| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mardiah Mardiah | -- | 1964 | 2023-11-23 06:44:45 | | | |
| 2 | Wendy Huang | Meta information modification | 1964 | 2023-11-23 12:41:58 | | |
Video Upload Options
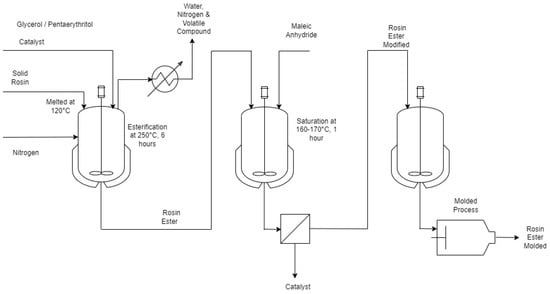
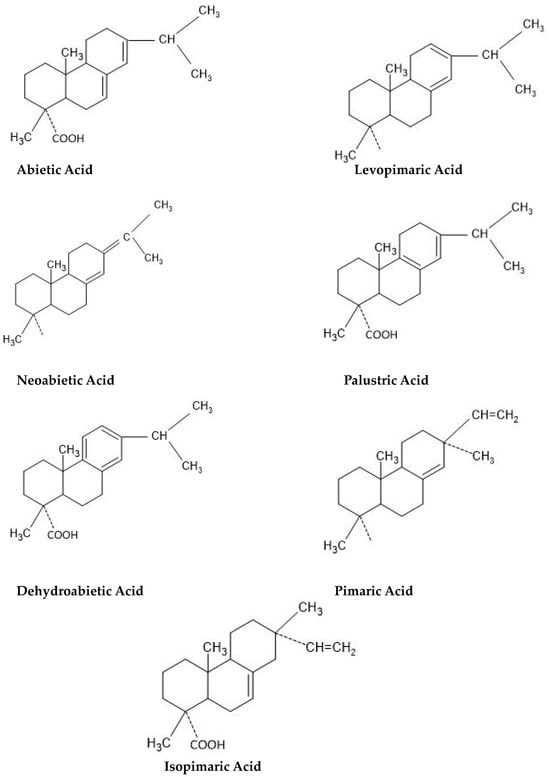
Rosin is a compound that originates from pine trees. It is obtained after the volatile component (turpentine) has been distilled. Rosin is translucent and its color ranges from brilliant yellow to brown. The substance exhibits insolubility in water but is soluble in a variety of organic solvents including alcohol, ether, acetone, benzene, chloroform, turpentine, and others. Gum rosin is an important agricultural commodity which is widely used as a raw material for various industries. However, gum rosin has low stability, crystallizes easily, and tends to oxidize. This is due to carboxyl groups and conjugated double bonds in gum rosin’s structure. Therefore, to reduce these weaknesses, it is necessary to modify the rosin compound to achieve better stability via the esterification process.
1. Introduction
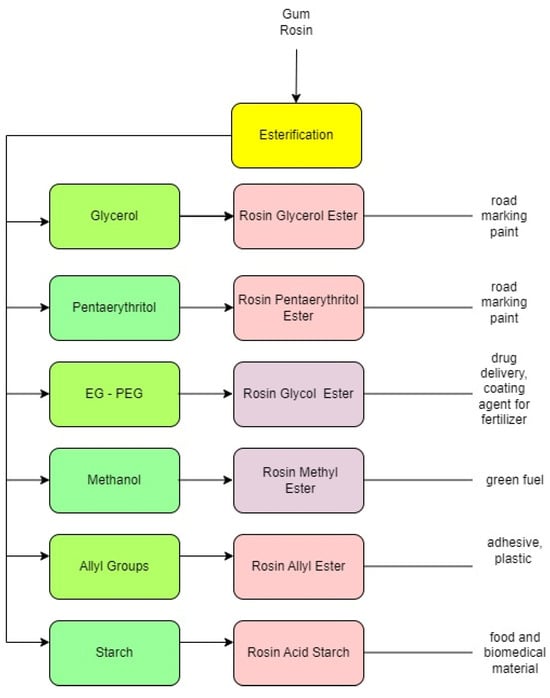
2. Rosin-Glycerol
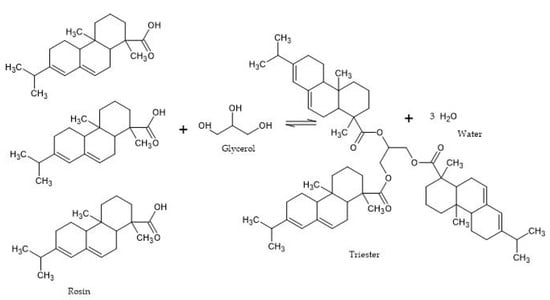
| Product | Reaction Time (h) | Temperature (°C) | Molar Ratio (Rosin/Glycerol) | Catalyst | Acid Value (mg KOH g−1) | Conversion Rate (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Rosin glyceride | 3.5 | 269 | 1.32 | No catalyst | 66.54 | 58.58 | [31] |
| 3 | 240 | 11% (wt) | Ni/Zeolite | 33.94 | 82.86% | [13] | |
| 1 | 260 | 1.5 (mass ratio) |
ZSM-5 | 69.72 | 58.99 | [20] | |
| 2 | 38.58 | 77.31 | |||||
| 4 | 20.90 | 87.71 | |||||
| 6 | 13.05 | 92.32 | |||||
| 8 | 11.08 | 93,48 | |||||
| 10 | 10.66 | 93.73 | |||||
| 1 | 260 | 1.5 (mass ratio) |
LaZSM-5 | 72.67 | 65.25 | ||
| 2 | 50.63 | 82.22 | |||||
| 4 | 22.62 | 90.69 | |||||
| 6 | 19.12 | 93.75 | |||||
| 8 | 16.83 | 95.10 | |||||
| 10 | 15.15 | 98.09 | |||||
| 2.5 | 240 | 2 | Fe3O4/MOF-5 | 92.6 | [21] | ||
| 3.5 | 269 | 1.32 | ZnO | 10.23 | 93.63 | [31] | |
| 3.5 | 269 | 1.32 | CO2 pressure of 3.95 MPa | 8.45 | 94.74 |
3. Conclusions
Rosin with its carboxyl group and conjugate double bonds has an important role in the esterification process. By using reactants or ester agents such as glycerol, specific rosin ester will be produced. The challenge in esterification is how to reduce the acid value or increase the rosin ester conversion, such as using heterogeneous catalysts. One of the advantages is that it can be separated. However, if the catalyst is dispersed during the reaction, it is not easy to separate. Meanwhile, the use of heterogeneous catalysts is a prospective way to increase the conversion of the reaction and reduce the acid value of the rosin. Rosin is a source of raw materials that are renewable and may show potential in the future.
