Despite the limited geographic range of Eranthis plants, it is possible to search for active substances, develop methods for biological and chemical synthesis of the isolated substances, and create a finished therapeutic substance based on them. Seven out of ~14 species found in Asia and parts of Europe have been studied to various degrees. Here, data are presented on the diversity of sets of chromones, furochromones, triterpene saponins, coumarins, and other classes of secondary metabolites of Eranthis species according to the literature. For new compounds—isolated from Eranthis for the first time—structural formulas are also provided.
- Eranthis
- chromone
- furochromone
- triterpene saponin
- coumarin
- biological activity
1. Introduction

2. Phytocomponents Identified in Eranthis Plants and Their Chemotaxonomic Significance
2.1. Chromones
| ID No. | Compound | Source Eranthis Species | Reference |
|---|---|---|---|
| Chromones | |||
| 1 | 8,11-dihydro-5-hydroxy-2,9-dihydroxymethyl-4H-pyrano [2,3-g][1] benzoxepin-4-one |
E. cilicica (T *) | [21] |
| 2 | Eranthin (5-hydroxy-9-hydroxymethyl-2-methyl-8,11-dihydro-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (R) | [22] |
| 3 | Eranthin-β-D-glucoside (9-{[(β-D-glucopyranosvl)oxy]methyl}-8,11-dihydro-5-hydroxy-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (R, T) | [22][23] |
| 4 | Eranthin 9-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) E. hyemalis (T) |
[21][23] |
| 5 | Eranthin β-D-gentiobioside (9-{[(β-D-gentiobiosyl)oxy]methyl}-8,11-dihydro-5-hydroxy-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (T) | [23] |
| 6 | 2-C-Hydroxyeranthin β-D-glucopyranoside (9-{[(β-D-glucopyranosyl)oxy]methyl}-8,11-dihydro-5-hydroxy-2-(hydroxymethyl-4H-pyrano[2,3-g][1]benzoxepin-4-one) |
E. hyemalis (T) | [23] |
| 7 | 9-[(O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl)oxy]methyl-8,11-dihydro-5,9-dihydroxy-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one | E. cilicica (T) | [21] |
| 8 | 8,11-dihydro-5,9-dihydroxy-9-hydroxymethyl-2-methyl-4H-pyrano[2,3-g][1]benzoxepin-4-one | E. cilicica (T) | [21] |
| 9 | 5,7-dihydroxy-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-2-methyl-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 10 | 5,7-dihydroxy-2-hydroxymethyl-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 11 | 7-[(β-D-glucopyranosyl)oxy]-5-hydroxy-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-2-methyl-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 12 | 7-[(β-D-glucopyranosyl)oxy]-5-hydroxy-2-hydroxymethyl-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-4H-1-benzopyran-4-one | E. cilicica (T) | [21] |
| 13 | 7,8-Secoeranthin β-D-glucoside (8-{(2E)-4-[(β-D-glucopyranosyl)oxy]-3-methylbut-2-enyl}-5,7-dihydroxy-2-methyl-4H-1-benzopyran-4-one) |
E. hyemalis (T) | [23] |
| 14 | 2-C-Hydroxy-7,8-secoeranthin β-D-glucoside (8-{(2E)-4-[(β-D-glucopyranosyl)oxy]-3-methylbut-2-enyl}-5,7-dihydroxy-2-(hydroxymethyl)-4H-1-benzopyran-4-one) |
E. hyemalis (T) | [23] |
