Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrey Sergeevich Erst | -- | 4079 | 2023-11-22 10:36:19 | | | |
| 2 | Sirius Huang | Meta information modification | 4079 | 2023-11-23 02:07:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Erst, A.S.; Petrova, N.V.; Kaidash, O.A.; Wang, W.; Kostikova, V.A. The Genus Eranthis. Encyclopedia. Available online: https://encyclopedia.pub/entry/51910 (accessed on 07 February 2026).
Erst AS, Petrova NV, Kaidash OA, Wang W, Kostikova VA. The Genus Eranthis. Encyclopedia. Available at: https://encyclopedia.pub/entry/51910. Accessed February 07, 2026.
Erst, Andrey S., Natalia V. Petrova, Olga A. Kaidash, Wei Wang, Vera A. Kostikova. "The Genus Eranthis" Encyclopedia, https://encyclopedia.pub/entry/51910 (accessed February 07, 2026).
Erst, A.S., Petrova, N.V., Kaidash, O.A., Wang, W., & Kostikova, V.A. (2023, November 22). The Genus Eranthis. In Encyclopedia. https://encyclopedia.pub/entry/51910
Erst, Andrey S., et al. "The Genus Eranthis." Encyclopedia. Web. 22 November, 2023.
Copy Citation
Despite the limited geographic range of Eranthis plants, it is possible to search for active substances, develop methods for biological and chemical synthesis of the isolated substances, and create a finished therapeutic substance based on them. Seven out of ~14 species found in Asia and parts of Europe have been studied to various degrees. Here, data are presented on the diversity of sets of chromones, furochromones, triterpene saponins, coumarins, and other classes of secondary metabolites of Eranthis species according to the literature. For new compounds—isolated from Eranthis for the first time—structural formulas are also provided.
Eranthis
chromone
furochromone
triterpene saponin
coumarin
biological activity
1. Introduction
According to molecular and morphological data, the tribe Cimicifugeae Torrey & Gray belongs to the family Ranunculaceae Juss. and includes four recognized genera and ~49 species: Actaea L. (32 species), Anemonopsis Siebold et Zucc. (one species), Beesia Balf. f. et W. W. Sm. (two species), and Eranthis Salisb. (14 species) [1][2][3]. Most of these species occur mainly in the northern hemisphere and are perennial herbs [4]. The taxonomic position of the genera Eranthis and Beesia has been a matter of systematic uncertainty within the tribal rank in the Ranunculaceae family. According to morphological information, Beesia has been assigned to three different tribes (Helleboreae DC., Actaeeae Spach, and Trollieae Schröd.) by intuitive taxonomic techniques but has seldom been included in cladistic analyses [5][6][7]. By contrast, the Eranthis genus has consistently been assigned to the Helleboreae tribe or as the only genus to the tribe Eranthideae T. Duncan & Keener in morphological classifications but always has been a sister taxon to plants of the Actaeeae tribe in cladistic analyses [8]. The genus Eranthis consists of 8–14 species growing in southern Europe and temperate Asia [9][10][11]. Traditionally, the genus has been subdivided into two sections: Eranthis sect. Eranthis and E. sect. Shibateranthis (Nakai) Tamura [12]. The type section Eranthis is characterized by plants with tubers, yellow sepals, and emarginate or slightly bilobate upper petal margins without pseudonectaries (Figure 1) [6][11]. The section Eranthis in Europe includes E. hyemalis (L.) Salisb. and E. bulgarica (Stef.) Stef., whereas in Southwest and West Asia, it includes E. cilicica Schott et Kotschy, E. kurdica Rukšāns, E. longistipitata Regel, and E. iranica Rukšāns et Zetterl. [13][14][15][16]. The section Shibateranthis has long-lived tubers, white sepals, and bilobate or forked petal margins with pseudonectaries (Figure 1) [6][17]. Representatives of this section have a natural geographic range in temperate North and East Asia (E. albiflora Franch., E. byunsanensis B.Y. Sun, E. lobulata W.T.Wang, E. pinnatifida Maxim., E. pungdoensis B.U. Oh, E. sibirica DC., E. stellata Maxim., and E. tanhoensis Erst) [10][11].

Figure 1. Species of the genus Eranthis. (A) E. longistipitata, (B) E. cilicica, (C) E. hyemalis, (D) E. sibirica, (E) E. tanhoensis, and (F) E. stellata.
Plants of the tribe Cimicifugeae are some of the richest sources of various active ingredients and of therapeutic and health-promoting substances. The value of the constituents has been confirmed by many years of use in East Asian countries in folk medicine. Thus, it is important to integrate new technologies into research on Cimicifugeae, both for the sustainable use of pharmaceutical resources from Cimicifugeae and for a search for new compounds with potential clinical efficacy and fewer adverse effects [18][19][20]. In the tribe Cimicifuga, representatives of the genus Actaea are the most frequently studied plants in the world of science. Nonetheless, little is known about the chemical profile and biological activity of other representatives of Cimicifugeae: Beesia and Anemonopsis. In recent decades, new information has been obtained about the chemical profiles of (and biological effects of extracts and individual compounds from) Eranthis species, which are early flowering geophytes with a limited geographic range.
2. Phytocomponents Identified in Eranthis Plants and Their Chemotaxonomic Significance
2.1. Chromones
Since the 1960s, from some Eranthis species, a series of substances has been isolated that represents an important class of oxygen-containing heterocyclic compounds that are derivatives of benzo-γ-pyrone: chromones. Their isolation has been performed by various chromatographic methods, and the structures of individual compounds have been investigated by 1-dimensional (H-NMR) and 2-dimensional nuclear magnetic resonance (C-NMR) spectroscopy. In structure, chromones are similar to flavonoids and coumarins but are substantially less common in the wild. Chromones can give rise to hydroxy- and methoxy-derivatives and can attach a sugar moiety, whereas after condensation with benzene, pyran, or furan rings, they can be transformed into a variety of benzo-, pyrano-, or furochromones, respectively. Compounds from the class “simple furochromones and chromones” are most often found in Eranthis species; chromones have been detected in underground parts, whereas furochromones have been found in underground and aboveground parts (Table 1).
