Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed F. Zayed | -- | 1623 | 2023-11-22 20:56:18 | | | |
| 2 | Wendy Huang | Meta information modification | 1623 | 2023-11-23 12:37:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zayed, M.F. Methods of Preparation of Quinoxalines. Encyclopedia. Available online: https://encyclopedia.pub/entry/51944 (accessed on 27 February 2026).
Zayed MF. Methods of Preparation of Quinoxalines. Encyclopedia. Available at: https://encyclopedia.pub/entry/51944. Accessed February 27, 2026.
Zayed, Mohamed F.. "Methods of Preparation of Quinoxalines" Encyclopedia, https://encyclopedia.pub/entry/51944 (accessed February 27, 2026).
Zayed, M.F. (2023, November 22). Methods of Preparation of Quinoxalines. In Encyclopedia. https://encyclopedia.pub/entry/51944
Zayed, Mohamed F.. "Methods of Preparation of Quinoxalines." Encyclopedia. Web. 22 November, 2023.
Copy Citation
Quinoxaline is a fused heterocycle system of a benzene ring and pyrazine ring. It has earned considerable attention due to its importance in the field of medicinal chemistry. The system is of extensive importance due to its comprehensive array of biological activities. Quinoxaline derivatives have been used as anticancer, anticonvulsant, anti-inflammatory, antidiabetic, antioxidant, antibacterial, anti-TB, antimalarial, antiviral, anti-HIV, and many other uses. Variously substituted quinoxalines are significant therapeutic agents in the pharmaceutical industry.
quinoxaline
preparation
traditional chemistry pathway
green chemistry pathway
catalyst
1. Introduction
Heterocycles containing nitrogen have great importance in the pharmaceutical field including uses in drug discovery, synthesis, and development processes [1][2][3]. Diazine heterocycles are central components of several drug candidates [4][5]. The benzo-diazene systems of quinoxalines, cinnolines, quinazolines, phthalazines, naphthalenes, and quinolines are used in the preparation of various drugs [6][7]. They are also used in several research studies for the discovery of new drugs [8][9]. Among these heterocycles, quinoxaline plays an essential role in drug discovery and production [10][11][12]. Quinoxaline is a benzopyrazine system with the molecular formula C8H6N2 [13]. It is formed of a benzene ring fused to the six-membered pyrazine ring [14]. It is a low-melting solid (29–30 °C), soluble in water, and a weak base (pKa = 0.56) [15]. Several studies were performed and displayed a wide range of pharmacological activities for quinoxaline derivatives (Figure 1) [16][17][18][19][20][21][22][23][24][25][26][27][28][29][30]. Additionally, quinoxalines are used for crop protection as a component of insecticides, herbicides, and fungicides [31][32][33][34][35]. Quinoxalines were linked to a metal center such as ruthenium or another heterocyclic moiety such as indole to be used as dyes in solar cell preparation, fluorescent materials, organic semiconductors, and inhibitors of corrosion in metals [36][37][38]. There are many commercially available quinoxalines that have an essential role in the pharmaceutical and industrial market [39][40].
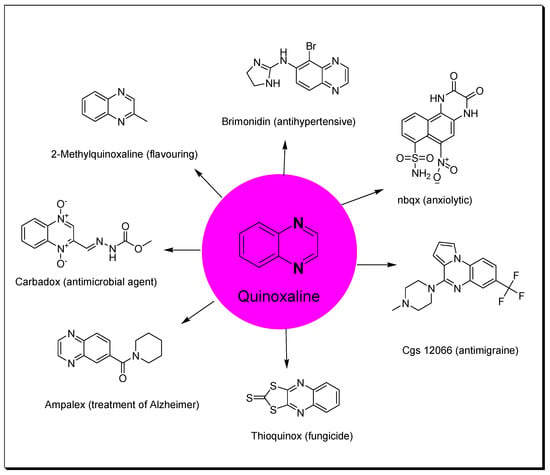
Figure 1. Quinoxaline and examples of its pharmacological activities.
Due to the massive synthetic importance and the various therapeutic activities of quinoxaline derivatives, several attempts have been made by many researchers to prepare a library of these molecules [41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56]. The methods of preparation of quinoxalines can be divided into two pathways:
-
The traditional chemistry pathway, which is based on the condensation between o-phenylenediamines and dicarbonyl compounds in the presence of special conditions such as organic solvents, high temperatures, long times, or strong catalysts. Additionally, the reaction yield may be low and side products may be produced. These types of reactions have negative effects on the environment.
-
The green chemistry pathway, which is a cost-effective pathway through using green chemistry methodologies to produce quinoxalines. This pathway is characterized by using an environmentally friendly recyclable catalyst, a low cost, lower consumption of energy, one-pot synthesis, no side products, short time, and high yield. It can be performed in an aqueous medium at room temperature or by the microwave reactor.
