Rosin is a compound that originates from pine trees. It is obtained after the volatile component (turpentine) has been distilled. Rosin is translucent and its color ranges from brilliant yellow to brown. The substance exhibits insolubility in water but is soluble in a variety of organic solvents including alcohol, ether, acetone, benzene, chloroform, turpentine, and others. Gum rosin is an important agricultural commodity which is widely used as a raw material for various industries. However, gum rosin has low stability, crystallizes easily, and tends to oxidize. This is due to carboxyl groups and conjugated double bonds in gum rosin’s structure. Therefore, to reduce these weaknesses, it is necessary to modify the rosin compound to achieve better stability via the esterification process.
1. Introduction
The production of sustainable materials is a highly significant problem that future generations must address. The supply of fossil fuels for energy production and plastic manufacturing is limited. It will be reduced during the next century. Environmental concerns combined with the decrease of oil reserves have sparked a surge in interest in developing green materials generated from renewable natural resources. Rosin, also known as colophony, is a natural substance obtained from pine trees that is biodegradable and abundant with a wide range of applications
[1]. Indonesia, China, and Brazil are the main producers of pine sap, which is used to make turpentine (resin) and gum rosin
[2]. Indonesia had a total pine-based production capacity of 141.150 tons in 2022
[3].
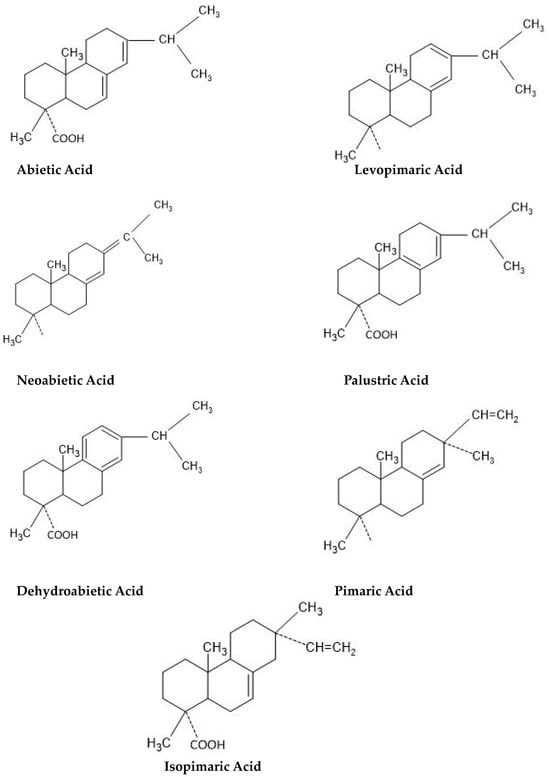
Rosin comprises a complex mixture of organic acids with large molecular weights and neutral materials. Rosin acids are monocarboxylic acids derived from alkylated hydrophenanthrene nuclei that form most of the rosin. Rosin has small parts that are not acidic or neutral. These are made up of high molecular weight aldehydes, alcohols, esters, and hydrocarbons that have structures like the rosin acids. They may differ in relative proportions depending on the origin of the rosin and the degree to which it has been processed. Rosin is composed of 10% neutral and 90% acidic elements. Rosin has the chemical reactivity of a monocarboxylic acid in general. The structures of the rosin acids are shown in
Figure 1 [4][5].
Figure 1. Structure of rosin acids [4]. Structure of rosin acids [5].
Rosin is classified into numerous categories based on the portion of the tree from which it is derived, namely gum rosin, tall oil rosin, and wood rosin. Gum rosin is obtained from living pine trees, while tall oil rosin is produced as a by-product of paper pulp. Meanwhile, wood rosin is recovered from tree stumps
[5][6]. Rosin has a variety of uses, including for antifouling
[6][7], adhesives
[7][8], cosmetics
[8][9], and adsorbent
[9][10]. Because of their biocompatibility, rosin and its by-products are being investigated in the pharmaceutical industry as an encapsulating agent for drug delivery
[10][11]. Gum rosin is also used in solder flux
[11][12] and road-marking paint
[2][12][2,13].