| Furochromones | |||
| 15 | Visnagin (4-methoxy-7-methyl-5H-furo[3,2-g]chromen-5-one) |
E. hyemalis E. longistipitata (L) |
[24][25] |
| 16 | Khellin (4,9-dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-one) |
E. hyemalis E. longistipitata (L) |
[24][25] |
| 17 | Khellol (7-(hydroxymethyl)-4-methoxyfuro[3,2-g]chromen-5-one) |
E. pinnatifida (L, St) | [26] |
| 18 | Khellol glucoside (khellinin; 7-hydroxymethyl-4-methoxy-5H-furo [3,2-g]][1]benzopyran-5-one glucoside) |
E. hyemalis (L, F) | [27] |
| 19 | Norkhellol (4-hydroxy-7-(hydroxymethyl)-5H-furo[3,2-g][1]benzopyran-5-one) |
E. pinnatifida (L, St) | [26] |
| 20 | Norammiol (4-hydroxy-7(hydroxymethyl)-9-methoxy-5H-furo[3,2-g][1]-benzopyran-5-one) |
E. pinnatifida (L, St) | [26] |
| 21 | 7-[(O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl)oxy]methyl-4-hydroxy-5H-furo[3,2-g][1]benzopyran-5-one | E. cilicica (T) | [21] |
| 22 | Methoxsalen (9-methoxyfuro[3,2-g]chromen-7-one) |
E. longistipitata (L) | [25] |
| 23 | Cimifugin (2S)-7-(hydroxymethyl)-2-(2-hydroxypropan-2-yl)-4-methoxy-2,3-dihydrofuro[3,2g]chromen-5-one) |
E. pinnatifida (L, St) E. cilicica (T) E. longistipitata (L) |
[21][25][26] |
| 24 | Norcimifugin (2S)-4-hydroxy-7-(hydroxymethyl)-2-(2-hydroxypropan-2-yl)-2,3-dihydrofuro[3,2-g]-chromen-5-one) |
E. pinnatifida (L, St) | [26] |
| 25 | Cimifugin β-D-glucopyranoside (7-{[(β-D-glucopyranosy1)oxy]methyl}-2,3-dihydro-2-(l-hydroxy-1-methylethyl)-4-methoxy-5H-furo[3,2-g][1]benzopyran-5-one) |
E. hyemalis (T) | [23] |
| 26 | 5-O-Methylvisammioside (4-O-β-D-glucosyl-5-O-methylvisamminol) |
E. longistipitata (L) | [25] |
| 27 | Visamminol-3′-O-glucoside (4-hydroxy-2-(2-hydroxypropan-2- yl)-methyl-2,3-dihydrofuro[3,2-g] chromen-5-one) |
E. longistipitata (L) | [25] |
| Triterpene saponins | |||
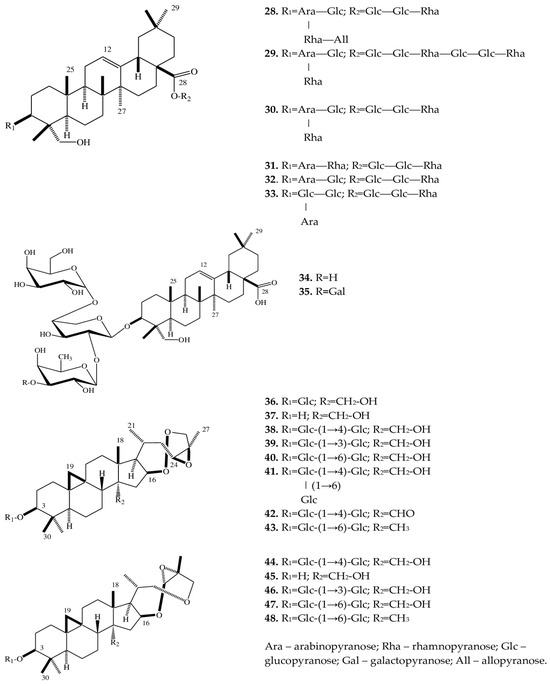
| 28 | Eranthisaponin A | E. cilicica (T) | [28] |
| (3β-[(O-β-D-allopyranosyl-(1→3)-O-α-L-rhamnopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranosyl)oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl- (1→6)-β-D-glucopyranoside) |
|||
| 29 | Eranthisaponin B (3β-[(O-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl-(1→4)-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl- (1→6)-β-D-glucopyranoside) |
E. cilicica (T) | [28] |
| 30 | 3β-[(O-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside |
E. cilicica (T) | [28] |
| 31 | 23-Hydroxy-3β-[(O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranosyl)oxy]olean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [28] |
| 32 | 3β-[(O-β-D-glucopyranosyl-(1→4)-α-L-arabinopyranosyl)oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [28] |
| 33 | 3β-[(O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranosyl)oxy]-23-hydroxyolean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [28] |
| 34 | 3β-[(O-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid |
E. cilicica (T) | [29] |
| 35 | 3β-[(O-β-D-galactopyranosyl-(1→3)-O-α-L-rhamnopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→4)]-α-L-arabinopyranosyl) oxy]-23-hydroxyolean-12-en-28-oic acid | E. cilicica (T) | [29] |