Table 1. Chemical constituents of the genus Eranthis (all classes of metabolites identified to date: vertical subdivisions in the table).
| ID No. | Compound | Source Eranthis Species | Reference |
|---|---|---|---|
| Chromones | |||
| 1 | 8,11-dihydro-5-hydroxy-2,9-dihydroxymethyl-4H-pyrano [2,3-g][1] benzoxepin-4-one |
E. cilicica (T *) | [21] |
| 2 | Eranthin (5-hydroxy-9-hydroxymethyl-2-methyl-8,11-dihydro-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (R) | [22] |
| 3 | Eranthin-β-D-glucoside (9-{[(β-D-glucopyranosvl)oxy]methyl}-8,11-dihydro-5-hydroxy-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (R, T) | [22][23] |
| 4 | Eranthin 9-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) E. hyemalis (T) |
[21][23] |
| 5 | Eranthin β-D-gentiobioside (9-{[(β-D-gentiobiosyl)oxy]methyl}-8,11-dihydro-5-hydroxy-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (T) | [23] |
| 6 | 2-C-Hydroxyeranthin β-D-glucopyranoside (9-{[(β-D-glucopyranosyl)oxy]methyl}-8,11-dihydro-5-hydroxy-2-(hydroxymethyl-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (T) | [23] |
| 7 | 9-[(O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl)oxy]methyl-8,11-dihydro-5,9-dihydroxy-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one | E. cilicica (T) | [21] |
| 8 | 8,11-dihydro-5,9-dihydroxy-9-hydroxymethyl-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one | E. cilicica (T) | [21] |
| 9 | 5,7-dihydroxy-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-2-methyl-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 10 | 5,7-dihydroxy-2-hydroxymethyl-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 11 | 7-[(β-D-glucopyranosyl)oxy]-5-hydroxy-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-2-methyl-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 12 | 7-[(β-D-glucopyranosyl)oxy]-5-hydroxy-2-hydroxymethyl-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 13 | 7,8-Secoeranthin β-D-glucoside (8-{(2E)-4-[(β-D-glucopyranosyl)oxy]-3-methylbut-2-enyl}-5,7-dihydroxy-2-methyl-4H-1-benzopyran-4-one) |
E. hyemalis (T) | [23] |
| 14 | 2-C-Hydroxy-7,8-secoeranthin β-D-glucoside (8-{(2E)-4-[(β-D-glucopyranosyl)oxy]-3-methylbut-2-enyl}-5,7-dihydroxy-2-(hydroxymethyl)-4H-1-benzopyran-4-one) |
E. hyemalis (T) | [23] |
| Furochromones | |||
| 15 | Visnagin (4-methoxy-7-methyl-5H-furo[3,2-g]chromen-5-one) |
E. hyemalis E. longistipitata (L) |
[24][25] |
| 16 | Khellin (4,9-dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-one) |
E. hyemalis E. longistipitata (L) |
[24][25] |
| 17 | Khellol (7-(hydroxymethyl)-4-methoxyfuro[3,2-g]chromen-5-one) |
E. pinnatifida (L, St) | [26] |
| 18 | Khellol glucoside (khellinin; 7-hydroxymethyl-4-methoxy-5H-furo [3,2-g]][1]benzopyran-5-one glucoside) |
E. hyemalis (L, F) | [27] |
| 19 | Norkhellol (4-hydroxy-7-(hydroxymethyl)-5H-furo[3,2-g][1]benzopyran-5-one) |
E. pinnatifida (L, St) | [26] |
| 20 | Norammiol (4-hydroxy-7(hydroxymethyl)-9-methoxy-5H-furo[3,2-g][1]-benzopyran-5-one) |
E. pinnatifida (L, St) | [26] |
| 21 | 7-[(O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl)oxy]methyl-4-hydroxy-5H-furo[3,2-g][1]benzopyran-5-one | E. cilicica (T) | [21] |
| 22 | Methoxsalen (9-methoxyfuro[3,2-g]chromen-7-one) |
E. longistipitata (L) | [25] |
| 23 | Cimifugin (2S)-7-(hydroxymethyl)-2-(2-hydroxypropan-2-yl)-4-methoxy-2,3-dihydrofuro[3,2g]chromen-5-one) |
E. pinnatifida (L, St) E. cilicica (T) E. longistipitata (L) |
[21][25][26] |
| 24 | Norcimifugin (2S)-4-hydroxy-7-(hydroxymethyl)-2-(2-hydroxypropan-2-yl)-2,3-dihydrofuro[3,2-g]-chromen-5-one) |
E. pinnatifida (L, St) | [26] |
| 25 | Cimifugin β-D-glucopyranoside (7-{[(β-D-glucopyranosy1)oxy]methyl}-2,3-dihydro-2-(l-hydroxy-1-methylethyl)-4-methoxy-5H-furo[3,2-g][1]benzopyran-5-one) |
E. hyemalis (T) | [23] |
| 26 | 5-O-Methylvisammioside (4-O-β-D-glucosyl-5-O-methylvisamminol) |
E. longistipitata (L) | [25] |
| 27 | Visamminol-3′-O-glucoside (4-hydroxy-2-(2-hydroxypropan-2- yl)-methyl-2,3-dihydrofuro[3,2-g] chromen-5-one) |
E. longistipitata (L) | [25] |
| Triterpene saponins | |||
| 28 | Eranthisaponin A | E. cilicica (T) | [28] |
| (3β-[(O-β-D-allopyranosyl-(1→3)-O-α-L-rhamnopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranosyl)oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl- (1→6)-β-D-glucopyranoside) |
|||
| 29 | Eranthisaponin B (3β-[(O-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl-(1→4)-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl- (1→6)-β-D-glucopyranoside) |
E. cilicica (T) | [28] |
| 30 | 3β-[(O-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside |
E. cilicica (T) | [28] |
| 31 | 23-Hydroxy-3β-[(O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranosyl)oxy]olean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [28] |
| 32 | 3β-[(O-β-D-glucopyranosyl-(1→4)-α-L-arabinopyranosyl)oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [28] |
| 33 | 3β-[(O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranosyl)oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [28] |
| 34 | 3β-[(O-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid |
E. cilicica (T) | [29] |
| 35 | 3β-[(O-β-D-galactopyranosyl-(1→3)-O-α-L-rhamnopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid | E. cilicica (T) | [29] |
| 36 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl β-D-glucopyranoside | E. cilicica (T) | [29] |
| 37 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartane-3β,28-diol | E. cilicica (T) | [29] |
