Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mohamed F. Zayed and Version 2 by Wendy Huang.
Quinoxaline is a fused heterocycle system of a benzene ring and pyrazine ring. It has earned considerable attention due to its importance in the field of medicinal chemistry. The system is of extensive importance due to its comprehensive array of biological activities. Quinoxaline derivatives have been used as anticancer, anticonvulsant, anti-inflammatory, antidiabetic, antioxidant, antibacterial, anti-TB, antimalarial, antiviral, anti-HIV, and many other uses. Variously substituted quinoxalines are significant therapeutic agents in the pharmaceutical industry.
- quinoxaline
- preparation
- traditional chemistry pathway
- green chemistry pathway
- catalyst
1. Introduction
Heterocycles containing nitrogen have great importance in the pharmaceutical field including uses in drug discovery, synthesis, and development processes [1][2][3][1,2,3]. Diazine heterocycles are central components of several drug candidates [4][5][4,5]. The benzo-diazene systems of quinoxalines, cinnolines, quinazolines, phthalazines, naphthalenes, and quinolines are used in the preparation of various drugs [6][7][6,7]. They are also used in several research studies for the discovery of new drugs [8][9][8,9]. Among these heterocycles, quinoxaline plays an essential role in drug discovery and production [10][11][12][10,11,12]. Quinoxaline is a benzopyrazine system with the molecular formula C8H6N2 [13]. It is formed of a benzene ring fused to the six-membered pyrazine ring [14]. It is a low-melting solid (29–30 °C), soluble in water, and a weak base (pKa = 0.56) [15]. Several studies were performed and displayed a wide range of pharmacological activities for quinoxaline derivatives (Figure 1) [16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Additionally, quinoxalines are used for crop protection as a component of insecticides, herbicides, and fungicides [31][32][33][34][35][31,32,33,34,35]. Quinoxalines were linked to a metal center such as ruthenium or another heterocyclic moiety such as indole to be used as dyes in solar cell preparation, fluorescent materials, organic semiconductors, and inhibitors of corrosion in metals [36][37][38][36,37,38]. There are many commercially available quinoxalines that have an essential role in the pharmaceutical and industrial market [39][40][39,40].
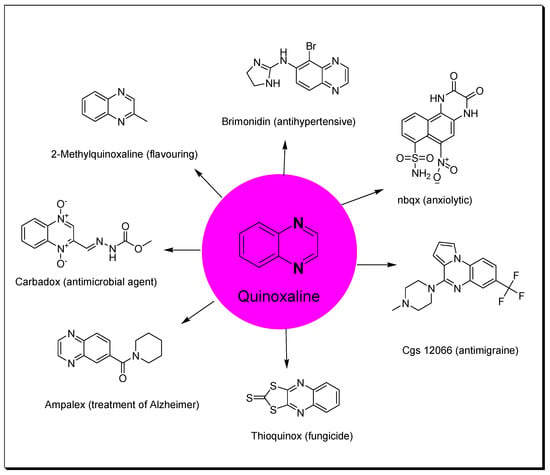
Figure 1.
Quinoxaline and examples of its pharmacological activities.
Due to the massive synthetic importance and the various therapeutic activities of quinoxaline derivatives, several attempts have been made by many researchers to prepare a library of these molecules [41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. The methods of preparation of quinoxalines can be divided into two pathways:
-
The traditional chemistry pathway, which is based on the condensation between o-phenylenediamines and dicarbonyl compounds in the presence of special conditions such as organic solvents, high temperatures, long times, or strong catalysts. Additionally, the reaction yield may be low and side products may be produced. These types of reactions have negative effects on the environment.
-
The green chemistry pathway, which is a cost-effective pathway through using green chemistry methodologies to produce quinoxalines. This pathway is characterized by using an environmentally friendly recyclable catalyst, a low cost, lower consumption of energy, one-pot synthesis, no side products, short time, and high yield. It can be performed in an aqueous medium at room temperature or by the microwave reactor.
2. Traditional Chemistry Pathway
2.1. Condensation of o-Phenylenediamine and 1,2-Dicarbonyl Derivatives
Korner and Hinsberg in 1884 synthesized the first derivative of quinoxaline through a condensation of o-phenylenediamine with a 1,2-dicarbonyl derivative. Various derivatives were obtained from this reaction (Scheme 1) [41] [76].
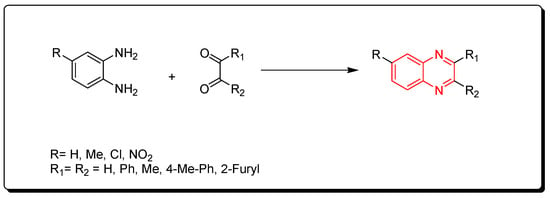

