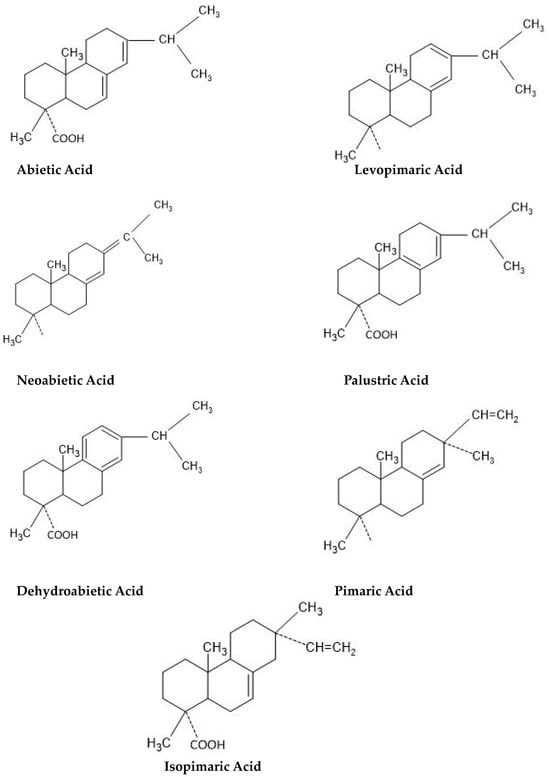
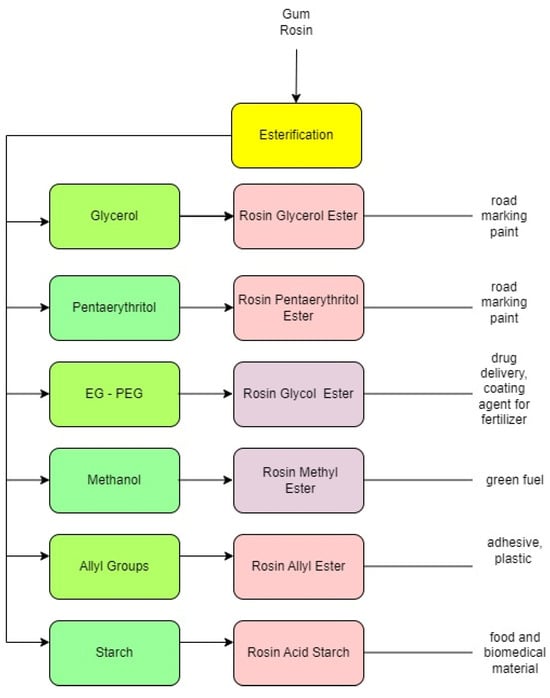
2. Rosin-Glycerol
Glycerol esterification with carboxylic acids from rosin (mostly abietic acid) is a common reaction in industry (for up to 80–90 years) [
27]. Rosin glyceride is widely employed as one of the most important rosin modification products. Rosin glyceride, also known as gum ester, is formed when rosin interacts with glycerol. The higher the quality of gum esters, the lighter the color. Gum esters have a refractive index of 1.545, a relative density of 1.095, an acid value of less than 10 mg KOH g
−1, and a softening point over 80 °C. Gum esters are soluble in aliphatic and aromatic hydrocarbon solvents, as well as terpenes, esters, hydrocarbons, ketones, and the majority of essential oils, but are insoluble in water and alcohols of low molecular weight [
21].
Glycerol, also known as 1,2,3-propanetriol, is a very viscous and dense polyalcohol with strong hygroscopic properties. It finds many uses in industries such as cosmetics, food, pharmaceuticals, and chemicals [
28]. This product is obtained as a by-product of biodiesel manufacturing [
29]. Biodiesel production is still in its infancy worldwide. Meanwhile, market prices for glycerol are declining rapidly and are over-available [
30]. Its utilization is expected to increase as glycerol-based processes.
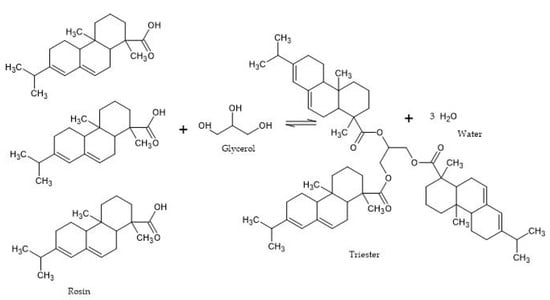
The following is the reaction mechanism between rosin and glycerol (Figure 4). Glycerol, which has three OH groups, allows it to react with an acid group of rosin to form three ester groups and triggers a process of dehydration/release of water molecules. The most common method used to produce esters is the reaction of a carboxylic acid with an alcohol with the release of water. Since the presence of water in the reacting mixture tends to shift the equilibrium away from rosin ester, water was continuously removed during the reaction.
Figure 4. Mechanism of rosin reaction with glycerol (processed from [
22]).
The esterification reaction can be carried out with or without a catalyst, but the conversion will be low if done without a catalyst (Table 1). The expected rosin ester with a low acid value is obtained by heating the rosin with glycerol at a temperature of 250–290 °C.