2. Traditional Chemistry Pathway
2.1. Condensation of o-Phenylenediamine and 1,2-Dicarbonyl Derivatives
Korner and Hinsberg in 1884 synthesized the first derivative of quinoxaline through a condensation of o-phenylenediamine with a 1,2-dicarbonyl derivative. Various derivatives were obtained from this reaction (Scheme 1) [41].
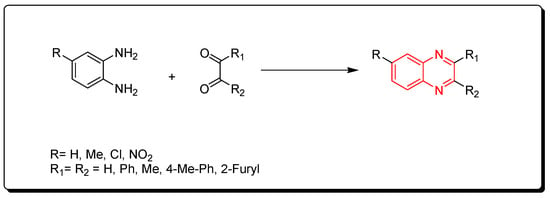
Scheme 1. Synthesis of quinoxaline by the condensation technique: diamine (1 mmol), dicarbonyl (1 mmol), glycerol (5 mL), water (2 mL), 90 °C, 4–6 min, yield (85–91%).
2.2. O-Phenylenediamine and In Situ Produced 1,2-Dicarbonyls
Quinoxalines were synthesized via catalytic iodine, which was used to accelerate the oxidative cyclization cascade between different 1,2-diamino compounds and hydroxyl ketones (Scheme 2) [42].
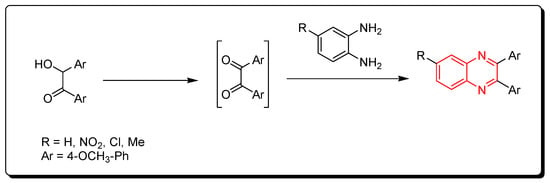
Scheme 2. Synthesis of quinoxaline from o-phenylenediamine and in situ generated 1,2-dicarbonyl derivatives: o-phenylenediamine (1 mmol), hydroxyl ketone (1 mmol), I2 (0.25 mmol), DMSO (2 mL), RT, 12 h, yield (80–90%).
2.3. Metal-Catalyzed Cyclization of Imines and Azides
Ketimines and azides were used to create quinoxalines. This is a metal-catalyzed cyclization reaction that produces quinoxaline derivatives (Scheme 3) [43][44][45].
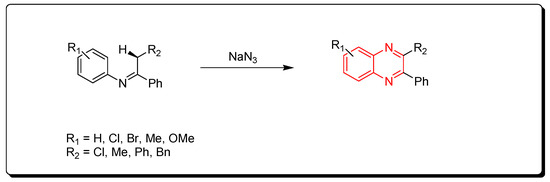
Scheme 3. Synthesis of quinoxalines from imines and azides: imine (1 mmol), sodium azide (3 mmol), (diacetoxyiodo)benzene (3 mmol), CuO (1 mmol), ethylacetate, Rt, 16 h, yield (35–80%).
2.4. Cyclocondensation of o-Phenylenediamine and Aromatic Alkynes
Quinoxalines were synthesized via cyclocondensation of phenylene diamine and aromatic alkynes in the presence of Cu(OAc)2 as a catalyst (Scheme 4) [46].
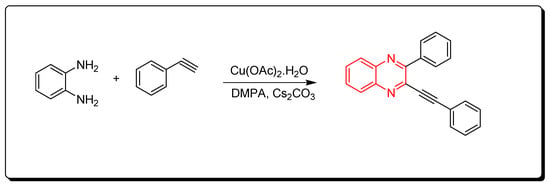
Scheme 4. Synthesis of quinoxalines from aromatic alkynes and amines: o-phenylenedia mine (0.25 mmol) in toluene, phenyl acetylene (1 mmol), Cs2CO3 (0.75 mmol), Cu(OAc)2.H2O (10 mol % from the o-phenylenediamine), DMPA (0.75 mmol), 70 °C, 8 h, yield (86%).
2.5. Cyclocondensation of o-Phenylenediamine and Nitro-Olefins
Using CuBr2 as a catalyst, phenylenediamine and nitro-olefins reacted to produce quinoxalines (Scheme 5) [47].
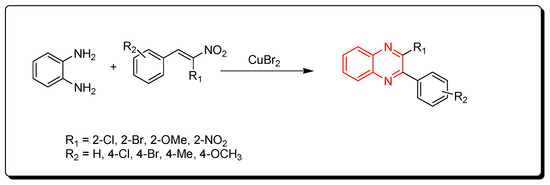
Scheme 5. Synthesis of quinoxalines from nitro-olefins and amines: phenylenediamine (1 mmol), nitro-olefins (1 mmol), CuBr2 (1 mmol), ethanol, 110 °C, 2–4 h, yield (35–90%).