| 36 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl β-D-glucopyranoside | E. cilicica (T) | [29] |
| 37 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartane-3β,28-diol | E. cilicica (T) | [29] |
| 38 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 39 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 40 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 41 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→4)-O-[β-D-glucopyranosyl (1→6)]-β-D-glucopyranoside |
E. cilicica (T) | [29] |
| 42 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-28-oxo-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 43 | (23R,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 44 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 45 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartan-3β,28-diol | E. cilicica (T) | [29] |
| 46 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 47 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-28-hydroxy-9,19-cylcoartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| 48 | (23S,24R,25R)-16β,23:23,26:24,25-triepoxy-9,19-cycloartan-3β-yl O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside | E. cilicica (T) | [29] |
| Alkaloids | |||
| 49 | Corytuberine (2,10-dimethoxy-6α-aporphine-1,11-diol) |
E. hyemalis (T; Ap) | [30] |
| Coumarins | |||
| 50 | 5,7-Dihydroxy-4-methylcoumarin | E. longistipitata (L) | [25] |
| 51 | Scoparone (6,7-dimethoxycoumarin) |
E. longistipitata (L) | [25] |
| 52 | Fraxetin (7,8-dihydroxy-6-methoxycoumarin) |
E. longistipitata (L) | [25] |
| 53 | Luvangetin (10-methoxy-2,2-dimethylpyrano[3,2-g]chromen-8-one) |
E. longistipitata (L) | [25] |
| Flavonoids | |||
| 54 | Quercetin | E. longistipitata (L) E. stellata (L) E. tanhoensis (L) |
[25][31][32] |
| 55 | Isoquercitrin (quercetin-3-O-β-D-glucoside) |
E. longistipitata (L) | [25][32] |
| 56 | Hyperoside (quercetin 3-O-β-D-galactoside) |
E. longistipitata (L) | [25][32] |
| 57 | Reynoutrin (quercetin-3-O-β-D-xylopyranoside) |
E. longistipitata (L) | [25][32] |
| 58 | Quercetin-6-O-β-D-xylopyranosyl-β-D- glucopyranoside |
E. longistipitata (L) | [25][32] |
| 59 | Quercetin-3-sambubioside (quercetin-3-O-[β-D-xylosyl-(1→2)-β-D-glucoside]) |
E. longistipitata (L) | [25][32] |
| 60 | Peltatoside (quercetin-3-(6-O-α-L-arabinopyranosyl)-β-D- glucopyranoside)) |
E. longistipitata (L) | [25][32] |
| 61 | Rutin (quercetin 3-O-β-D-rutinoside) |
E. longistipitata (L) | [25][32] |
| 62 | Kaempferol | E. longistipitata (L) E. stellata (L) E. tanhoensis (L) |
[25][31][32] |
| 63 | Juglalin (kaempferol 3-O-α-L-arabinopyranoside) |
E. longistipitata (L) | [25][32] |
| 64 | Trifolin (kaempferol-3-O-β-D-galactoside) |
E. longistipitata (L) | [25][32] |
| 65 | Aromadendrin [(+)-dihydrokaempferol] |
E. longistipitata (L) | [25][32] |
| 66 | Vitexin (apigenin 8-C-glucoside) |
E. sibirica (L) | [31] |
| 67 | Orientin (luteolin-8-C-glucoside) |
E. sibirica (L) | [31] |