Aside from the numerous advantages of rosin, the current issue is the high content of abietic acid in rosin, which causes a low softening point, has a brittle property, and leads to a dark appearance. The typical abietic acid content of gum rosin in Indonesia is 30–40%. The amount of abietic acid in gum rosin affects its quality, as it causes rosin to crystallize quickly when it is cooled from its liquid form. Crystallization causes problems in rosin production and industries that use rosin, and as such, abietic acid must be minimized to produce higher quality rosin by changing the acid to a modified form that keeps the rosin stable
[13][14].
The structure of rosin has a carboxyl group and a conjugated double bond, and those play a big role in its reactivity. As a result, rosin is chemically modified to improve its stability and useful characteristics. Esterification-maleation
[14][15], hydrogenation
[15][16], dimerization
[16][17], isomerization, and disproportionation
[17][18] are all strategies for improving the oxidative stability of rosin
[18][19].
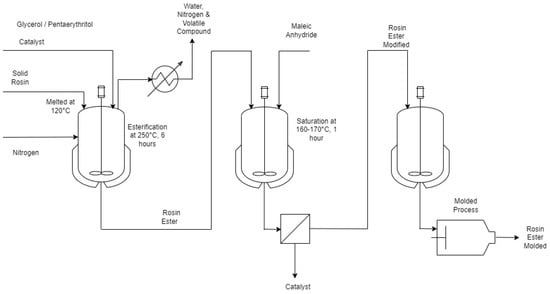
Esterification is a typical modification procedure commonly used by industry and is a simple process (
Figure 2). Rosin esters are formed when rosin is esterified with an alcohol or polyol. Alcohols and polyols such as glycerol, pentaerythritol, etyhlene glycol are widely used for industrial application, while PEG, methanol, allyl groups, and starch are also being continuously improved. Esterification can reduce the acid value of rosin, raise its thermal stability, and improve its resistance to both acids and alkalis. As a result, the raw material’s properties allow it to be employed in more applications
[19][20].
Figure 2. Flowsheet of esterification of gum rosin (processed from [2][20][21]). Flowsheet of esterification of gum rosin (processed from [2,21,22]).
The esterification process is marked by a decrease in the acid value of rosin, which enhances esterification conversion. Since rosin contains abietic acid, it will change to rosin ester when it is esterified. The rosin ester will confirm by Fourier transform infrared spectroscopy. Stretching O–H carboxylic acids is at a frequency of 3600–2500 cm
−1 and C=O, which is an ester group, is at a frequency of 1750–1720 cm
−1.
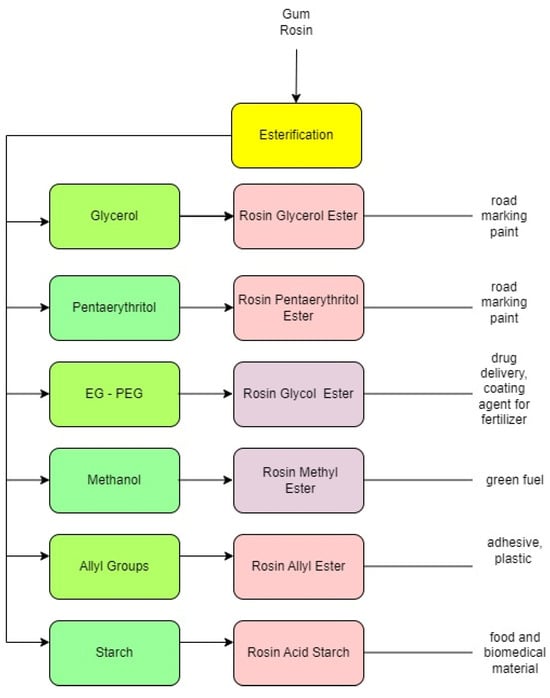
The rosin ester product depends on the type of reactant used (
Figure 3). For example, if methanol is used as a reactant, the rosin ester can take the form of a methyl ester, which has fuel-like properties
[22][23]. If glycerol and pentaerythritol are used as a reactant, a solid rosin ester will be formed with a high softening point that could exceed 100 °C. This product is suitable for raw material of road marking paint
[2][19][2,20]. When PEG is used in the reaction, the rosin ester will dissolve in water and this product can be used as drug delivery
[23][24]. Esterification with an allyl group can enhance properties of biomass-based polymer material. The ester can be used as a coating or a glue adhesion agent
[24][25]. The product using starch can be used as food packaging
[25][26].