2.6. Cyclocondensation of Aromatic Amines and DMF
A new strategy for the preparation of pyrrol [1,2-a]quinoxaline derivatives was described by using ferric chloride as a Lewis acid and an initiator for a straightforward reaction. DMF solvent was used as a source of carbon (Scheme 6) [48].
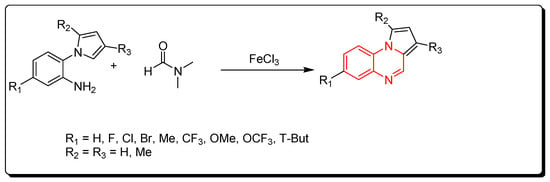
Scheme 6. Synthesis of quinoxalines from amines and DMF in Fe-mediated catalyst: aniline derivative (0.3 mmol), DMF (2 mL), FeCl3 (0.3 mmol), TBPB (0.9 mmol), 120 °C, 5–12 h, yield (40–97%).
3. Green Chemistry Pathway
3.1. Clay-10 Based Method
This is a green synthetic pathway for the synthesis of quinoxalines. It is environmentally friendly with no traditional limitations such as high temperature, expensive reagents, low yield, and contamination. Clay is a cheap material, green reagent, and is continuously available. This reaction is performed by mixing the two reagents with bentonite K-10 at room temperature, then it is flowed on a celite pad and ethanol. The mixture is concentrated to 5 mL and diluted with 10 mL of water. The reaction is allowed to stand for 1 h. The clay can be recovered after formation of the product as pure crystals and can be used five times again. This method agrees with the green chemistry protocol, and it is recommended for the synthesis of different quinoxaline derivatives to avoid the problems of the traditional pathway. The reaction is shown in Scheme 7 [49].
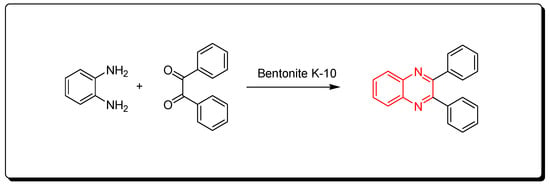
Scheme 7. Synthesis of quinoxalines by one-pot cascade method: o-phenylene-diamine (1 mmol), benzil (1 mmol), bentonite K-10 (3 gm), ethanol 50 mL, RT, 20 min, yield (95%).
3.2. Zinc Triflate Catalyst
Zinc triflate is a zinc salt of trifluoromethanesulfonic acid. It is an ecologically friendly and highly effective catalyst. It is one of the green chemistry catalysts. The reactions performed by using zinc triflate catalyst can be completed without solvent (solvent-free) using a microwave-assisted reactor or by using acetonitrile solvent. Quinoxaline derivatives were prepared by the reaction of o-phenylenediamine and α-diketones using a zinc triflate catalyst at room temperature in acetonitrile. This reaction produced a yield up to 90% (Scheme 8) [50].
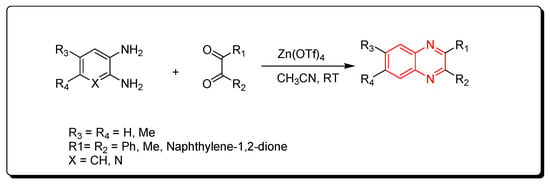
Scheme 8. Synthesis of quinoxaline by using zinc triflate catalyst: diamine (1.1 mmol), dicarbonyl (1 mmol), Zn(OTf)4 (0.2 mmol), CH3CN (5 mL), RT, yield (85–91%).
3.3. Phosphate-Based Catalyst
The phosphate catalysts include monoammonium phosphate (MAP), diammonium phosphate (DAP), and triple super phosphate (TSP), which is a constituent of fertilizer that mainly consists of monocalcium phosphate Ca(H2PO4)2. The needed amount from this type of catalyst is a minute amount (0.006 gm) for performing the one molar equivalent reaction. The resulting product is crystallized from ethanol while the catalyst is recovered from the reaction by simple filtration, washing with hot ethanol, and drying for 6 h (Scheme 9) [51].
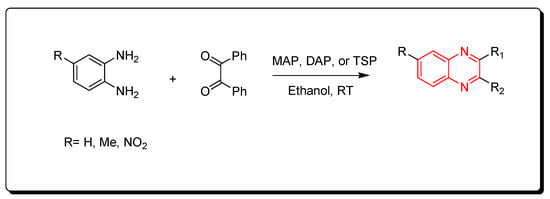
Scheme 9. Synthesis of quinoxaline by using phosphate-based catalyst: amine (1 mmol), benzil (1 mmol), MAP, DAP, or TSP (0.0006 gm), ethanol, RT, yield (85–91%).