| E. stellata (L) | |||
| 68 | Carlinoside (luteolin 6-C-β-D-glucopyranoside-8-C-α-L- |
E. longistipitata (L) | [25][32] |
| arabinopyranoside) | |||
| 69 | Cianidanol [(+)-catechin] |
E. longistipitata (L) | [25][32] |
| 70 | Auriculoside (7,3,5′-trihydroxy-4′-methoxyflavan-3′-glucoside) |
E. longistipitata (L) | [25][32] |
| 71 | 6-Methoxytaxifolin | E. longistipitata (L) | [25][32] |
| 72 | Aspalathin | E. longistipitata (L) | [25][32] |
| 73 | Phloridzin (phloretin-2′-O-β-glucoside) |
E. longistipitata (L) | [25][32] |
| 74 | Phloretin (dihydroxy naringenin) |
E. longistipitata (L) | [25][32] |
| Cinnamic acids | |||
| 75 | Chlorogenic acid (3-O-caffeoylquinic acid) |
E. sibirica (L) E. stellata (L) E. tanhoensis (L) |
[31] |
| 76 | Caffeic acid (3,4-dihydroxycinnamic acid) |
E. sibirica (L) E. stellata (L) |
[31] |
| Phenolic acids | |||
| 77 | Salicylic acid (3-tert-2-butyl-2-hydroxy-6-methylbenzoic acid) |
E. sibirica (L) E. tanhoensis (L) |
[31] |
| 78 | Gentisic acid (2,5-dihydroxybenzoic acid) |
E. stellata (L) | [31] |
| Fatty acids and their derivatives | |||
| 79 | Myristic acid (14:0) | E. hyemalis (S) | [33] |
| 80 | Pentadecylic acid (15:0) | E. hyemalis (S) | [33] |
| 81 | Palmitic acid (16:0) | E. hyemalis (S) | [33] |
| 82 | 16-Hydroxyhexadecanoic acid | E. longistipitata (L) | [25] |
| 83 | cis-5-Hexadecenoic acid (16:1 Δ5cis) | E. hyemalis (S) | [33] |
| 84 | Palmitoleic acid (16:1 Δ9cis) | E. longistipitata (L) | [25] |
| 85 | cis-9-Octadecanoic acid (18:0 Δ9cis) | E. hyemalis (S) | [33] |
| 86 | cis-Vaccenic acid (18:1 Δ11cis) | E. hyemalis (S) | [33] |
| 87 | Linoleic acid (18:2 Δ9cis, 12cis) | E. hyemalis (S) | [33] |
| 88 | 9-oxo-ODA (9-Oxo-trans-10, trans-12-octadecadienoic acid) |
E. longistipitata (L) | [25] |
| 89 | (+/−)13-HODE | E. longistipitata (L) | [25] |
| (13-hydroxyoctadecadienoic acid) | |||
| 90 | Corchorifatty acid F (9,12,13-trihydroxy-10(E),15(Z)-octadecadienoic acid) | E. longistipitata (L) | [25] |
| 91 | α-Linolenic acid (18:3 Δ9cis, 12cis, 15cis) | E. hyemalis (S) E. longistipitata (L) |
[25][33] |
| 92 | Linolenic acid ethyl ester | E. longistipitata (L) | [25] |
| 93 | α-Eleostearic acid (18:3 Δ9cis, 11trans, 13trans) | E. longistipitata (L) | [25] |
| 94 | Pinolenic acid (18:3 Δ5cis, 9cis, 12cis) | E. longistipitata (L) | [25] |
| 95 | 13(S)-HOTrE (13-OH-cis-9, trans-11, cis-15-octadecatrienoic acid) |
E. longistipitata (L) | [25] |
| 96 | (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | E. longistipitata (L) | [25] |
| 97 | 12-Oxo-phytodienoic acid | E. longistipitata (L) | [25] |
| 98 | 9S,13R-12-Oxo-phytodienoic acid | E. longistipitata (L) | [25] |
| 99 | Arachidic acid (20:0) | E. hyemalis (S) | [33] |
| 100 | cis-5-Eicosenoic acid (20:1 Δ5cis) | E. hyemalis (S) | [33] |
| 101 | Gondoic acid (20:1 Δ11cis) | E. hyemalis (S) | [33] |
| 102 | Keteleeronic acid (20:2 Δ5cis, 11cis) | E. hyemalis (S) | [33] |
| 103 | cis-11,14-Eicosadienoic acid (20:2 Δ11cis, 14cis) | E. hyemalis (S) | [33] |