| 38 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 39 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 40 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 41 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→4)-O-[β-D-glucopyranosyl (1→6)]-β-D-glucopyranoside |
E. cilicica (T) | [29] |
| 42 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-oxo-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 43 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 44 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 45 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartan-3β,28-diol | E. cilicica (T) | [29] |
| 46 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 47 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 48 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| Alkaloids | |||
| 49 | Corytuberine (2,10-dimethoxy-6α-aporphine-1,11-diol) |
E. hyemalis (T; Ap) | [30] |
| Coumarins | |||
| 50 | 5,7-Dihydroxy-4-methylcoumarin | E. longistipitata (L) | [25] |
| 51 | Scoparone (6,7-dimethoxycoumarin) |
E. longistipitata (L) | [25] |
| 52 | Fraxetin (7,8-dihydroxy-6-methoxycoumarin) |
E. longistipitata (L) | [25] |
| 53 | Luvangetin (10-methoxy-2,2-dimethylpyrano[3,2-g]chromen-8-one) |
E. longistipitata (L) | [25] |
| Flavonoids | |||
| 54 | Quercetin | E. longistipitata (L) E. stellata (L) E. tanhoensis (L) |
[25][31][32] |
| 55 | Isoquercitrin (quercetin-3-O-β-D-glucoside) |
E. longistipitata (L) | [25][32] |
| 56 | Hyperoside (quercetin 3-O-β-D-galactoside) |
E. longistipitata (L) | [25][32] |
| 57 | Reynoutrin (quercetin-3-O-β-D-xylopyranoside) |
E. longistipitata (L) | [25][32] |
| 58 | Quercetin-6-O-β-D-xylopyranosyl-β-D- glucopyranoside |
E. longistipitata (L) | [25][32] |
| 59 | Quercetin-3-sambubioside (quercetin-3-O-[β-D-xylosyl-(1→2)-β-D-glucoside]) |
E. longistipitata (L) | [25][32] |
| 60 | Peltatoside (quercetin-3-(6-O-α-L-arabinopyranosyl)-β-D- glucopyranoside)) |
E. longistipitata (L) | [25][32] |
| 61 | Rutin (quercetin 3-O-β-D-rutinoside) |
E. longistipitata (L) | [25][32] |
| 62 | Kaempferol | E. longistipitata (L) E. stellata (L) E. tanhoensis (L) |
[25][31][32] |
| 63 | Juglalin (kaempferol 3-O-α-L-arabinopyranoside) |
E. longistipitata (L) | [25][32] |
| 64 | Trifolin (kaempferol-3-O-β-D-galactoside) |
E. longistipitata (L) | [25][32] |
| 65 | Aromadendrin [(+)-dihydrokaempferol] |
E. longistipitata (L) | [25][32] |
| 66 | Vitexin (apigenin 8-C-glucoside) |
E. sibirica (L) | [31] |
| 67 | Orientin (luteolin-8-C-glucoside) |
E. sibirica (L) | [31] |
| E. stellata (L) | |||
| 68 | Carlinoside (luteolin 6-C-β-D-glucopyranoside-8-C-α-L- |
E. longistipitata (L) | [25][32] |
| arabinopyranoside) | |||
| 69 | Cianidanol [(+)-catechin] |
E. longistipitata (L) | [25][32] |
| 70 | Auriculoside (7,3,5′-trihydroxy-4′-methoxyflavan-3′-glucoside) |
E. longistipitata (L) | [25][32] |
| 71 | 6-Methoxytaxifolin | E. longistipitata (L) | [25][32] |
| 72 | Aspalathin | E. longistipitata (L) | [25][32] |
| 73 | Phloridzin (phloretin-2′-O-β-glucoside) |
E. longistipitata (L) | [25][32] |
| 74 | Phloretin (dihydroxy naringenin) |
E. longistipitata (L) | [25][32] |
| Cinnamic acids | |||
| 75 | Chlorogenic acid (3-O-caffeoylquinic acid) |
E. sibirica (L) E. stellata (L) E. tanhoensis (L) |
[31] |
| 76 | Caffeic acid (3,4-dihydroxycinnamic acid) |
E. sibirica (L) E. stellata (L) |
[31] |
| Phenolic acids | |||
| 77 | Salicylic acid (3-tert-2-butyl-2-hydroxy-6-methylbenzoic acid) |
E. sibirica (L) E. tanhoensis (L) |
[31] |
| 78 | Gentisic acid (2,5-dihydroxybenzoic acid) |
E. stellata (L) | [31] |
| Fatty acids and their derivatives | |||
| 79 | Myristic acid (14:0) | E. hyemalis (S) | [33] |
| 80 | Pentadecylic acid (15:0) | E. hyemalis (S) | [33] |
| 81 | Palmitic acid (16:0) | E. hyemalis (S) | [33] |
| 82 | 16-Hydroxyhexadecanoic acid | E. longistipitata (L) | [25] |
| 83 | cis-5-Hexadecenoic acid (16:1 Δ5cis) | E. hyemalis (S) | [33] |
| 84 | Palmitoleic acid (16:1 Δ9cis) | E. longistipitata (L) | [25] |
| 85 | cis-9-Octadecanoic acid (18:0 Δ9cis) | E. hyemalis (S) | [33] |
| 86 | cis-Vaccenic acid (18:1 Δ11cis) | E. hyemalis (S) | [33] |
| 87 | Linoleic acid (18:2 Δ9cis, 12cis) | E. hyemalis (S) | [33] |
| 88 | 9-oxo-ODA (9-Oxo-trans-10, trans-12-octadecadienoic acid) |
E. longistipitata (L) | [25] |
| 89 | (+/−)13-HODE | E. longistipitata (L) | [25] |
| (13-hydroxyoctadecadienoic acid) | |||
| 90 | Corchorifatty acid F (9,12,13-trihydroxy-10(E),15(Z)-octadecadienoic acid) | E. longistipitata (L) | [25] |
| 91 | α-Linolenic acid (18:3 Δ9cis, 12cis, 15cis) | E. hyemalis (S) E. longistipitata (L) |
[25][33] |
| 92 | Linolenic acid ethyl ester | E. longistipitata (L) | [25] |
| 93 | α-Eleostearic acid (18:3 Δ9cis, 11trans, 13trans) | E. longistipitata (L) | [25] |
| 94 | Pinolenic acid (18:3 Δ5cis, 9cis, 12cis) | E. longistipitata (L) | [25] |
| 95 | 13(S)-HOTrE (13-OH-cis-9, trans-11, cis-15-octadecatrienoic acid) |
E. longistipitata (L) | [25] |
| 96 | (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | E. longistipitata (L) | [25] |
| 97 | 12-Oxo-phytodienoic acid | E. longistipitata (L) | [25] |
| 98 | 9S,13R-12-Oxo-phytodienoic acid | E. longistipitata (L) | [25] |
| 99 | Arachidic acid (20:0) | E. hyemalis (S) | [33] |
| 100 | cis-5-Eicosenoic acid (20:1 Δ5cis) | E. hyemalis (S) | [33] |
| 101 | Gondoic acid (20:1 Δ11cis) | E. hyemalis (S) | [33] |