3.4. Lanthanide-Based Catalyst
Cerium (IV) ammonium nitrate (CAN) is one of the lanthanide reagents. It has earned attention in synthetic reactions of organic chemistry due to its low cost, availability, miscibility in water, safety, and high reactivity. It is used in green chemistry due to its unique characters. The reaction between o-phenylenediamine and benzil derivatives in the presence of cerium (IV) ammonium nitrate (CAN) readily happens in 20 min without any side products at room temperature to produce a good yield reaching up to 98%. Additionally, it is performed in an aqueous medium. The CAN catalyst is mixed with acetonitrile or any aprotic solvent. It is one of the green chemistry protocols that are used for the synthesis of quinoxaline derivatives (Scheme 10) [52].
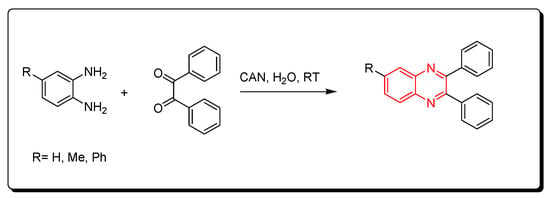
Scheme 10. Synthesis of quinoxaline by using lanthanide-based catalyst. Amine (1 mmol), benzil (1 mmol), CAN (5 mol), acetonitrile, RT, 20 min, yield (80–98%).
3.5. Fluorinated Alcohols Catalyst (HFIP)
Fluorinated alcohols are related to green chemistry catalysts. Recently, they have gained much attention due to their low nucleophilic characteristics, high polarity, their ability to strongly donate hydrogen bonds, and water solvation characteristics. They also can stabilize the helix conformation of proteins. The reaction between o-phenylenediamine and benzil derivatives in the presence of hexafluoroisopropanol (HFIP) was run at room temperature for one hour to produce quinoxaline derivatives with a 95% yield. It is a solvent-free reaction without side products and toxic solvents. Furthermore, the hexafluoroisopropanol can be recovered from the reaction without activity change. Therefore, it is a green chemistry pathway (Scheme 11) [52].
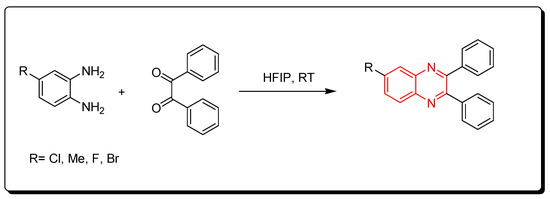
Scheme 11. Synthesis of quinoxaline by using fluorinated alcohols catalyst: amine (1 mmol), benzil (1 mmol), HFIP (5 mol), RT, 20 min, yield (95%).
3.6. Solid Acid Catalyst (TiO2-Pr-SO3H)
Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) is a green chemistry catalyst. It is a sulfonic acid nano porous titania resulting from the reaction of (3-mercaptopropyl) trimethoxysilane and titanium oxide. It can be recovered from the reaction without a change in the activity. It is used to catalyze the reaction between o-phenylenediamine and benzil derivatives at room temperature. The product yield was 95% and it needed only 10 min to be accomplished. This reaction can be performed in the presence of ethanol or in the absence of any solvent. It is a one-step reaction without side products (Scheme 12) [52].
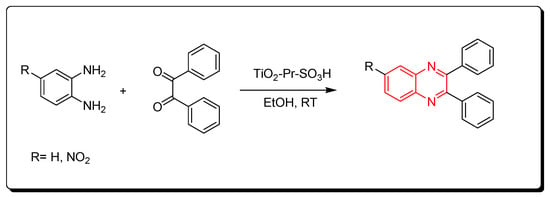
Scheme 12. Synthesis of quinoxaline by using solid acid catalyst: amine (1 mmol), benzil (1 mmol), TiO2-Pr-SO3H (1 mol), RT, 10 min, yield (95%).
4. Reaction of Quinoxalines
4.1. Intramolecular Arylation Using Lewis Acid Catalyst
Aryl derivatives of quinoxalines were produced via the reaction between dichloroquinoxalines and aryl derivatives using a Lewis acid catalyst (AlCl3). The previous method induced arylation via C–C bond formation (Scheme 13) [53].