| 104 | 15-OxoEDE (15-Oxo-cis-11,trans-13-eicosadienoic acid) |
E. longistipitata (L) | [25] |
| 105 | cis-5,11,14-Eicosatrienoic acid (20:3 Δ5cis, 11cis, 14cis) | E. hyemalis (S) | [33] |
| 106 | Behenic acid (22:0) | E. hyemalis (S) | [33] |
| 107 | Erucic acid (22:1 Δ13cis) | E. hyemalis (S) | [33] |
| 108 | cis-5,13-Docosadienoic acid (22:2 Δ5cis, 13cis) | E. hyemalis (S) | [33] |
| 109 | cis-13,16-Docosadienoic acid (22:2 Δ13cis, 16cis) | E. hyemalis (S) | [33] |
| 110 | cis-5,13,16-Docosatrienoic acid (22:3 Δ5cis, 13cis, 16cis) | E. hyemalis (S) | [33] |
| 111 | cis-10,13,16-Docosatrienoic acid (22:3 Δ10cis, 13cis, 16cis) | E. hyemalis (S) | [33] |
| Amino acids | |||
| 112 | D-(+)-Pyroglutamic acid | E. longistipitata (L) | [25] |
| 113 | D-(+)-Tryptophan | E. longistipitata (L) | [25] |
| 114 | Isoleucine | E. longistipitata (L) | [25] |
| 115 | L-Phenylalanine | E. longistipitata (L) | [25] |
| 116 | L-Tyrosine | E. longistipitata (L) | [25] |
| 117 | D-(−)-Glutamine | E. longistipitata (L) | [25] |
| Organic acids | |||
| 118 | Citric acid | E. longistipitata (L) | [25] |
| 119 | D-α-Hydroxyglutaric acid | E. longistipitata (L) | [25] |
| 120 | Gluconic acid | E. longistipitata (L) | [25] |
| Sugars | |||
| 121 | α-Lactose | E. longistipitata (L) | [25] |
| 122 | D-(+)-Galactose | E. longistipitata (L) | [25] |
| 123 | α.α-Trehalose | E. longistipitata (L) | [25] |
| Alcohols | |||
| 124 | D-(−)-Mannitol | E. longistipitata (L) | [25] |
| Phenylpropanoids | |||
| 125 | 6-Gingerol | E. longistipitata (L) | [25] |
| Lectins | |||
| 126 | EHL | E. hyemalis (R) | [34][35] |
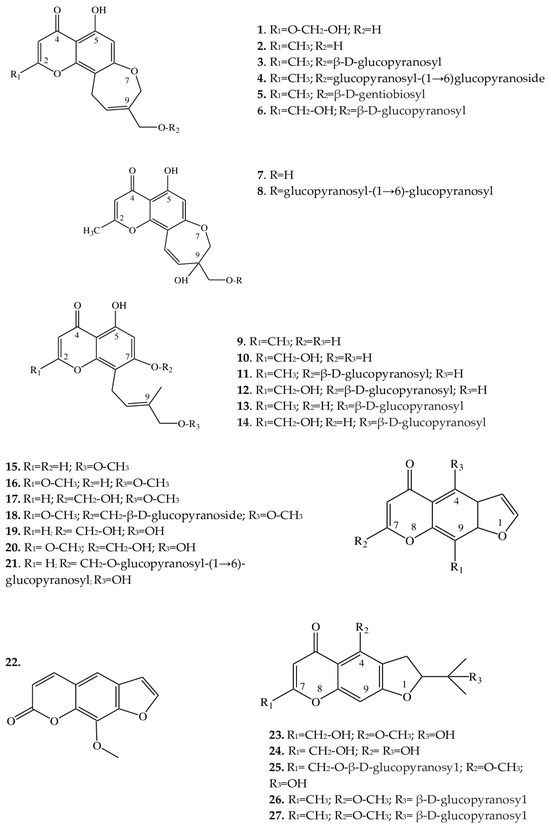
2.2. Furochromones
2.3. Triterpene Saponins
2.4. Alkaloids
2.5. Coumarins
2.6. Flavonoids
2.7. Phenolcarboxylic Acids
2.8. Fatty Acids
2.9. Lectins
2.10. Compounds from Other Classes
This entry is adapted from the peer-reviewed paper 10.3390/plants12223795
References
- Compton, J.A.; Culham, A.; Jury, S.L. Reclassification of Actaea to include Cimicifuga and Souliea (Ranunculaceae): Phytogeny inferred from morphology, nrDNA ITS, and cpDNA trnL-F sequence variation. Taxon 1998, 47, 593–634.
- Wang, W.; Li, R.Q.; Chen, Z.D. Systematic position of Asteropyrum (Ranunculaceae) inferred from chloroplast and nuclear sequences. Plant Syst. Evol. 2005, 255, 41–54.
- Yuan, Q.; Yang, Q.E. Tribal relationships of Beesia, Eranthis and seven other genera of Ranunculaceae: Evidence from cytological characters. Bot. J. Linn. Soc. 2006, 150, 267–289.
- Ling, Y.Y.; Xiang, K.L.; Peng, H.W.; Erst, A.S.; Lian, L.; Zhao, L.; Jabbour, F.; Wang, W. Biogeographic diversification of Actaea (Ranunculaceae): Insights into the historical assembly of deciduous broad-leaved forests in the Northern Hemisphere. Mol. Phylogenet. Evol. 2023, 186, 107870.
- Tamura, M. Morphology, ecology and phylogeny of the Ranunculaceae VII. Sci. Rep. Osaka Univ. 1968, 17, 41–56.
- Tamura, M. Eranthis. In Die Natürlichen Pflanzenfamilien; Duncker und Humblot: Berlin/Heidelberg, Germany, 1995; Volume 17, pp. 253–255.