References
- Zhang, X.; Ma, H.; Qin, W.; Guo, B.; Li, P. Antimicrobial and improved performance of biodegradable thermoplastic starch by using natural rosin to replace part of glycerol. Ind. Crops Prod. 2022, 178, 114613.
- Aqsha, A.; Winoto, H.P.; Adhi, T.P.; Adisasmito, S.; Ramli, Y.; Siddiq, L.; Pratama, F.B.; Ramdani, M.R.; Indarto, A. Sequential Esterification—Diels-Alder Reactions for Improving Pine Rosin Durability within Road Marking Paint. Molecules 2023, 28, 5236.
- Prakoso, T.; Hanley, J.; Soebianta, M.N.; Soerawidjaja, T.H.; Indarto, A. Synthesis of Terpineol from α-Pinene Using Low-Price Acid Catalyst. Catal. Lett. 2018, 148, 725.
- Maiti, S.; Ray, S.S.; Kundu, A.K. Rosin: A renewable resource for polymers and polymer chemicals. Prog. Polym. Sci. 1989, 14, 297–338.
- Silvestre, A.J.; Gandini, A. Rosin: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 67–88.
- Saat, A.M.; Yaakup, S.; Alaauldin, S.; Zawani, F.; Azaim, Z.; Isa, M.D.M.; Kamil, M.S.; Samsudin, S.; Iqbal, M. Performance of Rosin Modified Antifouling Coated on Mild Steel Surface at Various Immersion Orientation. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 5562–5565.
- Lee, S.; Lee, K.; Kim, Y.-W.; Shin, J. Preparation and characterization of a renewable pressure-sensitive adhesive system derived from ε-decalactone, l-lactide, epoxidized soybean oil, and rosin ester. ACS Sustain. Chem. Eng. 2015, 3, 2309–2320.
- Kim, J.H.; Min, H.J.; Park, K.; Kim, J. Preparation and evaluation of a cosmetic adhesive containing guar gum. Korean J. Chem. Eng. 2017, 34, 2236–2240.
- Li, W.; Yu-Yu, E.; Cheng, L.-Y.; Ding, M.; Li, W.-Y.; Diao, K.-S.; Liu, S.-G.; Li, K.; Lu, H.-Q.; Lei, F.-H. Rosin-based polymer@ silica core–shell adsorbent: Preparation, characterization, and application to melanoidin adsorption. LWT 2020, 132, 109937.
- Kumar, S.; Gupta, S.K. Rosin: A naturally derived excipient in drug delivery systems. Polim. Med. 2013, 43, 45–48.
- Phaphon, K.; Wacharasindhu, S.; Petsom, A. Preparation of PEG-rosin derivative for water soluble rosin flux. Solder. Surf. Mt. Technol. 2016, 28, 188–200.
- Mirabedini, S.; Zareanshahraki, F.; Mannari, V. Enhancing thermoplastic road-marking paints performance using sustainable rosin ester. Prog. Org. Coat. 2020, 139, 105454.