| 102 | Keteleeronic acid (20:2 Δ5cis, 11cis) | E. hyemalis (S) | [33] |
| 103 | cis-11,14-Eicosadienoic acid (20:2 Δ11cis, 14cis) | E. hyemalis (S) | [33] |
| 104 | 15-OxoEDE (15-Oxo-cis-11,trans-13-eicosadienoic acid) |
E. longistipitata (L) | [25] |
| 105 | cis-5,11,14-Eicosatrienoic acid (20:3 Δ5cis, 11cis, 14cis) | E. hyemalis (S) | [33] |
| 106 | Behenic acid (22:0) | E. hyemalis (S) | [33] |
| 107 | Erucic acid (22:1 Δ13cis) | E. hyemalis (S) | [33] |
| 108 | cis-5,13-Docosadienoic acid (22:2 Δ5cis, 13cis) | E. hyemalis (S) | [33] |
| 109 | cis-13,16-Docosadienoic acid (22:2 Δ13cis, 16cis) | E. hyemalis (S) | [33] |
| 110 | cis-5,13,16-Docosatrienoic acid (22:3 Δ5cis, 13cis, 16cis) | E. hyemalis (S) | [33] |
| 111 | cis-10,13,16-Docosatrienoic acid (22:3 Δ10cis, 13cis, 16cis) | E. hyemalis (S) | [33] |
| Amino acids | |||
| 112 | D-(+)-Pyroglutamic acid | E. longistipitata (L) | [25] |
| 113 | D-(+)-Tryptophan | E. longistipitata (L) | [25] |
| 114 | Isoleucine | E. longistipitata (L) | [25] |
| 115 | L-Phenylalanine | E. longistipitata (L) | [25] |
| 116 | L-Tyrosine | E. longistipitata (L) | [25] |
| 117 | D-(−)-Glutamine | E. longistipitata (L) | [25] |
| Organic acids | |||
| 118 | Citric acid | E. longistipitata (L) | [25] |
| 119 | D-α-Hydroxyglutaric acid | E. longistipitata (L) | [25] |
| 120 | Gluconic acid | E. longistipitata (L) | [25] |
| Sugars | |||
| 121 | α-Lactose | E. longistipitata (L) | [25] |
| 122 | D-(+)-Galactose | E. longistipitata (L) | [25] |
| 123 | α.α-Trehalose | E. longistipitata (L) | [25] |
| Alcohols | |||
| 124 | D-(−)-Mannitol | E. longistipitata (L) | [25] |
| Phenylpropanoids | |||
| 125 | 6-Gingerol | E. longistipitata (L) | [25] |
| Lectins | |||
| 126 | EHL | E. hyemalis (R) | [34][35] |
* Ap, aerial part; F, flowers; L, leaves; R, rhizome; S, seeds; St, stems; T, tubers.
Structurally, the chromones found in Eranthis species can be categorized into several classes (Figure 2). Structures of compounds 1–6 are similar in the carbon backbone—containing an oxepin ring—but differ from one another in substituents at positions C-2 and C-9. These substituents can be methyl and hydroxymethyl groups as well as mono- and diglycosides. These compounds have been registered in the underground parts of E. cilicica Schott & Kotschy and E. hyemalis (L.) Salisb. [21][22][23]. The first publications on the isolation of chromones of this subclass date back to the end of the 1970s, when 5-hydroxy-9-hydroxymethyl-2-methyl-8,11-dihydro-4H-pyrano[2,3-g][1]benzoxepin-4-one [named eranthin (2)] and its β-D-glucoside (3) were isolated [22].
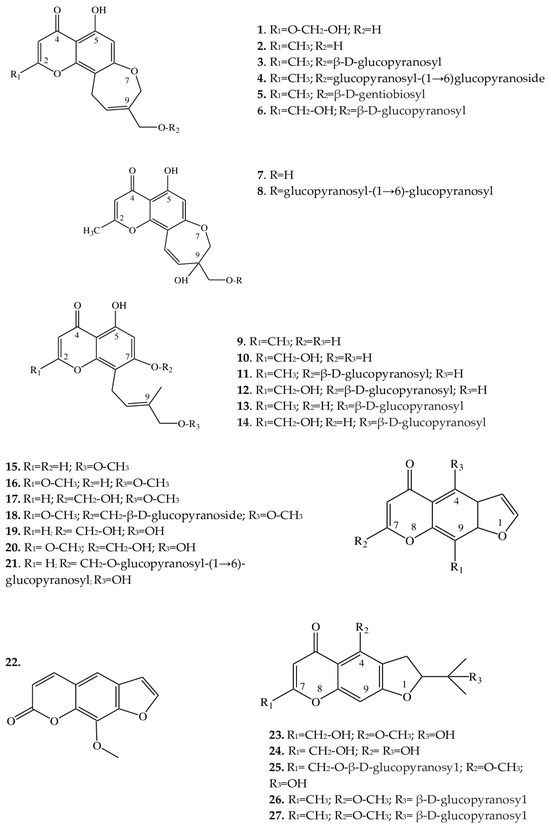
Figure 2. Structures of furochromones and chromones from Eranthis species.
Structures similar to this type (7 and 8), in contrast to the above compounds, have a displaced double bond [from C-9(10) to C-10(11)] on the oxepin ring; in addition, at position C-9, there is both an oxymethylene group bearing various substituents and a hydroxyl group. These compounds have so far been found only in the underground part of E. cilicica [21]. 1H and 13C nuclear magnetic resonance data have allowed us to identify the structural features of compounds 9–14 and to determine that their oxepin ring is open; a 4′-hydroxy-3′-methylbut-2′-enyl group has been found at position C-8. Compounds 11 and 12 additionally contain a D-glucose residue at the C-7 position, whereas compounds 13 and 14 contain it at the C-4′ position. These chromones have been detected in the underground parts of E. cilicica and E. hyemalis [21][22]. All of the above chromones are specific to the genus Eranthis.
2.2. Furochromones
These compounds of Eranthis species are formed by the condensation of a simple chromone with a furan ring at positions C-6 and C-7 and, in contrast to the aforementioned chromones, are relatively common in the plant kingdom. For instance, the first representative of this subclass of compounds called khellin (16) has long been used in folk medicine to relieve ureteral pain during colic. For the first time, khellin was found in a seed extract of Ammi visnaga (L.) Lam. and was isolated as far back as the end of the 19th century [36]. Currently, khellin’s ability to act directly on smooth-muscle fibers is widely used in clinical practice [37]. Khellin, aside from species of the genus Ammi, has been found in other representatives of the family Apiaceae Lindl., for example, in Dioscorea L. sp. and Pimpinella L. sp. [38][39][40], and among Eranthis species, in E. hyemalis and E. longistipitata Regel [24][25]. The diversity of the structures in the furochromone subclass, which includes khellin, is mostly determined by the presence of substituents at the C-4, C-7, and C-9 positions. At the C-4 position, methoxy or hydroxyl groups can serve as a substituent; at position C-7, methoxy groups and glucose; and at position C-9, a methoxy group, or—as in 15, 17, 19, and 21—the substituent may be absent. Khellol (17) represents an aglycone of khellol glucoside (18), in which the sugar moiety is attached at position C-7. In the genus Eranthis, most research on furochromones of this subclass has been conducted on samples of the aerial parts (leaves, stems, and flowers) of E. pinnatifida Maxim., E. hyemalis, and E. longistipitata [24][25][26][27], and only compound 21 has been detected in an underground part (tubers) of E. cilicica [21].