- Yang, Q.-E. Correction of karyotype of diploid Beesia calthifolia and discovery of a tetraploid cytotype. J. Syst. Evol. 1999, 37, 1–9.
- Compton, J.A.; Culham, A. Phylogeny and circumscription of tribe Actaeeae (Ranunculaceae). Syst. Bot. 2002, 27, 502–511.
- Lee, C.S.; Yeau, S.H.; Lee, N.S. Taxonomic status and genetic variation of Korean endemic plants, Eranthis byunsanensis and Eranthis pungdoensis (Ranunculaceae) based on nrDNA ITS and cpDNA sequences. J. Plant Biol. 2012, 55, 165–177.
- Park, S.Y.; Jeon, M.J.; Ma, S.H.; Wahlsteen, E.; Amundsen, K.; Kim, J.H.; Suh, J.K.; Chang, J.S.; Joung, Y.H. Phylogeny and genetic variation in the genus Eranthis using nrITS and cpIS singlenucleotide polymorphisms. Hortic. Environ. Biotechnol. 2019, 60, 239–252.
- Erst, A.S.; Sukhorukov, A.P.; Mitrenina, E.Y.; Skaptsov, M.V.; Kostikova, V.A.; Chernisheva, O.A.; Troshkina, V.; Kushunina, M.; Krivenko, D.A.; Ikeda, H.; et al. An integrative taxonomic approach reveals a new species of Eranthis (Ranunculaceae) in North Asia. PhytoKeys 2020, 140, 75–100.
- Tamura, M. Eranthis and Shibateranthis. Acta Phytotax. Geobot. 1987, 38, 96–97.
- Stefanoff, B. Dopolnitelni materiali vrhu florata na Blgaria. Izv. Bot. Inst. 1943, 11, 155. (In Bulgarian)
- Rukšāns, J.; Zetterlund, H. Eranthis iranica (Ranunculaceae) Rukšāns & Zetterlund new species of winter aconite from Iran. Intern. Rock Gard. 2018, 108, 2–19.
- Erst, A.S.; Tashev, A.N.; Bancheva, S.T. New record of Eranthis bulgarica Stef. (Ranunculaceae) for the flora of Serbia. Syst. Notes Mater. P. N. Krylov Herb. Tomsk. State Univ. 2020, 121, 32–36.
- Rukšāns, J. Eranthis kurdica (Ranunculaceae) Rukšāns—A new species of winter aconite (Eranthis, Ranunculaceae) from Iran. Intern. Rock Gard. 2022, 151, 2–18.
- Huang, Z.; Zhang, X. Floral nectaries and pseudonectaries in Eranthis (Ranunculaceae): Petal development, micromorphology, structure and ultrastructure. Protoplasma 2022, 259, 1283–1300.
- Hao, D.C.; Gu, X.J.; Xiao, P.G.; Liang, Z.G.; Xu, L.J.; Peng, Y. Recent advance in chemical and biological studies of Cimicifugeae pharmaceutical resources. Chin. Herb. Med. 2013, 5, 81–95.
- Hao, D.C.; Gu, X.J.; Xiao, P.G. Chemical and biological studies of Cimicifugeae pharmaceutical resources. In Medicinal Plants: Chemistry, Biology and Omics; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 8; pp. 293–340.
- Hao, D.C.; He, C.N.; Shen, J.; Xiao, P.G. Anticancer chemodiversity of Ranunculaceae medicinal plants: Molecular mechanisms and functions. Curr. Genom. 2017, 18, 39–59.
- Kuroda, M.; Uchida, S.; Watanabe, K.; Mimaki, Y. Chromones from the tubers of Eranthis cilicica and their antioxidant activity. Phytochemistry 2009, 70, 288–293.
- Junior, P. Eranthin and eranthin-β-D-glucoside: Two new chromones from Eranthis hiemalis. Phytochemistry 1979, 18, 2053–2054.
- Kopp, B.; Kubelka, E.; Reich, C.; Robien, W.; Kubelka, W. 4-H-Chromenone glycosides from Eranthis hyemalis (L.) Salisbury. Helv. Chim. Acta. 1991, 74, 611–616.
- Harborne, J.B. Biochemistry of Phenolic Compounds; Academic Press: New York, NY, USA, 1964; 88p.