- Dewajani, H.; Chumaidi, A.; Iswara, M.A.I.; Khasanah, R.; Agustina, T.D. Synthesis ester gum through esterification reaction of rosin and gliserol using zeolite modified by nickel as catalyst. AIP Conf. Proc. 2019, 2097, 030037.
- Prakoso, T.; Kumalasari, I.; Jiwandaru, B.; Soerawidjaja, T.H.; Azis, M.M.; Indarto, A. Synthesis of Maleic-Modified Rosin Ester from Pine Rosin. In IOP Conference Series: Materials Science and Engineering, Volume 1143, International Seminar on Chemical Engineering Soehadi Reksowardojo (STKSR 2020), Bandung, Indonesia, 16–17 November 2020; IOP Publishing Ltd.: Bristol, UK, 2021; p. 012071.
- Li, Q.; Gong, S.; Yan, J.; Hu, H.; Shu, X.; Tong, H.; Cai, Z. Synthesis and kinetics of hydrogenated rosin dodecyl ester as an environmentally friendly plasticizer. J. Renew. Mater. 2020, 8, 289.
- Liu, S.; Xie, C.; Yu, S.; Liu, F. Dimerization of rosin using Brønsted–Lewis acidic ionic liquid as catalyst. Catal. Commun. 2008, 9, 2030–2034.
- Wang, L.; Chen, X.; Liang, J.; Chen, Y.; Pu, X.; Tong, Z. Kinetics of the catalytic isomerization and disproportionation of rosin over carbon-supported palladium. Chem. Eng. J. 2009, 152, 242–250.
- Lu, Y.J.; Xu, R.S.; Zhao, Z.D.; Zhang, P.H.; Wang, M.X. Recent progress on derivation and chemical modification of rosin acids. Adv. Mater. Res. 2013, 785, 1111–1116.
- Hardhianti, M.P.W.; Rochmadi; Azis, M.M. Kinetic studies of esterification of rosin and pentaerythritol. Processes 2021, 10, 39.
- Wang, X.; Guo, F.; Yu, Y.; Liu, Z.; Wang, Y.; Sun, H.; Liu, X.; Xue, Y.; Wei, X.; Guo, S. Study on the Synthesized Rosin Glyceride over LaZSM-5 Zeolite Catalyst Synthesized by the in Situ Method. ACS Omega 2020, 5, 31543–31550.
- Zhou, D.; Wang, L.; Chen, X.; Wei, X.; Liang, J.; Tang, R.; Xu, Y. Reaction mechanism investigation on the esterification of rosin with glycerol over annealed Fe3O4/MOF-5 via kinetics and TGA-FTIR analysis. Chem. Eng. J. 2020, 401, 126024.
- García, D.F.; Bustamante, F.; Villa, A.L.; Alarcón, E.A. Esterification of rosin with methyl alcohol for fuel applications. Rev. Fac. Ing. Univ. Antioq. 2021, 100, 10–20.
- Morkhade, D.M.; Nande, V.S.; Barabde, U.V.; Kamble, M.U.; Patil, A.T.; Joshi, S.B. A comparative study of aqueous and organic-based films and coatings of PEGylated rosin derivative. Drug Dev. Ind. Pharm. 2008, 34, 24–32.
- Lu, Y.; Zhao, Z.; Chen, Y.; Wang, J.; Xu, S.; Gu, Y. Synthesis of allyl acrylpimarate by microwave irradiation and phase-transfer catalytic reaction and its UV-curing reactions as a new monomer. Prog. Org. Coat. 2017, 109, 9–21.
- Lin, R.; Li, H.; Long, H.; Su, J.; Huang, W. Structure and characteristics of lipase-catalyzed rosin acid starch. Food Hydrocoll. 2015, 43, 352–359.
- Ladero, M.; de Gracia, M.; Tamayo, J.J.; de Ahumada, I.L.; Trujillo, F.; Garcia-Ochoa, F. Kinetic modelling of the esterification of rosin and glycerol: Application to industrial operation. Chem. Eng. J. 2011, 169, 319–328.
- Ladero, M.; de Gracia, M.; Trujillo, F.; Garcia-Ochoa, F. Phenomenological kinetic modelling of the esterification of rosin and polyols. Chem. Eng. J. 2012, 197, 387–397.
- Quispe, C.A.; Coronado, C.J.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493.
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223.
- Sun, S.; Cheng, X.; Ma, M.; Liu, Y.; Wang, G.; Yu, H.; Liu, S.; Yu, S. High-efficient esterification of rosin and glycerol catalyzed by novel rare earth Lewis acidic ionic liquid: Reaction development and mechanistic study. J. Taiwan Inst. Chem. Eng. 2021, 127, 1–6.
- Zhou, D.; Wang, L.; Chen, X.; Wei, X.; Liang, J.; Zhang, D.; Ding, G. A novel acid catalyst based on super/subcritical CO2-enriched water for the efficient esterification of rosin. R. Soc. Open Sci. 2018, 5, 171031.
- Li, Y.; Yu, S.; Zhang, H.; Zhang, J. Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester. Open Chem. 2021, 19, 938–944.