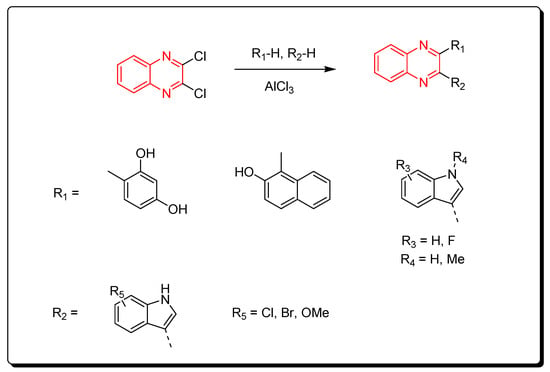
Scheme 13. Synthesis of quinoxalines derivatives by AlCl3-induced arylation of dichloroquinoxalines: dichloroquinoxaline (1 mmol), R1-H (1 mmol), R2-H (1 mmol), AlCl3 (2.2 mmol), DCE, 80 °C, 60 min, yield (87–85%).
4.2. Intramolecular Cyclization of Quinoxalines
Substituted pyrrolo[2,3-b]quinoxaline from allyl-3-chloroquinoxaline-2-ylamine having terminal alkene and aromatic amine derivatives was prepared using a Pd-mediated catalyst Pd(OAc)2 (Scheme 14) [54].
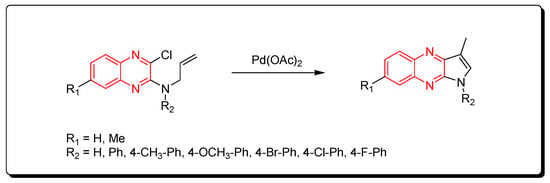
Scheme 14. Synthesis of quinoxalines derivatives by Pd-mediated catalyst: amine (1 mmol), Pd(OAc)2 (0.015 mmol), K2CO3 (3 mmol), DMF, 100 °C, 2 h, yield (80–91%).
References
- Mabrouk, R.R.; Abdallaha, A.E.; Mahdy, H.A.; El-Kalyoubi, S.A.; Kamal, O.J.; Abdelghany, T.M.; Zayed, M.F.; Alshaeri, H.K.; Alasmari, M.M.; El-Zahabi, M.A. Design, Synthesis, and Biological Evaluation of New Potential Unusual Modified Anticancer Immunomodulators for Possible Non-Teratogenic Quinazoline-Based Thalidomide Analogs. Int. J. Mol. Sci. 2023, 24, 12416.
- Ihmaid, S.; Ahmed, H.E.A.; Zayed, M.F. The Design and Development of Potent Small Molecules as Anticancer Agents Targeting EGFR TK and Tubulin Polymerization. Int. J. Mol. Sci. 2018, 19, 408.
- El-Helby, A.A.; Ayyad, A.R.; Zayed, M.F.; Abulkhir, S.H.; Elkady, H.; El-Adl, K. Design, synthesis, in silico ADMET profile and GABA-A docking of novel phthalazines as potent anticonvulsants. Arch. Pharm. 2019, 352, 1800373.
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases. Sci. Pharm. 2023, 91, 18.
- El-Zahabia, M.A.; Bamanie, H.F.; Ghareeb, S.; Alshaeri, K.H.; Alasmari, M.M.; Muostafa, M.; Al-Marzoki, Z.; Zayed, M.F. Design, Synthesis, Molecular Modeling andAnti-Hyperglycemic Evaluation of Quinazoline-Sulfonylurea Hybrids as Peroxisome Proliferator-Activated Receptor Gamma (PPAR) and Sulfonylurea Receptor (SUR) Agonists. Int. J. Mol. Sci. 2022, 23, 9605.
- Zayed, M.F.; Ibrahim, S.; Habib, E.E.; Hassan, M.H.; Ahmed, S.; Rateb, H.S. Design, synthesis, antimicrobial and anti-biofilm evaluation, and molecular docking of new substituted fluoroquinazolinones. J. Med. Chem. 2019, 15, 657–673.
- Ghorab, M.M.; Abdel-Kader, M.S.; Alqahtani, A.S.; Soliman, A.M. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J. Enzym. Inhib. Med. Chem. 2021, 36, 218–237.
- Zayed, M.F.; Ayyad, R.R. Some Novel Anticonvulsant Agents Derived from Phthalazinedione. Arzneimittelforschung 2012, 62, 532–536.
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Analgesic and Anti-Inflammatory Agents. ChemEngineering 2022, 6, 94.
- Abulkhair, S.H.; Elmeligie, S.; Ghiaty, A.; El-Morsy, A.; Bayoumi, H.A.; Ahmed, A.E.H.; El-Adl, K.; Zayed, M.F.; Hassan, H.M.; Akl, E.N.; et al. In vivo- and in silico-driven identification of novel synthetic quinoxalines as anticonvulsants and AMPA inhibitors. Arch. Pharm. 2021, 354, 2000449.
- Elhelby, A.A.; Ayyad, R.R.; Zayed, M.F. Synthesis and biological evaluation of some quinoxaline derivatives as anticonvulsant agents. Arzneimittelforschung 2011, 61, 379–381.