Scheme 1.
Synthesis of quinoxaline by the condensation technique: diamine (1 mmol), dicarbonyl (1 mmol), glycerol (5 mL), water (2 mL), 90 °C, 4–6 min, yield (85–91%).
2.2. O-Phenylenediamine and In Situ Produced 1,2-Dicarbonyls
Quinoxalines were synthesized via catalytic iodine, which was used to accelerate the oxidative cyclization cascade between different 1,2-diamino compounds and hydroxyl ketones (Scheme 2) [42][77].
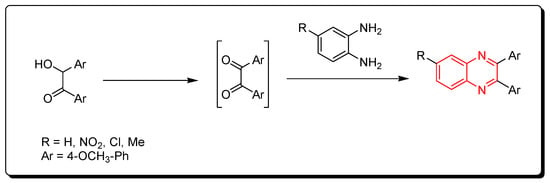

Scheme 2. Synthesis of quinoxaline from o-phenylenediamine and in situ generated 1,2-dicarbonyl derivatives: o-phenylenediamine (1 mmol), hydroxyl ketone (1 mmol), I2 (0.25 mmol), DMSO (2 mL), RT, 12 h, yield (80–90%).
2.3. Metal-Catalyzed Cyclization of Imines and Azides
Ketimines and azides were used to create quinoxalines. This is a metal-catalyzed cyclization reaction that produces quinoxaline derivatives (Scheme 3) [43][44][45][78,79,80].
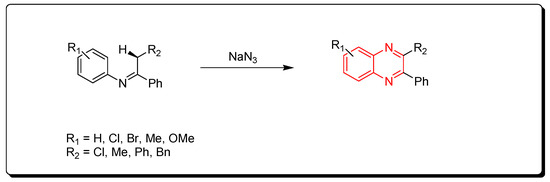

Scheme 3. Synthesis of quinoxalines from imines and azides: imine (1 mmol), sodium azide (3 mmol), (diacetoxyiodo)benzene (3 mmol), CuO (1 mmol), ethylacetate, Rt, 16 h, yield (35–80%).
2.4. Cyclocondensation of o-Phenylenediamine and Aromatic Alkynes
Quinoxalines were synthesized via cyclocondensation of phenylene diamine and aromatic alkynes in the presence of Cu(OAc)2 as a catalyst (Scheme 4) [46][81].
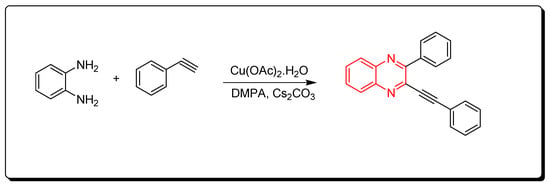

