O administration is via inhalation utilizing a simple face mask, laryngeal mask airway or an endotracheal tube. In accordance with the European Society of Anesthesiology Task Force on Nitrous Oxide, N
O is used at lower concentrations (30 to 50% with oxygen) for sedation in surgical and dental procedures and up 70% for general anesthesia with associated unconsciousness and immobility [
]. N
O is the least potent inhalational anesthetic, as defined by the minimum alveolar concentration (MAC), which prevents a movement response (immobility) during a painful (e.g., surgical) stimulus. Currently, non-competitive inhibition of the NMDA receptors, specifically the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and kainite forms, is considered the main molecular target for N
]. These receptors comprise ligand-gated ion channels that are activated by glutamate.
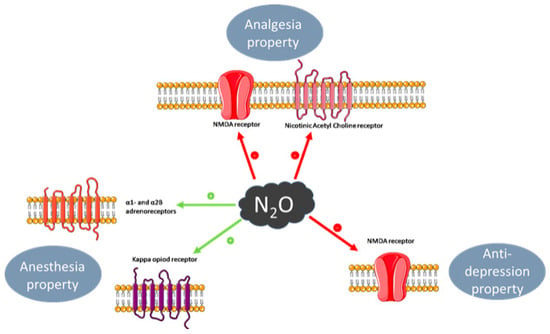
Analgesia is defined as insensibility to pain without loss of consciousness, and a property of general anesthesia [
43]. The analgesic and anti-nociceptive effect of N
2O involves the opioidergic system, by antagonism of the kappa opioid receptor, and the subsequent regulation of GABAergic and noradrenergic systems. In the periaqueductal grey (PAG) area of the midbrain, which is responsible for modulation of descending pain, blockade of these opioid receptors ablates nitrous-oxide-mediated analgesia, itself also partially reversed by the opioid receptor antagonist naloxone [
47]. Corticotrophin releasing factor from the hypothalamus is also released in response to N
2O [
48] and causes activation of opiodergic neurons in the PAG with release of endogenous opioids such as dynorphins, which also activate kappa opioid receptors [
49].
3.4. Anxiolytic Effect
Anxiolytics are used to prevent or treat anxiety symptoms or disorders and include the benzodiazepine class of drugs. The anxiolytic effect of N
2O involves activation of the gamma-aminobutyric acid type A (GABAA) receptor through its benzodiazepine binding site, though a direct effect is uncertain [
51,
52]. However, any such effect is considered minimal compared to the effect on NMDA receptors [
53].
3.5. Anti-depressant Effect
N
2O’s purported anti-depressant effect [
54], which is a comparatively more recent and ongoing area of exploration, is mediated through non-competitive inhibition of NMDA receptors, and is considered analogous to that of ketamine and similarly short-lived [
54,
55]. The latter property perhaps hinders its use clinically as an anti-depressant. Other purported molecular targets and effects include the regulation of Brain-derived neurotrophic factor (BDNF) which has a role in synaptic plasticity, synaptogenesis and neurogenesis [
56,
57], contributing to its anti-depressant effect, as opposed to neuronal atrophy and synaptic loss, as seen with stress and depression.
4. Laboratory Medicine
4.1. Direct N2O Measurement
N
2O may affect driving behavior and may cause fatal car accidents. As such, detection is an important issue. N
2O has a very short half-life of a few minutes [
58], as the uptake and elimination curves are comparable [
59]. N
2O elimination is mainly pulmonary. When exposure ends, exhaled air concentration declines rapidly, from 66–70% to 6–9% at 5 min and to 2–4% at 30 min during normoventilation. The elimination is slower in cases of hypoventilation [
60]. Thus, the measurement of N
2O in exhaled air is not routinely usable for patients presenting to the emergency department due to the time gap between consumption and admission. The issue is the same for toxicology screening: indeed, for police roadside controls, this appears to be difficult due to the time gap between arrest and sample collection.
Additionally, there are technical difficulties concerning N
2O measurement in biological fluids. First, gas chromatography–mass spectrometry (GC-MS) can be used, but has limitations, including the challenge of finding an optimal internal standard, the lack of sensitivity and the potential risk of leaks during sampling, extraction and analysis. Headspace-GC-MS, which is a method in which the sample is placed in a hermetically-sealed, gas-tight container, could be promising but need further studies to be used in laboratory medicine [
61]. Infrared Spectroscopy techniques are sensitive methods to measure N
2O in air, but not on biological matrices [
62].
4.2. Impact on Metabolism
4.2.1. Cobalamin and One Carbon Metabolism
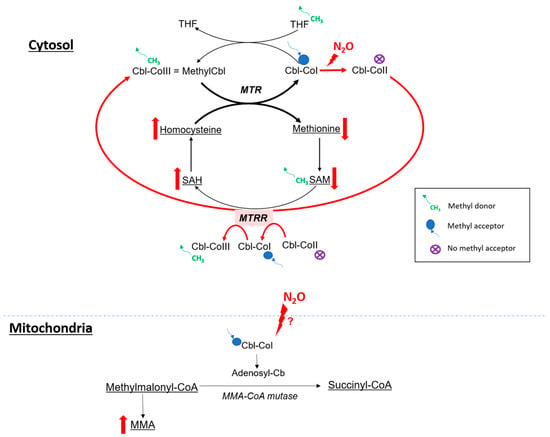
The clinical presentation of N
2O intoxication is related to the functional impairment of vitamin B12, also called cobalamin (
Figure 3). Indeed, N
2O is a powerful oxidant agent: it leads to the oxidation of the cobalt ion of cobalamin(I) [
63], resulting in the formation of cobalamin(II), unable to accept methyl groups. This results in a decrease in the formation of methylcobalamin, which is a cofactor for methionine synthase (MS or MTR).