- Erst, A.S.; Chernonosov, A.A.; Petrova, N.V.; Kulikovskiy, M.S.; Maltseva, S.Y.; Wang, W.; Kostikova, V.A. Investigation of chemical constituents of Eranthis longistipitata (Ranunculaceae): Coumarins and furochromones. Int. J. Mol. Sci. 2022, 23, 406.
- Wada, H.; Gaino, M.; Saito, S. Furochromones of Erantis pinnatifida. Phytochemistry 1974, 13, 297–299.
- Egger, K. Khellolglucosid in Eranthis hiemalis. Z. Naturforsch. 1961, 16, 697–702.
- Watanabe, K.; Mimaki, Y.; Sakuma, C.; Sashida, Y. Eranthisaponins A and B, two new bisdesmosidic triterpene saponins from the tubers of Eranthis cilicica. J. Nat. Prod. 2003, 66, 879–882.
- Watanabe, K.; Mimaki, Y.; Fukaya, H.; Matsuo, Y. Cycloartane and oleanane glycosides from the tubers of Eranthis cilicica. Molecules 2019, 24, 69.
- Slavík, J.; Bochořáková, J.; Slavíková, L. Occurrence of magnoflorine and corytuberine in some wild or cultivated plants of Czechoslovakia. Coll. Czechosl. Chem. Commun. 1987, 52, 804–812.
- Kostikova, V.A.; Erst, A.S.; Kuznetsov, A.A.; Gureyeva, I.I. Levels of phenolic compounds in leaves of Eranthis sibirica, E. stellata, and E. tanhoensis (Ranunculaceae). Ukr. J. Ecol. 2020, 10, 232–237.
- Kostikova, V.A.; Chernonosov, A.A.; Kuznetsov, A.A.; Petrova, N.V.; Krivenko, D.A.; Chernysheva, O.A.; Wang, W.; Erst, A.S. Identification of Flavonoids in the Leaves of Eranthis longistipitata (Ranunculaceae) by Liquid Chromatography with High—Resolution Mass Spectrometry (LC-HRMS). Plants 2021, 10, 2146.
- Aitzetmüller, K. An unusual fatty acid pattern in Eranthis seed oil. Lipids 1996, 31, 201–205.
- Cammue, B.P.; Peeters, B.; Peumans, W.J. Isolation and partial characterization of an N-acetylgalactosaminespecific lectin from winter-aconite (Eranthis hyemalis) root tubes. Biochem. J. 1985, 227, 949–955.
- Kumar, M.A.; Timm, D.E.; Neet, K.E.; Owen, W.G.; Peumans, W.J.; Rao, A. Characterization of the lectin from the bulbs of Eranthis hyemalis (winter aconite) as an inhibitor of protein synthesis. J. Biol. Chem. 1993, 268, 25176–25183.
- Edwards, A.M.; Howell, J.B.L. The chromones: History, chemistry and clinical development. A tribute to the work of Dr. R. E. C. Altounyan. Clin. Exp. Allergy 2000, 30, 756–774.
- Vanguru, M.; Merugu, R.; Garimella, S.; Laxminarayana, E. A review on the synthetic methodologies of chromones. Asian J. Pharm. Clin. Res. 2018, 11, 9–16.
- Luthria, D.L.; Banerji, A. Biosynthesis of furanochromones in Pimpinella monoica. Proc. Indian Acad. Sci. (Chem. Sci.) 1994, 106, 1149–1156.
- Badr, J.M.; Hadad, G.M.; Nahriry, K.; Hassanean, H.A. Validated HPLC method for simultaneous estimation of khellol glucoside, khellin and visnagin in Ammi visnaga L. fruits and pharmaceutical preparations. Nat. Prod. Res. 2015, 29, 593–601.
- Adimcilar, V.; Beyazit, N.; Erim, F.B. Khellin and visnagin in different organs of Ammi visnaga and Ammi majus. Nat. Prod. Res. 2023, 37, 164–166.
- Basnet, P.; Kadota, S.; Manandhar, K.; Manandhar, M.D.; Namba, T. Constituents of Boenninghausenia albiflora: Isolation and identification of some coumarins. Planta Med. 1993, 59, 384–386.
- Wu, A.; Lu, J.; Zhong, G.; Lu, L.; Qu, Y.; Zhang, C. Xanthoxin (8-methoxypsoralen): A review of its chemistry, pharmacology, pharmacokinetics, and toxicicity. Phytother. Res. 2022, 36, 3805–3832.
