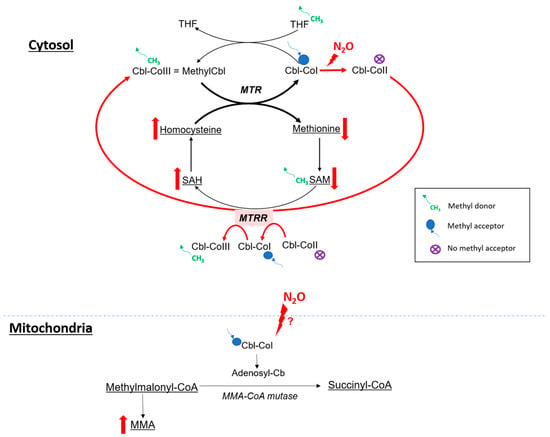
4.2.2. N2O and Oxidative Stress
4.2.3. Homocysteine and Oxidative Stress
4.3. Indirect Biomarkers of N2O Intoxication
4.3.1. Vitamin B12
4.3.2. Plasma Homocysteine
4.3.3. Plasma MMA
4.3.4. Plasma Methionine
4.3.5. Oxidative Stress Markers
4.3.6. Others Biological Parameters to Consider
5. Conclusions
References
- (PDF) Recreational Use of Nitrous Oxide: A Growing Concern for Europe. Available online: https://www.researchgate.net/publication/366138268_Recreational_use_of_nitrous_oxide_a_growing_concern_for_Europe (accessed on 18 October 2023).
- Lassen, H.C.; Henriksen, E.; Neukirch, F.; Kristensen, H.S. Treatment of tetanus; severe bone-marrow depression after prolonged nitrous-oxide anaesthesia. Lancet 1956, 270, 527–530.
- Sund Kristensen, H.; Berthelsen, P.G. Risus sardonicus and laughing gas–when nitrous oxide lost its innocence. Acta Anaesthesiol. Scand. 1994, 38, 751–752.
- Layzer, R.B. Myeloneuropathy after prolonged exposure to nitrous oxide. Lancet 1978, 2, 1227–1230.
- Layzer, R.B.; Fishman, R.A.; Schafer, J.A. Neuropathy following abuse of nitrous oxide. Neurology 1978, 28, 504–506.
- Jastak, J.T. Nitrous oxide and its abuse. J. Am. Dent. Assoc. 1991, 122, 48–52.
- Winstock, A.R.; Ferris, J.A. Nitrous oxide causes peripheral neuropathy in a dose dependent manner among recreational users. J. Psychopharmacol. 2020, 34, 229–236.
- Zhang, J.; Xie, D.; Zou, Y.; Yu, X.; Ji, Y.; Wang, C.; Lv, X.; Zhou, N.; Jiang, X.; Wang, K.; et al. Key Characteristics of Nitrous Oxide-Induced Neurological Disorders and Differences Between Populations. Front. Neurol. 2021, 12, 627183.
- Fang, X.; Yu, M.; Zheng, D.; Gao, H.; Li, W.; Ma, Y. Electrophysiologic Characteristics of Nitrous-Oxide-Associated Peripheral Neuropathy: A Retrospective Study of 76 Patients. J. Clin. Neurol. 2023, 19, 44–51.
- Berling, E.; Fargeot, G.; Aure, K.; Tran, T.H.; Kubis, N.; Lozeron, P.; Zanin, A. Nitrous oxide-induced predominantly motor neuropathies: A follow-up study. J. Neurol. 2022, 269, 2720–2726.
- Dohrn, C.S.; Lichtor, J.; Coalson, D.W.; Uitvlugt, A.; de Wit, H.; Zacny, J.P. Reinforcing effects of extended inhalation of nitrous oxide in humans. Drug Alcohol Depend. 1993, 31, 265–280.
- Dj, W.; Jp, Z. Within- and between-subject variability in the reinforcing and subjective effects of nitrous oxide in healthy volunteers. Drug Alcohol Depend. 2001, 64, 85–96.
- Dj, W.; Jp, Z. Analysis of the reinforcing and subjective effects of different doses of nitrous oxide using a free-choice procedure. Drug Alcohol Depend. 2002, 66, 93–103.
- Carter, A.; Capps, B.; Hall, W. Addiction Neurobiology: Ethical and Social Implications; EMCDDA: Lisbon, Portugal, 2009.
- Walsh, K.; Das, R.K.; Kamboj, S.K. The Subjective Response to Nitrous Oxide is a Potential Pharmaco-Endophenotype for Alcohol Use Disorder: A Preliminary Study with Heavy Drinkers. Int. J. Neuropsychopharmacol. 2017, 20, 346–350.
- Radparvar, S. The Clinical Assessment and Treatment of Inhalant Abuse. Perm. J. 2023, 27, 99–109.
- Urban, B.W.; Bleckwenn, M. Concepts and correlations relevant to general anaesthesia. Br. J. Anaesth. 2002, 89, 3–16.
- Sanders, R.D.; Weimann, J.; Maze, M. Biologic effects of nitrous oxide: A mechanistic and toxicologic review. Anesthesiology 2008, 109, 707–722.