- Iazzetti, A.; Fabrizi, G.; Goggiamani, A.; Marrone, F.; Sferrazza, A.; Ullah, K. Synthesis of Functionalized 3H-pyrrolo- Quinoxalines via Gold-Catalyzed Intramolecular Hydroamination of Alkynes. Molecules 2023, 28, 5831.
- Sharma, A.; Deep, A.; Marwaha, M.; Marwaha, R.K. Quinoxaline: A chemical moiety with spectrum of interesting biological activities. Mini Rev. Med. Chem. 2022, 22, 927–948.
- Suthar, K.S.; NChundawat, S.N.; Singh, P.G.; Padrón, M.J.; Jhala, K.Y. Quinoxaline: A comprehension of current pharmacological advancement in medicinal chemistry. Eur. J. Med. Chem. Rep. 2022, 5, 100040.
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline Derivatives as Antiviral Agents: A Systematic Review. Molecules 2020, 25, 2784.
- Patinote, C.; Raevens, S.; Baumann, A.; Pellegrin, E.; Bonnet, P.-A.; Deleuze-Masquéfa, C. triazoloquinoxaline as Novel Scaffold in the Imiqualines Family: Candidates with Cytotoxic Activities on Melanoma Cell Lines. Molecules 2023, 28, 5478.
- Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Fillová, N.; Moreau, S.; Baylot, V.; Desplat, V. Synthesis, Crystal Structure and Anti-Leukemic Activity of (E)-Pyrroloquinoxalin-4-yl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one. Molbank 2023, 2023, M1691.
- Yang, Q.; Wang, H.; Wang, X.; Lei, Y. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis Reactions. Molecules 2023, 28, 5030.
- Matveevskaya, V.V.; Pavlov, D.I.; Kovrizhina, A.R.; Sukhikh, T.S.; Sadykov, E.H.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khlebnikov, A.I.; Potapov, A.S. Experimental and Computational Investigation of the Oxime Bond Stereochemistry in c-Jun N-terminal Kinase 3 Inhibitors 11H-Indenoquinoxalin-11-one Oxime and Tryptanthrin-6-oxime. Pharmaceutics 2023, 15, 1802.
- Dumur, F. Recent Advances on Quinoxaline-Based Photoinitiators of Polymerization. Catalysts 2023, 13, 718.
- Yang, Q.; Wang, B.; Wu, M.; Lei, Y.-Z. Recent Developments in Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules 2023, 28, 2513.
- Kumar, A.; Singh, A.K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.P.; Pathak, P.; Grishina, M.; et al. Nitrogen Containing Heterocycles as Anticancer Agents: A Medicinal Chemistry Perspective. Pharmaceuticals 2023, 16, 299.
- Zhdankina, A.A.; Tikhonov, D.I.; Logvinov, S.V.; Plotnikov, M.B.; Khlebnikov, A.I.; Kolosova, N.G. Suppression of Age-Related Macular Degeneration-like Pathology by c-Jun N-Terminal Kinase Inhibitor IQ-1S. Biomedicines 2023, 11, 395.
- Pooladian, F.; Crawford, P.W.; Kessler, J.M.; Casey, G.R.; Ragain, C.M. Reduction Potential Predictions for Thirty-Seven 1,4-di-N-Oxide Quinoxaline-2-Carboxamide Derivatives with Anti-Tuberculosis Activity. Compounds 2023, 3, 83–95.
- Goel, K.K.; Hussain, A.; Altamimi, M.A.; Rajput, S.K.; Sharma, P.P.; Kharb, R.; Mahdi, W.A.; Imam, S.S.; Alshehri, S.; Alnemer, O.A.; et al. Identification of Potential Antitubulin Agents with Anticancer Assets from a Series of Imidazoquinoxaline Derivatives: In Silico and In Vitro Approaches. Molecules 2023, 28, 802.
- Maltsev, D.V.; Skripka, M.O.; Spasov, A.A.; Vassiliev, P.M.; Perfiliev, M.A.; Divaeva, L.N.; Zubenko, A.A.; Morkovnik, A.S.; Klimenko, A.I.; Miroshnikov, M.V.; et al. Design, Synthesis and Pharmacological Evaluation of Novel C2,C3-Quinoxaline Derivatives as Promising Anxiolytic Agents. Int. J. Mol. Sci. 2022, 23, 14401.
- Fan, D.; Liu, P.; Jiang, Y.; He, X.; Zhang, L.; Wang, L.; Yang, T. Discovery and SAR Study of Quinoxaline–Arylfuran Derivatives as a New Class of Antitumor Agents. Pharmaceutics 2022, 14, 2420.