Scheme 4. Synthesis of quinoxalines from aromatic alkynes and amines: o-phenylenedia mine (0.25 mmol) in toluene, phenyl acetylene (1 mmol), Cs2CO3 (0.75 mmol), Cu(OAc)2.H2O (10 mol % from the o-phenylenediamine), DMPA (0.75 mmol), 70 °C, 8 h, yield (86%).
2.5. Cyclocondensation of o-Phenylenediamine and Nitro-Olefins
Using CuBr2 as a catalyst, phenylenediamine and nitro-olefins reacted to produce quinoxalines (Scheme 5) [47][82].
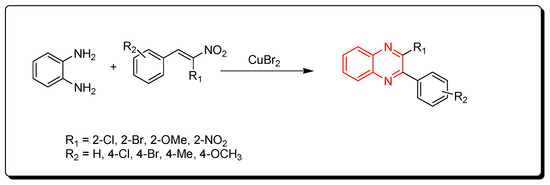

Scheme 5.
Synthesis of quinoxalines from nitro-olefins and amines: phenylenediamine (1 mmol), nitro-olefins (1 mmol), CuBr2 (1 mmol), ethanol, 110 °C, 2–4 h, yield (35–90%).
2.6. Cyclocondensation of Aromatic Amines and DMF
A new strategy for the preparation of pyrrol [1,2-a]quinoxaline derivatives was described by using ferric chloride as a Lewis acid and an initiator for a straightforward reaction. DMF solvent was used as a source of carbon (Scheme 6) [48][83].
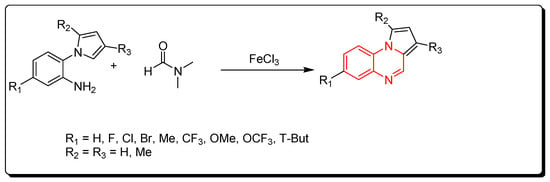

Scheme 6. Synthesis of quinoxalines from amines and DMF in Fe-mediated catalyst: aniline derivative (0.3 mmol), DMF (2 mL), FeCl3 (0.3 mmol), TBPB (0.9 mmol), 120 °C, 5–12 h, yield (40–97%).
3. Green Chemistry Pathway
3.1. Clay-10 Based Method
This is a green synthetic pathway for the synthesis of quinoxalines. It is environmentally friendly with no traditional limitations such as high temperature, expensive reagents, low yield, and contamination. Clay is a cheap material, green reagent, and is continuously available. This reaction is performed by mixing the two reagents with bentonite K-10 at room temperature, then it is flowed on a celite pad and ethanol. The mixture is concentrated to 5 mL and diluted with 10 mL of water. The reaction is allowed to stand for 1 h. The clay can be recovered after formation of the product as pure crystals and can be used five times again. This method agrees with the green chemistry protocol, and it is recommended for the synthesis of different quinoxaline derivatives to avoid the problems of the traditional pathway. The reaction is shown in Scheme 7 [49]Scheme 7 [84].
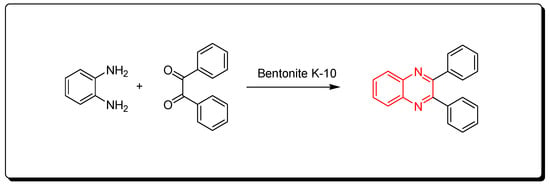

Scheme 7.
Synthesis of quinoxalines by one-pot cascade method: o-phenylene-diamine (1 mmol), benzil (1 mmol), bentonite K-10 (3 gm), ethanol 50 mL, RT, 20 min, yield (95%).
3.2. Zinc Triflate Catalyst
Zinc triflate is a zinc salt of trifluoromethanesulfonic acid. It is an ecologically friendly and highly effective catalyst. It is one of the green chemistry catalysts. The reactions performed by using zinc triflate catalyst can be completed without solvent (solvent-free) using a microwave-assisted reactor or by using acetonitrile solvent. Quinoxaline derivatives were prepared by the reaction of o-phenylenediamine and α-diketones using a zinc triflate catalyst at room temperature in acetonitrile. This reaction produced a yield up to 90% (Scheme 8) [50][85].
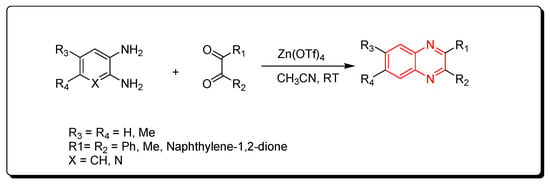