- Rosen, M.A. Nitrous oxide for relief of labor pain: A systematic review. Am. J. Obstet. Gynecol. 2002, 186, S110–S126.
- Sawamura, S.; Obara, M.; Takeda, K.; Maze, M.; Hanaoka, K. Corticotropin-releasing factor mediates the antinociceptive action of nitrous oxide in rats. Anesthesiology 2003, 99, 708–715.
- Hang, A.; Wang, Y.; He, L.; Liu, J. The role of the dynorphin/κ opioid receptor system in anxiety. Acta Pharmacol. Sin. 2015, 36, 783–790.
- Ohashi, Y.; Guo, T.; Orii, R.; Maze, M.; Fujinaga, M. Brain stem opioidergic and GABAergic neurons mediate the antinociceptive effect of nitrous oxide in Fischer rats. Anesthesiology 2003, 99, 947–954.
- Emmanouil, D.E.; Quock, R.M. Advances in understanding the actions of nitrous oxide. Anesth. Prog. 2007, 54, 9–18.
- Yamakura, T.; Harris, R.A. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology 2000, 93, 1095–1101.
- Nagele, P.; Duma, A.; Kopec, M.; Gebara, M.A.; Parsoei, A.; Walker, M.; Janski, A.; Panagopoulos, V.N.; Cristancho, P.; Miller, J.P.; et al. Nitrous Oxide for Treatment-Resistant Major Depression: A Proof-of-Concept Trial. Biol. Psychiatry 2015, 78, 10–18.
- Kalmoe, M.C.; Janski, A.M.; Zorumski, C.F.; Nagele, P.; Palanca, B.J.; Conway, C.R. Ketamine and nitrous oxide: The evolution of NMDA receptor antagonists as antidepressant agents. J. Neurol. Sci. 2020, 412, 116778.
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79.
- Rantamäki, T.; Yalcin, I. Depression and antidepressant action-from molecules to networks. Cell Tissue Res. 2019, 377, 1–4.
- Molloy, M.J.; Latto, I.P.; Rosen, M. Analysis of nitrous oxide concentrations in whole blood: An evaluation of an equilibration technique. Br. J. Anaesth. 1973, 45, 556–562.
- Salanitre, E.; Rackow, H.; Greene, L.T.; Klonymus, D.; Epstein, R.M. Uptake and excretion of subanesthetic concentrations of nitrous oxide in man. Anesthesiology 1962, 23, 814–822.
- Einarsson, S.; Stenqvist, O.; Bengtsson, A.; Houltz, E.; Bengtson, J.P. Nitrous oxide elimination and diffusion hypoxia during normo- and hypoventilation. Br. J. Anaesth. 1993, 71, 189–193.
- Poli, D.; Gagliano-Candela, R.; Strisciullo, G.; Colucci, A.P.; Strada, L.; Laviola, D.; Goldoni, M.; Mutti, A. Nitrous oxide determination in postmortem biological samples: A case of serial fatal poisoning in a public hospital. J. Forensic Sci. 2010, 55, 258–264.
- Heusler, H. Quantitative analysis of common anaesthetic agents. J. Chromatogr. 1985, 340, 273–319.
- Frasca, V.; Riazzi, B.S.; Matthews, R.G. In vitro inactivation of methionine synthase by nitrous oxide. J. Biol. Chem. 1986, 261, 15823–15826.
- Wrońska-Nofer, T.; Nofer, J.-R.; Jajte, J.; Dziubałtowska, E.; Szymczak, W.; Krajewski, W.; Wąsowicz, W.; Rydzyński, K. Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N2O). Mutat. Res. 2012, 731, 58–63.
- Robert, K.; Nehmé, J.; Bourdon, E.; Pivert, G.; Friguet, B.; Delcayre, C.; Delabar, J.-M.; Janel, N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology 2005, 128, 1405–1415.
- Grzych, G.; Deheul, S.; Gernez, E.; Davion, J.-B.; Dobbelaere, D.; Carton, L.; Kim, I.; Guichard, J.C.; Girot, M.; Humbert, L.; et al. Comparison of biomarker for diagnosis of nitrous oxide abuse: Challenge of cobalamin metabolic parameters, a retrospective study. J. Neurol. 2023, 270, 2237–2245.
- Frontiera, M.S.; Stabler, S.P.; Kolhouse, J.F.; Allen, R.H. Regulation of methionine metabolism: Effects of nitrous oxide and excess dietary methionine. J. Nutr. Biochem. 1994, 5, 28–38.
- Gernez, E.; Bennis, A.; Diesnis, R.; Niguet, J.P.; Grzych, G. Awareness of health care related to nitrous oxide abuse for diagnosis, treatment and follow-up. Ir. J. Med. Sci. 2023, 192, 383–388.
- Oussalah, A.; Julien, M.; Levy, J.; Hajjar, O.; Franczak, C.; Stephan, C.; Laugel, E.; Wandzel, M.; Filhine-Tresarrieu, P.; Green, R.; et al. Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis. J. Clin. Med. 2019, 8, 551.