Recently, new compounds not previously found in Eranthis species were discovered in samples of E. longistipitata from Central Asia (Kyrgyzstan): methoxsalen (22), 5-O-methylvisammioside (26), and visamminol-3′-O-glucoside (27) [25]. Methoxsalen (22) is often seen in the plant extracts of such families as Apiaceae, Rutaceae Juss., Fabaceae Lindl., and Brassicaceae Burnett [41][42], whereas the last two compounds of this subclass (26 and 27) have been registered only in extracts from an underground part of Saposhnikovia divaricata (Turcz.) Shischk. (Apiaceae) [43][44][45].
Because Eranthis species synthesize chromones during normal physiological processes, preliminary conclusions have been made that the genus Eranthis is closest to the genera Cimicifuga and Actaea (in whose extracts, chromones have also been found), and not Helleborus L. (for example), whose species do not synthesize chromones but are distinguished by the accumulation of cardenolides and bufadienolides [23][46].
2.3. Triterpene Saponins
A phytochemical analysis of a methanol extract from tubers of E. cilicica has revealed two new bisdesmosidic triterpenes, named eranthisaponins A (28) and B (29) [28]. The new saponins are based on the structural backbone of hederagenin, which is a triterpenoid first isolated from seeds and leaves of Hedera helix L. [47]. A distinctive feature of eranthisaponin A (28) is a branched tetraglycoside attached at the C-3 position of the aglycone, whereas a feature of eranthisaponin B (29) is a linear hexaglycoside attached at the C-28 position of the aglycone. Such sugar forms in triterpene saponins have not been described previously. In addition, as one of the substituents, eranthisaponin A (28) contains D-allopyranose: a monosaccharide that is extremely rare in plant saponins [28].
Furthermore, in Eranthis plants, a number of known triterpene saponins (30–35) have been discovered that are (just as eranthisaponins B and A) based on the backbone of hederagenin with substituents at positions C-3 and C-28; extremely rarely (only in 31), the substituent (a hydroxyl group) is located at the C-23 position. Other substituents include di- and triglycosides composed of glucose, arabinose, and rhamnose residues.
The research continued by K. Watanabe with coauthors [29] has allowed to subsequently isolate a new oleanane glycoside (34) from the tubers of E. cilicica. Another oleanane glycoside (35) had been discovered earlier in the underground part of Anemone coronaria L. (Ranunculaceae) [48]. These substances (34 and 35) contain triglycosides only at position C-3 of the carbohydrate part of the molecule. All of the above triterpene saponins belong to the oleanan type.
In the same study [29], when fractionating a methanol extract from the tubers of E. cilicica, Watanabe et al. isolated several cycloartane-type compounds (36–48). There were 13 such triterpene saponins, all of which had not been characterized before. Compounds 37 and 45 are aglycones of 36 and 44, respectively. The new compounds can be categorized into two very similar subclasses: 36–43 and 44–48. In terms of their structure, rings A–D are similar, and differences lie in rings E and F (Figure 3).
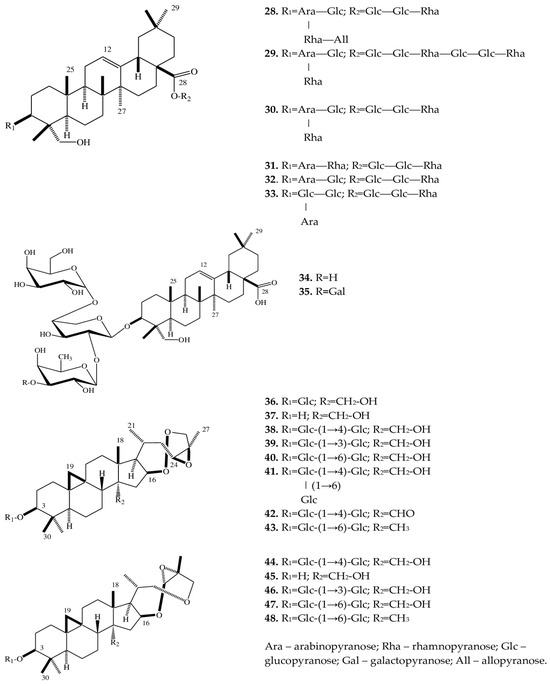
Figure 3. Structures of triterpene saponins of Eranthis species.
The compounds of both subclasses differ among themselves in the presence of various sugar moieties at the C-3 position of the aglycone as well as in the presence of a hydroxy, oxo, or methyl group at position C-28.
2.4. Alkaloids
In the tubers and aerial parts of E. hyemalis, trace amounts of an alkaloid called corytuberine (49) have been found [30].
2.5. Coumarins
In ongoing studies on E. longistipitata, coumarins have been discovered in aqueous-ethanol extracts from the leaves of this species: this class of compounds was registered in the genus Eranthis for the first time [25] but is widespread in the family Ranunculaceae [49]. 5,7-Dihydroxy-4-methylcoumarin (50), scoparone (51), and fraxetin (52) are affiliated with the subclass “simple coumarins”, which are based on a coumarin molecule with substituents in the form of methyl, hydroxy, and methoxy groups. Luvangetin (53) can be assigned to linear pyranocoumarins, in which—aside from various substituents—a pyran ring is present in the backbone.