Scheme 8.
Synthesis of quinoxaline by using zinc triflate catalyst: diamine (1.1 mmol), dicarbonyl (1 mmol), Zn(OTf)
4
(0.2 mmol), CH
3
CN (5 mL), RT, yield (85–91%).
3.3. Phosphate-Based Catalyst
The phosphate catalysts include monoammonium phosphate (MAP), diammonium phosphate (DAP), and triple super phosphate (TSP), which is a constituent of fertilizer that mainly consists of monocalcium phosphate Ca(H2PO4)2. The needed amount from this type of catalyst is a minute amount (0.006 gm) for performing the one molar equivalent reaction. The resulting product is crystallized from ethanol while the catalyst is recovered from the reaction by simple filtration, washing with hot ethanol, and drying for 6 h (Scheme 9) [51][86].
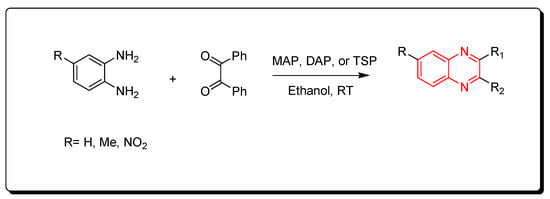

Scheme 9.
Synthesis of quinoxaline by using phosphate-based catalyst: amine (1 mmol), benzil (1 mmol), MAP, DAP, or TSP (0.0006 gm), ethanol, RT, yield (85–91%).
3.4. Lanthanide-Based Catalyst
Cerium (IV) ammonium nitrate (CAN) is one of the lanthanide reagents. It has earned attention in synthetic reactions of organic chemistry due to its low cost, availability, miscibility in water, safety, and high reactivity. It is used in green chemistry due to its unique characters. The reaction between o-phenylenediamine and benzil derivatives in the presence of cerium (IV) ammonium nitrate (CAN) readily happens in 20 min without any side products at room temperature to produce a good yield reaching up to 98%. Additionally, it is performed in an aqueous medium. The CAN catalyst is mixed with acetonitrile or any aprotic solvent. It is one of the green chemistry protocols that are used for the synthesis of quinoxaline derivatives (Scheme 10) [52][87].
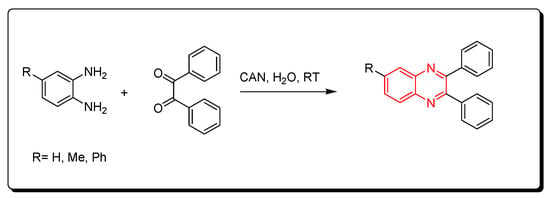

Scheme 10.
Synthesis of quinoxaline by using lanthanide-based catalyst. Amine (1 mmol), benzil (1 mmol), CAN (5 mol), acetonitrile, RT, 20 min, yield (80–98%).
3.5. Fluorinated Alcohols Catalyst (HFIP)
Fluorinated alcohols are related to green chemistry catalysts. Recently, they have gained much attention due to their low nucleophilic characteristics, high polarity, their ability to strongly donate hydrogen bonds, and water solvation characteristics. They also can stabilize the helix conformation of proteins. The reaction between o-phenylenediamine and benzil derivatives in the presence of hexafluoroisopropanol (HFIP) was run at room temperature for one hour to produce quinoxaline derivatives with a 95% yield. It is a solvent-free reaction without side products and toxic solvents. Furthermore, the hexafluoroisopropanol can be recovered from the reaction without activity change. Therefore, it is a green chemistry pathway (Scheme 11) [52][87].
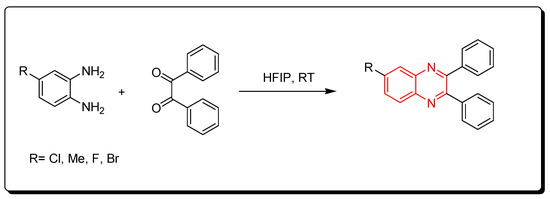