- González-González, A.; Sánchez-Sánchez, O.; Krauth-Siegel, R.L.; Bolognesi, M.L.; Gớmez-Escobedo, R.; Nogueda-Torres, B.; Vázquez-Jiménez, L.K.; Saavedra, E.; Encalada, R.; Espinoza-Hicks, J.C.; et al. In Vitro and In Silico Analysis of New n-Butyl and Isobutyl Quinoxaline-7-carboxylate 1,4-di-N-oxide Derivatives against Trypanosoma cruzi as Trypanothione Reductase Inhibitors. Int. J. Mol. Sci. 2022, 23, 13315.
- Bouali, N.; Hammouda, M.B.; Ahmad, I.; Ghannay, S.; Thouri, A.; Dbeibia, A.; Patel, H.; Hamadou, W.S.; Hosni, K.; Snoussi, M.; et al. Multifunctional Derivatives of Spiropyrrolidine Tethered Indeno-Quinoxaline Heterocyclic Hybrids as Potent Antimicrobial, Antioxidant and Antidiabetic Agents: Design, Synthesis, In Vitro and In Silico Approaches. Molecules 2022, 27, 7248.
- Aksenov, A.V.; Arutiunov, N.A.; Aksenov, D.A.; Samovolov, A.V.; Kurenkov, I.A.; Aksenov, N.A.; Aleksandrova, E.A.; Momotova, D.S.; Rubin, M. A Convenient Way to Quinoxaline Derivatives through the Reaction of 2-(3-Oxoindolin-2-yl)-2-phenylacetonitriles with Benzene-1,2-diamines. Int. J. Mol. Sci. 2022, 23, 11120.
- Bhat, Z.R.; Kumar, M.; Sharma, N.; Yadav, U.P.; Singh, T.; Joshi, G.; Pujala, B.; Raja, M.; Chatterjee, J.; Tikoo, K.; et al. In Vivo Anticancer Evaluation of 6b, a Non-Covalent Imidazoquinoxaline-Based Epidermal Growth Factor Receptor Inhibitor against Human Xenograft Tumor in Nude Mice. Molecules 2022, 27, 5540.
- Syam, Y.M.; Anwar, M.M.; Abd El-Karim, S.S.; Elokely, K.M.; Abdelwahed, S.H. New Quinoxaline-Based Derivatives as PARP-1 Inhibitors: Design, Synthesis, Antiproliferative, and Computational Studies. Molecules 2022, 27, 4924.
- Montero, V.; Montana, M.; Khoumeri, O.; Correard, F.; Estève, M.-A.; Vanelle, P. Synthesis, In Vitro Antiproliferative Activity, and In Silico Evaluation of Novel Oxiranyl-Quinoxaline Derivatives. Pharmaceuticals 2022, 15, 781.
- Kumar, N.; Inwati, G.K.; Ahmed, E.M.; Lal, C.; Makwana, B.; Yadav, V.K.; Islam, S.; Ahn, H.-J.; Yadav, K.K.; Jeon, B.-H. Modified 7-Chloro-11H-indenoquinoxaline Heterocyclic System for Biological Activities. Catalysts 2022, 12, 213.
- Guillon, J.; Savrimoutou, S.; Albenque-Rubio, S.; Pinaud, N.; Moreau, S.; Desplat, V. Synthesis, Crystal Structure and Anti-Leukemic Activity of 1,3-Dihydro-1--2H-benzimidazol-2-one. Molbank 2022, 2022, M1333.
- Sharma, B.K.; Shaikh, A.M.; Chacko, S.; Kamble, M.R. Synthesis, spectral, electrochemical and theoretical investigation of indoloquinoxaline dyes derived from anthraquinone for n–type materials. J. Chem. Sci. 2017, 129, 483–494.
- Peppas, A.; Sokalis, D.; Perganti, D.; Schnakenburg, G.; Falaras, P.; Philippopoulos, I.A. Sterically demanding pyridine-quinoline anchoring ligands as building blocks for copper(i)-based dye-sensitized solar cell (DSSC) complexes. Dalton Trans. 2022, 51, 15049.
- Abu-Hashem, A.A.; Al-Hussain, S.A. Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazoquinoxaline and Thienopyrimidine from Isatin Derivatives as Anticancer Agents. Molecules 2022, 27, 835.
- Liakhov, S.A.; Schepetkin, I.A.; Karpenko, O.S.; Duma, H.I.; Haidarzhy, N.M.; Kirpotina, L.N.; Kovrizhina, A.R.; Khlebnikov, A.I.; Bagryanskaya, I.Y.; Quinn, M.T. Novel c-Jun N-Terminal Kinase (JNK) Inhibitors with an 11H-Indenoquinoxalin-11-one Scaffold. Molecules 2021, 26, 5688.