2.6. Flavonoids
In contrast to the sets of chromones and triterpene saponins, the set of flavonoids in Eranthis species mainly contains known substances. For instance, a study on the aqueous-ethanol extracts from the leaves of four Eranthis species has led to the identification of several flavonoids: quercetin (54) (E. longistipitata, E. stellata Maxim., and E. tanhoensis), kaempferol (62) (E. longistipitata, E. stellata, and E. tanhoensis), vitexin (66) (E. sibirica DC.), and orientin (67) (E. sibirica and E. stellata) [31]. In addition, in E. longistipitata, from the class of flavonols, researchers have identified isoquercitrin (55), hyperoside (56), reynoutrin (57), quercetin-3-sambubioside (59), peltatoside (60), rutin (61), juglalin (63), and trifolin (64); from flavanones, aromadendrin (65) and 6-methoxytaxifolin (71); from C-glycoside flavones, there is carlinoside (68); from the class of flavans, investigators have identified cianidanol (69) and auriculoside (70); and from chalcones, aspalathin (72), phloridzin (73), and phloretin (74) [32]. Flavonoids in the leaves of E. hyemalis are represented by the glycosides of quercetin and kaempferol, the detailed structures of which have not been determined [27]. The heterogeneity of the qualitative and quantitative profiles of flavonoids has been noted among the analyzed Eranthis species [11][31][32].
2.7. Phenolcarboxylic Acids
The investigation of this class of phenolic compounds is represented by a single publication covering only three Eranthis species and dealing with the identification of phenolcarboxylic acids that are widespread in nature: chlorogenic (75) (E. sibirica, E. stellata, and E. tanhoensis), caffeic (76) (E. sibirica and E. stellata), salicylic (77) (E. sibirica and E. tanhoensis), and gentisic (78) (E. stellata), whereas the concentration of caffeic acid (0.29–0.32 mg/g), chlorogenic acid (0.34–0.96 mg/g), and salicylic acid (0.25 mg/g) has proven to be the highest in E. sibirica [31]. Thus, there is evidence of variation in the profile and levels of phenolcarboxylic acids among these species [11][31][32].
2.8. Fatty Acids
To date, fatty acids in the leaves of E. longistipitata (82, 84, 88–98, and 104) and the composition of seed oil from E. hyemalis (79–81, 83, 85–87, 91, 99–103, and 105–111) have been determined [25][33].
Although the chemical composition of seed oil has been investigated only in E. hyemalis, this class of compounds deserves special attention because in most other genera of Ranunculaceae it is taxonomically significant. For instance, in most of Ranunculus L. species, hexadecadienoic acid (16:2n − 6) is dominant and constitutes 2–10% of seed oil. For the genera Pulsatilla Mill., Adonis L., and Aconitum L. and some Anemone L. species, the major fatty acid (up to 80% of total) is linoleic acid, whereas the relative abundance of eicosadienoic acid reaches 7–8% in some Anemone species, and its concentration in the species of Cimicifuga Wernisch., Helleborus L., Actaea L., and Caltha L. is the lowest [50]. In E. hyemalis, cis-13,16-docosadienoic acid (109) serves as a major fatty acid, constituting up to 57% of seed oil [33]. In terms of the total set of fatty acids in seed oil, E. hyemalis is close to the genera Cimicifuga and Actaea, but more detailed conclusions require additional investigation.
2.9. Lectins
The name lectin was proposed by W. Boyd in 1954 [51] for proteins that can agglutinate red blood cells and selectively bind to carbohydrates [52]. So far, more than 500 lectins have been isolated from higher and lower plants [53] and can accumulate in roots, leaves, fruits, seeds, and wood [54][55]. It is believed that they provide protection to plants from phytopathogenic microorganisms and phytophages, play a decisive role in the establishment of symbiotic relationships with nitrogen-fixing bacteria, and participate in the transport of hormones and glycoproteins [55][56][57].
To date, in the family Ranunculaceae, only in Clematis montana Buch.-Ham and Eranthis hyemalis have researchers demonstrated the presence of lectins. The E. hyemalis lectin, called EHL, was first isolated in the second half of the 1980s by B.P. Cammue [34]. This lectin represents the most widespread type of lectin among plants: ribosome-inactivating proteins [58][59][60]. EHL, just like other ribosome-inactivating proteins, consists of two chains: chain A is responsible for enzymatic activity, and chain B binds carbohydrates, thereby helping the lectin molecule get inside the cell. In terms of its specificity to carbohydrates, EHL belongs to type II, that is, it can bind to D-galactose and N-acetyl-D-galactosamine. The role of bound carbohydrates is probably to increase the water solubility of a given glycoprotein [61]. Structurally, EHL resembles the lectin of Bryonia dioica Jacq. (Cucurbitaceae), but the latter possesses moderate activity, its relative abundance does not exceed 0.4% of the total soluble protein, and this lectin is found in all vegetative organs [62]. On the contrary, EHL is located in underground organs, its relative abundance reaches 2% of the total amount of soluble proteins, and in terms of activity, EHL exceeds the lectin of B. dioica 20-fold [34]. The research of B.P. Cammue was expanded in 1993 by M.A. Kumar and coworkers, who characterized in detail physicochemical properties of the lectin and determined a part of amino acid sequence of chain A [35].
2.10. Compounds from Other Classes
In a leaf extract of E. longistipitata, by means of liquid chromatography combined with high-resolution mass spectrometry, the presence of amino acid–related compounds (112–117) has been established: D-(+)-pyroglutamic acid, D-(+)-tryptophan, isoleucine, L-phenylalanine, L-tyrosine, and D-(−)-glutamine; the researchers detected organic acids (118–120): citric acid, D-α-hydroxyglutaric acid, and gluconic acid; sugars (121–123): α-lactose, D-(+)-galactose, and α-trehalose; alcohol D-(−)-mannitol (124); and phenylpropanoid 6-gingerol (125) [25].
References
- Compton, J.A.; Culham, A.; Jury, S.L. Reclassification of Actaea to include Cimicifuga and Souliea (Ranunculaceae): Phytogeny inferred from morphology, nrDNA ITS, and cpDNA trnL-F sequence variation. Taxon 1998, 47, 593–634.
- Wang, W.; Li, R.Q.; Chen, Z.D. Systematic position of Asteropyrum (Ranunculaceae) inferred from chloroplast and nuclear sequences. Plant Syst. Evol. 2005, 255, 41–54.
- Yuan, Q.; Yang, Q.E. Tribal relationships of Beesia, Eranthis and seven other genera of Ranunculaceae: Evidence from cytological characters. Bot. J. Linn. Soc. 2006, 150, 267–289.
- Ling, Y.Y.; Xiang, K.L.; Peng, H.W.; Erst, A.S.; Lian, L.; Zhao, L.; Jabbour, F.; Wang, W. Biogeographic diversification of Actaea (Ranunculaceae): Insights into the historical assembly of deciduous broad-leaved forests in the Northern Hemisphere. Mol. Phylogenet. Evol. 2023, 186, 107870.
- Tamura, M. Morphology, ecology and phylogeny of the Ranunculaceae VII. Sci. Rep. Osaka Univ. 1968, 17, 41–56.
- Tamura, M. Eranthis. In Die Natürlichen Pflanzenfamilien; Duncker und Humblot: Berlin/Heidelberg, Germany, 1995; Volume 17, pp. 253–255.