Figure 3.
Agent of reactant in esterification.
2. Rosin-Glycerol
Glycerol esterification with carboxylic acids from rosin (mostly abietic acid) is a common reaction in industry (for up to 80–90 years)
[26][27]. Rosin glyceride is widely employed as one of the most important rosin modification products. Rosin glyceride, also known as gum ester, is formed when rosin interacts with glycerol. The higher the quality of gum esters, the lighter the color. Gum esters have a refractive index of 1.545, a relative density of 1.095, an acid value of less than 10 mg KOH g
−1, and a softening point over 80 °C. Gum esters are soluble in aliphatic and aromatic hydrocarbon solvents, as well as terpenes, esters, hydrocarbons, ketones, and the majority of essential oils, but are insoluble in water and alcohols of low molecular weight
[20][21].
Glycerol, also known as 1,2,3-propanetriol, is a very viscous and dense polyalcohol with strong hygroscopic properties. It finds many uses in industries such as cosmetics, food, pharmaceuticals, and chemicals
[27][28]. This product is obtained as a by-product of biodiesel manufacturing
[28][29]. Biodiesel production is still in its infancy worldwide. Meanwhile, market prices for glycerol are declining rapidly and are over-available
[29][30]. Its utilization is expected to increase as glycerol-based processes.
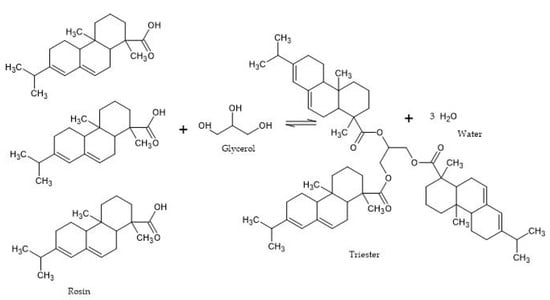
The following is the reaction mechanism between rosin and glycerol (
Figure 4). Glycerol, which has three OH groups, allows it to react with an acid group of rosin to form three ester groups and triggers a process of dehydration/release of water molecules. The most common method used to produce esters is the reaction of a carboxylic acid with an alcohol with the release of water. Since the presence of water in the reacting mixture tends to shift the equilibrium away from rosin ester, water was continuously removed during the reaction.
Figure 4. Mechanism of rosin reaction with glycerol (processed from [21]). Mechanism of rosin reaction with glycerol (processed from [22]).
The esterification reaction can be carried out with or without a catalyst, but the conversion will be low if done without a catalyst (
Table 1). The expected rosin ester with a low acid value is obtained by heating the rosin with glycerol at a temperature of 250–290 °C.
However, the use of liquid catalysts such as H
2SO
4 and H
3PO
4 in the production of rosin glycerides causes corrosion, is difficult to separate and is always environmentally harmful
[21][30][22,31]. Due to the widespread use of rosin ester products in manufacturing, such as in food, medicine, printing inks and pressure-sensitive adhesives, the esterification of rosin with alcohol on heterogeneous catalysts or solid catalysts is an important reaction for industry.
Previously, hydrothermal methods were employed for the synthesis of ZSM-5. Zeolite catalytic performance was examined using rosin and glycerol as reagents for esterification. The ZSM-5 zeolites that were created had a larger specific surface area and mesoporous volume than commercial ZSM-5 zeolites, indicating a faster esterification, lower product acid values, and increased stability. The obtained esterification percentage was 93.73%
[20][21].
Zeolite is utilized as a catalyst in several processes because of its high activity and selectivity. This is attributed to its excellent ion exchange performance, consistent pore structure, acidity, and great temperature persistence. Zeolite ZSM-5 is a catalyst that may be used for rosin and glycerol esterification processes since it its various advantages include not being corrosive to equipment, high temperature tolerance, simplicity of manufacture, high activity, and the capacity to be reused. Furthermore, solid granules of zeolite may be easily isolated from the reaction mixture
[20][21].