Scheme 11.
Synthesis of quinoxaline by using fluorinated alcohols catalyst: amine (1 mmol), benzil (1 mmol), HFIP (5 mol), RT, 20 min, yield (95%).
3.6. Solid Acid Catalyst (TiO2-Pr-SO3H)
Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) is a green chemistry catalyst. It is a sulfonic acid nano porous titania resulting from the reaction of (3-mercaptopropyl) trimethoxysilane and titanium oxide. It can be recovered from the reaction without a change in the activity. It is used to catalyze the reaction between o-phenylenediamine and benzil derivatives at room temperature. The product yield was 95% and it needed only 10 min to be accomplished. This reaction can be performed in the presence of ethanol or in the absence of any solvent. It is a one-step reaction without side products (Scheme 12) [52][87].
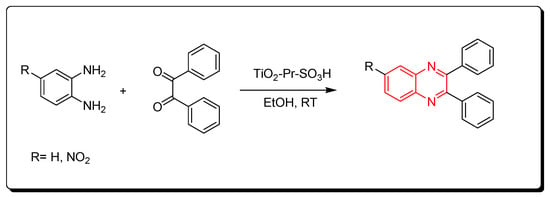

Scheme 12.
Synthesis of quinoxaline by using solid acid catalyst: amine (1 mmol), benzil (1 mmol), TiO
2
-Pr-SO
3
H (1 mol), RT, 10 min, yield (95%).
4. Reaction of Quinoxalines
4.1. Intramolecular Arylation Using Lewis Acid Catalyst
Aryl derivatives of quinoxalines were produced via the reaction between dichloroquinoxalines and aryl derivatives using a Lewis acid catalyst (AlCl3). The previous method induced arylation via C–C bond formation (Scheme 13) [53][88].
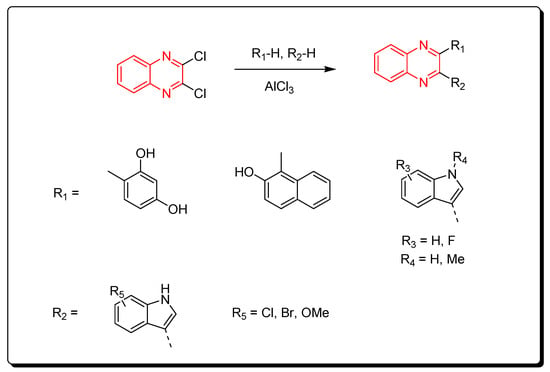

Scheme 13. Synthesis of quinoxalines derivatives by AlCl3-induced arylation of dichloroquinoxalines: dichloroquinoxaline (1 mmol), R1-H (1 mmol), R2-H (1 mmol), AlCl3 (2.2 mmol), DCE, 80 °C, 60 min, yield (87–85%).
4.2. Intramolecular Cyclization of Quinoxalines
Substituted pyrrolo[2,3-b]quinoxaline from allyl-3-chloroquinoxaline-2-ylamine having terminal alkene and aromatic amine derivatives was prepared using a Pd-mediated catalyst Pd(OAc)2 (Scheme 14) [54][89].
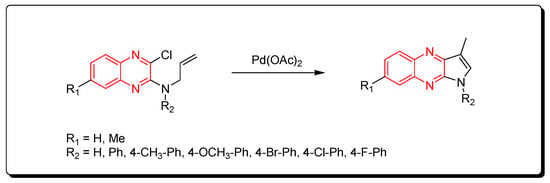

Scheme 14.
Synthesis of quinoxalines derivatives by Pd-mediated catalyst: amine (1 mmol), Pd(OAc)
2
(0.015 mmol), K
2
CO
3
(3 mmol), DMF, 100 °C, 2 h, yield (80–91%).