- Yang, Q.-E. Correction of karyotype of diploid Beesia calthifolia and discovery of a tetraploid cytotype. J. Syst. Evol. 1999, 37, 1–9.
- Compton, J.A.; Culham, A. Phylogeny and circumscription of tribe Actaeeae (Ranunculaceae). Syst. Bot. 2002, 27, 502–511.
- Lee, C.S.; Yeau, S.H.; Lee, N.S. Taxonomic status and genetic variation of Korean endemic plants, Eranthis byunsanensis and Eranthis pungdoensis (Ranunculaceae) based on nrDNA ITS and cpDNA sequences. J. Plant Biol. 2012, 55, 165–177.
- Park, S.Y.; Jeon, M.J.; Ma, S.H.; Wahlsteen, E.; Amundsen, K.; Kim, J.H.; Suh, J.K.; Chang, J.S.; Joung, Y.H. Phylogeny and genetic variation in the genus Eranthis using nrITS and cpIS singlenucleotide polymorphisms. Hortic. Environ. Biotechnol. 2019, 60, 239–252.
- Erst, A.S.; Sukhorukov, A.P.; Mitrenina, E.Y.; Skaptsov, M.V.; Kostikova, V.A.; Chernisheva, O.A.; Troshkina, V.; Kushunina, M.; Krivenko, D.A.; Ikeda, H.; et al. An integrative taxonomic approach reveals a new species of Eranthis (Ranunculaceae) in North Asia. PhytoKeys 2020, 140, 75–100.
- Tamura, M. Eranthis and Shibateranthis. Acta Phytotax. Geobot. 1987, 38, 96–97.
- Stefanoff, B. Dopolnitelni materiali vrhu florata na Blgaria. Izv. Bot. Inst. 1943, 11, 155. (In Bulgarian)
- Rukšāns, J.; Zetterlund, H. Eranthis iranica (Ranunculaceae) Rukšāns & Zetterlund new species of winter aconite from Iran. Intern. Rock Gard. 2018, 108, 2–19.
- Erst, A.S.; Tashev, A.N.; Bancheva, S.T. New record of Eranthis bulgarica Stef. (Ranunculaceae) for the flora of Serbia. Syst. Notes Mater. P. N. Krylov Herb. Tomsk. State Univ. 2020, 121, 32–36.
- Rukšāns, J. Eranthis kurdica (Ranunculaceae) Rukšāns—A new species of winter aconite (Eranthis, Ranunculaceae) from Iran. Intern. Rock Gard. 2022, 151, 2–18.
- Huang, Z.; Zhang, X. Floral nectaries and pseudonectaries in Eranthis (Ranunculaceae): Petal development, micromorphology, structure and ultrastructure. Protoplasma 2022, 259, 1283–1300.
- Hao, D.C.; Gu, X.J.; Xiao, P.G.; Liang, Z.G.; Xu, L.J.; Peng, Y. Recent advance in chemical and biological studies of Cimicifugeae pharmaceutical resources. Chin. Herb. Med. 2013, 5, 81–95.
- Hao, D.C.; Gu, X.J.; Xiao, P.G. Chemical and biological studies of Cimicifugeae pharmaceutical resources. In Medicinal Plants: Chemistry, Biology and Omics; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 8; pp. 293–340.
- Hao, D.C.; He, C.N.; Shen, J.; Xiao, P.G. Anticancer chemodiversity of Ranunculaceae medicinal plants: Molecular mechanisms and functions. Curr. Genom. 2017, 18, 39–59.
- Kuroda, M.; Uchida, S.; Watanabe, K.; Mimaki, Y. Chromones from the tubers of Eranthis cilicica and their antioxidant activity. Phytochemistry 2009, 70, 288–293.
- Junior, P. Eranthin and eranthin-β-D-glucoside: Two new chromones from Eranthis hiemalis. Phytochemistry 1979, 18, 2053–2054.
- Kopp, B.; Kubelka, E.; Reich, C.; Robien, W.; Kubelka, W. 4-H-Chromenone glycosides from Eranthis hyemalis (L.) Salisbury. Helv. Chim. Acta. 1991, 74, 611–616.
- Harborne, J.B. Biochemistry of Phenolic Compounds; Academic Press: New York, NY, USA, 1964; 88p.
- Erst, A.S.; Chernonosov, A.A.; Petrova, N.V.; Kulikovskiy, M.S.; Maltseva, S.Y.; Wang, W.; Kostikova, V.A. Investigation of chemical constituents of Eranthis longistipitata (Ranunculaceae): Coumarins and furochromones. Int. J. Mol. Sci. 2022, 23, 406.
- Wada, H.; Gaino, M.; Saito, S. Furochromones of Erantis pinnatifida. Phytochemistry 1974, 13, 297–299.
- Egger, K. Khellolglucosid in Eranthis hiemalis. Z. Naturforsch. 1961, 16, 697–702.
- Watanabe, K.; Mimaki, Y.; Sakuma, C.; Sashida, Y. Eranthisaponins A and B, two new bisdesmosidic triterpene saponins from the tubers of Eranthis cilicica. J. Nat. Prod. 2003, 66, 879–882.
- Watanabe, K.; Mimaki, Y.; Fukaya, H.; Matsuo, Y. Cycloartane and oleanane glycosides from the tubers of Eranthis cilicica. Molecules 2019, 24, 69.
- Slavík, J.; Bochořáková, J.; Slavíková, L. Occurrence of magnoflorine and corytuberine in some wild or cultivated plants of Czechoslovakia. Coll. Czechosl. Chem. Commun. 1987, 52, 804–812.
- Kostikova, V.A.; Erst, A.S.; Kuznetsov, A.A.; Gureyeva, I.I. Levels of phenolic compounds in leaves of Eranthis sibirica, E. stellata, and E. tanhoensis (Ranunculaceae). Ukr. J. Ecol. 2020, 10, 232–237.
- Kostikova, V.A.; Chernonosov, A.A.; Kuznetsov, A.A.; Petrova, N.V.; Krivenko, D.A.; Chernysheva, O.A.; Wang, W.; Erst, A.S. Identification of Flavonoids in the Leaves of Eranthis longistipitata (Ranunculaceae) by Liquid Chromatography with High—Resolution Mass Spectrometry (LC-HRMS). Plants 2021, 10, 2146.
- Aitzetmüller, K. An unusual fatty acid pattern in Eranthis seed oil. Lipids 1996, 31, 201–205.
- Cammue, B.P.; Peeters, B.; Peumans, W.J. Isolation and partial characterization of an N-acetylgalactosaminespecific lectin from winter-aconite (Eranthis hyemalis) root tubes. Biochem. J. 1985, 227, 949–955.