However, the use of liquid catalysts such as H
2SO
4 and H
3PO
4 in the production of rosin glycerides causes corrosion, is difficult to separate and is always environmentally harmful [
22,
31]. Due to the widespread use of rosin ester products in manufacturing, such as in food, medicine, printing inks and pressure-sensitive adhesives, the esterification of rosin with alcohol on heterogeneous catalysts or solid catalysts is an important reaction for industry.
Previously, hydrothermal methods were employed for the synthesis of ZSM-5. Zeolite catalytic performance was examined using rosin and glycerol as reagents for esterification. The ZSM-5 zeolites that were created had a larger specific surface area and mesoporous volume than commercial ZSM-5 zeolites, indicating a faster esterification, lower product acid values, and increased stability. The obtained esterification percentage was 93.73% [
21].
Zeolite is utilized as a catalyst in several processes because of its high activity and selectivity. This is attributed to its excellent ion exchange performance, consistent pore structure, acidity, and great temperature persistence. Zeolite ZSM-5 is a catalyst that may be used for rosin and glycerol esterification processes since it its various advantages include not being corrosive to equipment, high temperature tolerance, simplicity of manufacture, high activity, and the capacity to be reused. Furthermore, solid granules of zeolite may be easily isolated from the reaction mixture [
21].
Several researchers modified natural zeolite with nickel metal to boost catalytic activity. The highest conversion of 82.86% was achieved after 3 h at 240 °C and 11% ratio [
14]. La metal also added to the ZSM-5 zeolite [
21]. Several catalysts affect rosin esterification severity, as seen in
Table 1.
Table 1. Impact of various catalysts on the esterification process of rosin-glycerol.
| Product |
Reaction Time (h) |
Temperature (°C) |
Molar Ratio (Rosin/Glycerol) |
Catalyst |
Acid Value (mg KOH g−1) |
Conversion Rate (%) |
Ref. |
| Rosin glyceride |
3.5 |
269 |
1.32 |
No catalyst |
66.54 |
58.58 |
[32] |
| 3 |
240 |
11% (wt) |
Ni/Zeolite |
33.94 |
82.86% |
[14] |
| 1 |
260 |
1.5
(mass ratio) |
ZSM-5 |
69.72 |
58.99 |
[21] |
| 2 |
38.58 |
77.31 |
| 4 |
20.90 |
87.71 |
| 6 |
13.05 |
92.32 |
| 8 |
11.08 |
93,48 |
| 10 |
10.66 |
93.73 |
| 1 |
260 |
1.5
(mass ratio) |
LaZSM-5 |
72.67 |
65.25 |
| 2 |
50.63 |
82.22 |
| 4 |
22.62 |
90.69 |
| 6 |
19.12 |
93.75 |
| 8 |
16.83 |
95.10 |
| 10 |
15.15 |
98.09 |
| 2.5 |
240 |
2 |
Fe3O4/MOF-5 |
|
92.6 |
[22] |
| 3.5 |
269 |
1.32 |
ZnO |
10.23 |
93.63 |
[32] |
| 3.5 |
269 |
1.32 |
CO2 pressure of 3.95 MPa |
8.45 |
94.74 |
La-ZSM-5 has a greater acid value and esterification rate than ZSM-5, as demonstrated in
Table 1. Catalyzing the liquid phase esterification reaction requires acidification of the catalyst surface. Both the core of Bronsted acid and the center of Lewis acid can undergo the esterification reaction. The esterification reaction, on the other hand, is dependent on the Lewis acid center. Meanwhile, reaction byproducts such as ether and olefins are primarily generated in the Bronsted acid center. Based on existing catalyst characterization, La-ZSM-5 exhibits a higher concentration of Lewis acid sites compared to ZSM-5. As a result, La-ZSM-5 has higher esterification catalysis activities compared to ZSM-5 [
21].
The esterification reaction mechanism of rosin by glycerol using annealed Fe
3O
4/MOF-5 at temperatures ranging from ambient temperature to 900 °C was also examined [
22].
Table 1 summarizes the impact of the main response parameters. In general, room temperature annealing provided the best conditions for rosin esterification utilizing Fe
3O
4/MOF-5. The development of super/subcritical CO
2 and the presence of high temperature liquid water are investigated as environmentally friendly acid catalysts in the esterification process between rosin and glycerol [
32]. Experiments with response surface methods revealed that CO
2 pressure affects the yield, which is higher than that produced with a ZnO catalyst. However, the difference is barely 1%. Zinc oxide is also a common catalyst in the rosin esterification reaction. The abundance of oxygen vacancies on the surface of ZnO can boost the catalyst’s Lewis acidity and catalytic activity [
33].
Based on the above experiments that have been discussed and shown in Table 1, heterogeneous catalysts ZSM-5 modified by La metal achieve the highest conversions (up to 98%) but still require a higher reaction time.
Conclusions
Rosin with its carboxyl group and conjugate double bonds has an important role in the esterification process. By using reactants or ester agents such as glycerol, specific rosin ester will be produced. The challenge in esterification is how to reduce the acid value or increase the rosin ester conversion, such as using heterogeneous catalysts. One of the advantages is that it can be separated. However, if the catalyst is dispersed during the reaction, it is not easy to separate. Meanwhile, the use of heterogeneous catalysts is a prospective way to increase the conversion of the reaction and reduce the acid value of the rosin. Rosin is a source of raw materials that are renewable and may show potential in the future.