- Dai, J.; Chen, X.; Cheng, W.; Liu, X.; Fan, X.; Shen, Z.; Bi, K. A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of two active chromones from Saposhnikovia root in rat plasma and urine. J. Chromatogr. B 2008, 868, 13–19.
- Liu, R.; Wu, S.; Sun, A. Separation and purification of four chromones from radix saposhnikoviae by high-speed counter-current chromatography. Phytochem. Anal. 2008, 19, 206–211.
- Ma, S.Y.; Shi, L.G.; Gu, Z.B.; Wu, Y.L.; Wei, L.B.; Wei, Q.Q.; Gao, X.L.; Liao, N. Two new chromone glycosides from the roots of Saposhnikovia divaricata. Chem. Biodivers. 2018, 15, e1800253.
- Maior, M.C.; Dobrotă, C. Natural compounds with important medical potential found in Helleborus sp. Cent. Eur. J. Biol. 2013, 8, 272–285.
- Simonsen, J.L. The Terpenes, 2nd ed.; Cambridge University Press: Cambridge, UK, 1957; Volume 2, 654p.
- Mimaki, Y.; Watanabe, K.; Matsuo, Y.; Sakagami, H. Triterpene glycosides from the tubers of Anemone coronaria. Chem. Pharm. Bull. 2009, 57, 724–729.
- Budantsev, A.L. (Ed.) Plant Resources of Russia: Wild Flowering Plants, Their Component Composition and Biological Activity. T. 1: Families Magnoliaceae—Juglandaceae, Ulmaceae, Moraceae, Cannabaceae, Urticaceae; KMK: Saint Petersburg, Russia; Moscow, Russia, 2008; 421p. (In Russian)
- Aitzetmüller, K.; Tsevegsüren, N. Seed fatty acids, “front-end”-desaturases and chemotaxonomy—A case study in the Ranunculaceae. J. Plant Physiol. 1994, 143, 538–543.
- Boyd, W.C.; Shapleigh, E. Specific precipitating activity of plant agglutinins (lectins). Science 1954, 119, 419.
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53–62.
- Santos, A.F.S.; da Silva, M.D.C.; Napoleäo, T.H.; Paiva, P.M.G.; Correia, M.T.S.; Coelho, L.C.B.B. Lectins: Functions, structure, biological properties and potential applications. Curr. Top. Pept. Protein Res. 2014, 15, 41–62.
- Vann Damme, J.M.; Peumans, W.J.; Barre, A.; Rougé, P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998, 17, 575–692.
- Peumans, W.J.; Hao, Q.; Van Damme, E.J. Ribosome-inactivating proteins from plants: More than RNA N-glycosidases? FASEB J. 2001, 15, 1493–1506.
- Rao, K.; Rathore, K.S.; Hodges, T.K.; Fu, X.; Stoger, E.; Sudhakar, D.; Brown, D.P.; Powell, K.S.; Spence, J.; Gatehouse, A.M.; et al. Expression of snowdrop lectin (GNA) in transgenic rice plants confers resistance to rice brown planthopper. Plant J. 1998, 15, 469–477.
- Sharon, N.; Lis, H. Lectins, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007; p. 454.
- Park, W.B.; Han, S.K.; Lee, M.H.; Han, K.H. Isolation and characterization of lectins from stem and leaves of Korean mistletoe (Viscum album var. coloratum) by affinity chromatography. Arch. Pharmacol. Res. 1997, 20, 306–312.
- Yoon, T.J.; Yoo, Y.C.; Kang, T.B.; Shimazaki, K.; Song, S.K.; Lee, K.H.; Kim, S.H.; Park, C.H.; Azuma, I.; Kim, J.B. Lectins isolated from Korean mistletoe (Viscum album coloratum) induce apoptosis in tumor cells. Cancer Lett. 1999, 136, 33–40.
- Lyu, S.Y.; Park, S.M.; Choung, B.Y.; Park, W.B. Comparative study of Korean (Viscum album var. coloratum) and European mistletoes (Viscum album). Arch. Pharmacal Res. 2000, 23, 592–598.
- George, O.; Solscheid, C.; Bertolo, E.; Fell, J.; Lisgarten, D. Extraction and purification of the lectin found in the tubers of Eranthis hyemalis (winter aconite). J. Integr. OMICS 2011, 1, 268–271.
- Peumans, W.J.; Nsimba-Lubaki, M.; Carlier, A.R.; Van Driessche, E. A lectin from Bryonia dioica root stocks. Planta 1984, 160, 222–228.