Figure 3. Hypothetic impact of N
2O on metabolism. SAH: S-adenosyl-homocysteine, SAM: S-adenosyl-methionine, MTR: methionine synthase, MTRR: methionine synthase reductase, THF: tetrahydrofolate, MMA: methylmalonic acid. Red arrow for major pathway in case of cobalamin oxidation.
4.2.2. N2O and Oxidative Stress
N
2O has powerful oxidant properties. A study was conducted on 36 nurses occupationally exposed to anesthetics including N
2O during surgical procedures. Biological assessments revealed an increase in oxidative stress markers, including thiobarbituric acid-reactive substances (TBARS) and F2 isoprostanes. There was also a significant decrease in the activity of the antioxidant enzyme glutathione peroxidase (GPX), and an increase in the levels of reactive oxygen species (ROS) in peripheral blood leukocytes [
71].
4.2.3. Homocysteine and Oxidative Stress
Hyperhomocysteinemia also enhances oxidative stress. In a study conducted on CBS (cystathionine beta-synthase) deficient mice [
74], an inherited metabolic disease inducing severe hyperhomocysteinemia, several indicators of oxidative stress were notably increased. Lipid peroxidation markers, such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE), were elevated, as well as protein-associated carbonyl groups, indicating protein oxidation.
4.3. Indirect Biomarkers of N2O Intoxication
4.3.1. Vitamin B12
As N
2O leads to a functional vitamin B12 deficiency, the quantitative deficiency in vitamin B12 is secondary and inconsistent [
14]. Patients are also frequently supplemented with vitamin B12; increased levels of vitamin B12 can also be found in intoxicated patients. Consequently, it seems more pertinent to investigate functional markers of vitamin B12 in cases of N
2O intoxication, which are plasma MMA and plasma homocysteine.
4.3.2. Plasma Homocysteine
Plasma homocysteine is highly sensitive and can be used as a marker of recent N
2O consumption [
14] because it rapidly increases in case of consumption. However, homocysteine levels decrease rapidly and can return to physiological values within several days after the last N
2O consumption [
76]. This biomarker is also not specific to N
2O intoxication: plasma homocysteine increases in case of vitamin deficiency (vitamin B6, vitamin B9, vitamin B12), renal or hepatic injury, hypothyroidism and in certain metabolic diseases.
4.3.3. Plasma MMA
Plasma MMA is more specific than homocysteine in the exploration of vitamin B12 status because it does not depend of vitamin B6 and B9 status; but rise in case of renal insufficiency and in certain metabolic diseases. However, it is not a sensitive marker of N
2O abuse as its elevation is not consistent. Plasma MMA is correlated to the clinical severity [
14]; thus, it can be used as a marker of clinical severity of N
2O intoxication.
4.3.4. Plasma Methionine
Methionine has also been investigated as a potential biomarker in nitrous oxide intoxication. Indeed, the decrease in MS activity could lead to a decrease in production of methionine, which is involved in the formation of myelin.
4.3.5. Oxidative Stress Markers
Oxidative stress markers could be of interest in N2O intoxication. However, no studies have been conducted on patients with a recreational use but only on occupational exposure. Therefore, additional investigations are needed to determine whether these markers may be of interest as a consumption marker or as a marker of clinical severity.
4.3.6. Others Biological Parameters to Consider
Some biological parameters are crucial for the differential diagnosis of hyperhomocysteinemia, as this parameter is not specific of N
2O intoxication. Thus, renal (creatinine) and hepatic exploration (AST, ALT, alkaline phosphatase, GGT) should be performed, as well as vitamin assessment (vitamin B6, vitamin B9), to explore nutritional deficiencies [
77]. Cell blood count can be performed to investigate a potential anemia, although N
2O does not appear to cause macrocytic anemia [
23].
5. Conclusions
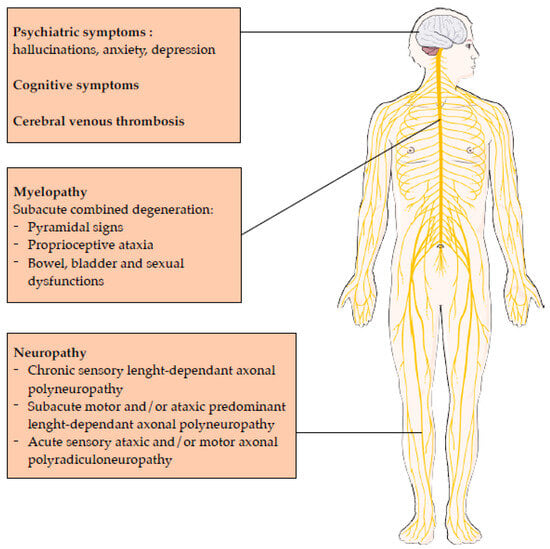
The recreational use of N2O has seen a significant increase in recent years, leading to a growing concern about its acute and chronic toxicity. There is a wide range of chronic manifestations including myelopathy, neuropathy, psychiatric manifestations, cognitive symptoms and cardiovascular effects. N2O interacts with neurotransmitter systems, leading to anesthetic, analgesic, anxiolytic and potential anti-depressant effects, with a potential dependance. Laboratory medicine plays a critical role in assessing N2O intoxication, with biomarkers such as plasma homocysteine, a marker of recent consumption, and plasma MMA, a marker of clinical gravity. Other biomarkers, like oxidative stress markers, could be interesting but need further investigations.