- Suwanhom, P.; Saetang, J.; Khongkow, P.; Nualnoi, T.; Tipmanee, V.; Lomlim, L. Synthesis, Biological Evaluation, and In Silico Studies of New Acetylcholinesterase Inhibitors Based on Quinoxaline Scaffold. Molecules 2021, 26, 4895.
- Aichhorn, S.; Himmelsbach, M.; Schofberger, W. Synthesis of quinoxalines or quinolin-8-amines from N-propargyl aniline derivatives employing tin and indium chlorides. Org. Biomol. Chem. 2015, 13, 9373–9380.
- Heravi, M.M.; Bakhtiari, K.; Tehrani, M.H.; Javadi, N.M.; Oskooie, A.H. Facile synthesis of quinoxaline derivatives using o-iodoxybenzoic acid (IBX) at room temperature. ARKIVOC 2006, XVI, 16–22.
- Jeganathan, M.; Dhakshinamoorthy, A.; Pitchumani, K. One-pot synthesis of 2- substituted quinoxalines using K10-montmorillonite as heterogeneous catalyst. Tetrahedron Lett. 2014, 55, 1616–1620.
- Chen, T.; Chen, X.; Wei, J.; Lin, D.; Xie, Y.; Zeng, W. Copper-catalyzed cascade Cycloamination of α-csp3–H Bond of N-aryl Ketimines with azides: Access to Quinoxalines. Org. Lett. 2016, 18, 2078–2081.
- Wang, W.; Shen, Y.; Meng, X.; Zhao, M.; Chen, Y.; Chen, B. Copper-catalyzed synthesis of quinoxalines with o-phenylenediamine and terminal alkyne in the presence of bases. Org. Lett. 2011, 13, 4514–4517.
- Chen, Y.; Li, K.; Zhao, M.; Li, Y.; Chen, B. Cu(II)-catalyzed synthesis of quinoxalines from o-phenylene diamines and nitroolefins. Tetrahedron Lett. 2013, 54, 1627–1630.
- Wang, X.; Liu, H.; Xie, C.; Zhou, F.; Chen, M. Terminal methyl as a one-carbon synthon: Synthesis of quinoxaline derivatives via radical-type transformation. New J. Chem. 2020, 44, 2465–2470.
- Hasaninejad, A.; Zare, A.; Shekouhy, M.; Moosavi-Zare, A.R. Bentonite clay K-10 as an efficient reagent for the synthesis of quinoxaline derivatives at room temperature. J. Chem. 2009, 6, S247–S253.
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; Volume 30.
- Subrahmanyam, S.C.; Narayanan, S. Synthesis of Quinoxalines in Presence of Zinc Triflate. Asian J. Chem. 2011, 23, 1331–1333.
- An, Z.; Wu, M.; Ni, J.; Qi, Z.; Yu, G.; Yan, R.; Zhao, L.-B. FeCl3-Catalyzed synthesis of pyrroloquinoxaline derivatives from 1-(2-aminophenyl)pyrroles through annulation and cleavage of cyclic ethers. Chem. Commun. 2017, 53, 11572–11575.
- Atghia, S.V.; Beigbaghlou, S.S. Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) as a new, highly efficient, and recyclable solid acid catalyst for the preparation of quinoxaline derivatives. J. Nanostruct. Chem. 2013, 3, 38.
- Kumar, K.S.; Rambabu, D.; Sandra, S.; Kapavarapu, R.; Krishna, R.G.G.; Rao, B.V.M.; Chatti, K.; Reddy, M.C.; Misra, P.; Pal, M. AlCl3 induced (hetero)arylation of 2,3-dichloroquinoxaline: A one-pot synthesis of mono/disubstituted quinoxalines as potential antitubercular agents. Bioorg. Med. Chem. 2012, 20, 1711–1722.
- Babu, V.P.; Mukherjee, S.; Deora, S.G.; Chennubhotla, S.K.; Medisetti, R.; Yellanki, S.; Kulkarni, P.P.; Sripelly, S.; Parsa, L.V.K.; Chatti, K.; et al. Ligand/PTCfree intramolecular Heck reaction: Synthesis of pyrroloquinoxalines and their evaluation against PDE4/luciferase/oral cancer cell growth in vitro and zebrafish in vivo. Org. Biomol. Chem. 2013, 11, 6680.
- DrugBank Online. Available online: https://go.drugbank.com/ (accessed on 28 September 2023).
- Kamble, A.A.; Kamble, R.R.; Kumbar, M.N.; Tegginamath, G. Pyridine-catalyzed synthesis of quinoxalines as anticancer and anti-tubercular agents. Med. Chem. Res. 2016, 25, 1163–1174.
More
Information
Subjects:
Chemistry, Medicinal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
8.6K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
23 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