- Kumar, M.A.; Timm, D.E.; Neet, K.E.; Owen, W.G.; Peumans, W.J.; Rao, A. Characterization of the lectin from the bulbs of Eranthis hyemalis (winter aconite) as an inhibitor of protein synthesis. J. Biol. Chem. 1993, 268, 25176–25183.
- Edwards, A.M.; Howell, J.B.L. The chromones: History, chemistry and clinical development. A tribute to the work of Dr. R. E. C. Altounyan. Clin. Exp. Allergy 2000, 30, 756–774.
- Vanguru, M.; Merugu, R.; Garimella, S.; Laxminarayana, E. A review on the synthetic methodologies of chromones. Asian J. Pharm. Clin. Res. 2018, 11, 9–16.
- Luthria, D.L.; Banerji, A. Biosynthesis of furanochromones in Pimpinella monoica. Proc. Indian Acad. Sci. (Chem. Sci.) 1994, 106, 1149–1156.
- Badr, J.M.; Hadad, G.M.; Nahriry, K.; Hassanean, H.A. Validated HPLC method for simultaneous estimation of khellol glucoside, khellin and visnagin in Ammi visnaga L. fruits and pharmaceutical preparations. Nat. Prod. Res. 2015, 29, 593–601.
- Adimcilar, V.; Beyazit, N.; Erim, F.B. Khellin and visnagin in different organs of Ammi visnaga and Ammi majus. Nat. Prod. Res. 2023, 37, 164–166.
- Basnet, P.; Kadota, S.; Manandhar, K.; Manandhar, M.D.; Namba, T. Constituents of Boenninghausenia albiflora: Isolation and identification of some coumarins. Planta Med. 1993, 59, 384–386.
- Wu, A.; Lu, J.; Zhong, G.; Lu, L.; Qu, Y.; Zhang, C. Xanthoxin (8-methoxypsoralen): A review of its chemistry, pharmacology, pharmacokinetics, and toxicicity. Phytother. Res. 2022, 36, 3805–3832.
- Dai, J.; Chen, X.; Cheng, W.; Liu, X.; Fan, X.; Shen, Z.; Bi, K. A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of two active chromones from Saposhnikovia root in rat plasma and urine. J. Chromatogr. B 2008, 868, 13–19.
- Liu, R.; Wu, S.; Sun, A. Separation and purification of four chromones from radix saposhnikoviae by high-speed counter-current chromatography. Phytochem. Anal. 2008, 19, 206–211.
- Ma, S.Y.; Shi, L.G.; Gu, Z.B.; Wu, Y.L.; Wei, L.B.; Wei, Q.Q.; Gao, X.L.; Liao, N. Two new chromone glycosides from the roots of Saposhnikovia divaricata. Chem. Biodivers. 2018, 15, e1800253.
- Maior, M.C.; Dobrotă, C. Natural compounds with important medical potential found in Helleborus sp. Cent. Eur. J. Biol. 2013, 8, 272–285.
- Simonsen, J.L. The Terpenes, 2nd ed.; Cambridge University Press: Cambridge, UK, 1957; Volume 2, 654p.
- Mimaki, Y.; Watanabe, K.; Matsuo, Y.; Sakagami, H. Triterpene glycosides from the tubers of Anemone coronaria. Chem. Pharm. Bull. 2009, 57, 724–729.
- Budantsev, A.L. (Ed.) Plant Resources of Russia: Wild Flowering Plants, Their Component Composition and Biological Activity. T. 1: Families Magnoliaceae—Juglandaceae, Ulmaceae, Moraceae, Cannabaceae, Urticaceae; KMK: Saint Petersburg, Russia; Moscow, Russia, 2008; 421p. (In Russian)
- Aitzetmüller, K.; Tsevegsüren, N. Seed fatty acids, “front-end”-desaturases and chemotaxonomy—A case study in the Ranunculaceae. J. Plant Physiol. 1994, 143, 538–543.
- Boyd, W.C.; Shapleigh, E. Specific precipitating activity of plant agglutinins (lectins). Science 1954, 119, 419.
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53–62.
- Santos, A.F.S.; da Silva, M.D.C.; Napoleäo, T.H.; Paiva, P.M.G.; Correia, M.T.S.; Coelho, L.C.B.B. Lectins: Functions, structure, biological properties and potential applications. Curr. Top. Pept. Protein Res. 2014, 15, 41–62.
- Vann Damme, J.M.; Peumans, W.J.; Barre, A.; Rougé, P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998, 17, 575–692.
- Peumans, W.J.; Hao, Q.; Van Damme, E.J. Ribosome-inactivating proteins from plants: More than RNA N-glycosidases? FASEB J. 2001, 15, 1493–1506.
- Rao, K.; Rathore, K.S.; Hodges, T.K.; Fu, X.; Stoger, E.; Sudhakar, D.; Brown, D.P.; Powell, K.S.; Spence, J.; Gatehouse, A.M.; et al. Expression of snowdrop lectin (GNA) in transgenic rice plants confers resistance to rice brown planthopper. Plant J. 1998, 15, 469–477.
- Sharon, N.; Lis, H. Lectins, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007; p. 454.
- Park, W.B.; Han, S.K.; Lee, M.H.; Han, K.H. Isolation and characterization of lectins from stem and leaves of Korean mistletoe (Viscum album var. coloratum) by affinity chromatography. Arch. Pharmacol. Res. 1997, 20, 306–312.
- Yoon, T.J.; Yoo, Y.C.; Kang, T.B.; Shimazaki, K.; Song, S.K.; Lee, K.H.; Kim, S.H.; Park, C.H.; Azuma, I.; Kim, J.B. Lectins isolated from Korean mistletoe (Viscum album coloratum) induce apoptosis in tumor cells. Cancer Lett. 1999, 136, 33–40.
- Lyu, S.Y.; Park, S.M.; Choung, B.Y.; Park, W.B. Comparative study of Korean (Viscum album var. coloratum) and European mistletoes (Viscum album). Arch. Pharmacal Res. 2000, 23, 592–598.
- George, O.; Solscheid, C.; Bertolo, E.; Fell, J.; Lisgarten, D. Extraction and purification of the lectin found in the tubers of Eranthis hyemalis (winter aconite). J. Integr. OMICS 2011, 1, 268–271.
- Peumans, W.J.; Nsimba-Lubaki, M.; Carlier, A.R.; Van Driessche, E. A lectin from Bryonia dioica root stocks. Planta 1984, 160, 222–228.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
657
Revisions:
2 times
(View History)
Update Date:
23 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