Several researchers modified natural zeolite with nickel metal to boost catalytic activity. The highest conversion of 82.86% was achieved after 3 h at 240 °C and 11% ratio
[13][14]. La metal also added to the ZSM-5 zeolite
[20][21]. Several catalysts affect rosin esterification severity, as seen in
Table 1.
Table 1.
Impact of various catalysts on the esterification process of rosin-glycerol.
| Product |
Reaction Time (h) |
Temperature (°C) |
Molar Ratio (Rosin/Glycerol) |
Catalyst |
Acid Value (mg KOH g−1) |
Conversion Rate (%) |
Ref. |
| Rosin glyceride |
3.5 |
269 |
1.32 |
No catalyst |
66.54 |
58.58 |
[31][32] |
| 3 |
240 |
11% (wt) |
Ni/Zeolite |
33.94 |
82.86% |
[13][14] |
| 1 |
260 |
1.5
(mass ratio) |
ZSM-5 |
69.72 |
58.99 |
[20][21] |
| 2 |
38.58 |
77.31 |
| 4 |
20.90 |
87.71 |
| 6 |
13.05 |
92.32 |
| 8 |
11.08 |
93,48 |
| 10 |
10.66 |
93.73 |
| 1 |
260 |
1.5
(mass ratio) |
LaZSM-5 |
72.67 |
65.25 |
| 2 |
50.63 |
82.22 |
| 4 |
22.62 |
90.69 |
| 6 |
19.12 |
93.75 |
| 8 |
16.83 |
95.10 |
| 10 |
15.15 |
98.09 |
| 2.5 |
240 |
2 |
Fe3O4/MOF-5 |
|
92.6 |
[21][22] |
| 3.5 |
269 |
1.32 |
ZnO |
10.23 |
93.63 |
[31][32] |
| 3.5 |
269 |
1.32 |
CO2 pressure of 3.95 MPa |
8.45 |
94.74 |
La-ZSM-5 has a greater acid value and esterification rate than ZSM-5, as demonstrated in
Table 1. Catalyzing the liquid phase esterification reaction requires acidification of the catalyst surface. Both the core of Bronsted acid and the center of Lewis acid can undergo the esterification reaction. The esterification reaction, on the other hand, is dependent on the Lewis acid center. Meanwhile, reaction byproducts such as ether and olefins are primarily generated in the Bronsted acid center. Based on existing catalyst characterization, La-ZSM-5 exhibits a higher concentration of Lewis acid sites compared to ZSM-5. As a result, La-ZSM-5 has higher esterification catalysis activities compared to ZSM-5
[20][21].
The esterification reaction mechanism of rosin by glycerol using annealed Fe
3O
4/MOF-5 at temperatures ranging from ambient temperature to 900 °C was also examined
[21][22].
Table 1 summarizes the impact of the main response parameters. In general, room temperature annealing provided the best conditions for rosin esterification utilizing Fe
3O
4/MOF-5. The development of super/subcritical CO
2 and the presence of high temperature liquid water are investigated as environmentally friendly acid catalysts in the esterification process between rosin and glycerol
[31][32]. Experiments with response surface methods revealed that CO
2 pressure affects the yield, which is higher than that produced with a ZnO catalyst. However, the difference is barely 1%. Zinc oxide is also a common catalyst in the rosin esterification reaction. The abundance of oxygen vacancies on the surface of ZnO can boost the catalyst’s Lewis acidity and catalytic activity
[32][33].
Based on the above experiments that have been discussed and shown in
Table 1, heterogeneous catalysts ZSM-5 modified by La metal achieve the highest conversions (up to 98%) but still require a higher reaction time.
3. Conclusions
Rosin with its carboxyl group and conjugate double bonds has an important role in the esterification process. By using reactants or ester agents such as glycerol, specific rosin ester will be produced. The challenge in esterification is how to reduce the acid value or increase the rosin ester conversion, such as using heterogeneous catalysts. One of the advantages is that it can be separated. However, if the catalyst is dispersed during the reaction, it is not easy to separate. Meanwhile, the use of heterogeneous catalysts is a prospective way to increase the conversion of the reaction and reduce the acid value of the rosin. Rosin is a source of raw materials that are renewable and may show potential in the future.