Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | JOYSTU DUTTA | -- | 6361 | 2023-11-17 14:54:19 | | | |
| 2 | Catherine Yang | Meta information modification | 6361 | 2023-11-20 02:01:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thakur, T.K.; Barya, M.P.; Dutta, J.; Mukherjee, P.; Thakur, A.; Swamy, S.L.; Anderson, J.T. Contaminant Removal in Different Constructed Wetland Types. Encyclopedia. Available online: https://encyclopedia.pub/entry/51757 (accessed on 07 February 2026).
Thakur TK, Barya MP, Dutta J, Mukherjee P, Thakur A, Swamy SL, et al. Contaminant Removal in Different Constructed Wetland Types. Encyclopedia. Available at: https://encyclopedia.pub/entry/51757. Accessed February 07, 2026.
Thakur, Tarun Kumar, Mahesh Prasad Barya, Joystu Dutta, Pritam Mukherjee, Anita Thakur, Singam Laxmana Swamy, James T. Anderson. "Contaminant Removal in Different Constructed Wetland Types" Encyclopedia, https://encyclopedia.pub/entry/51757 (accessed February 07, 2026).
Thakur, T.K., Barya, M.P., Dutta, J., Mukherjee, P., Thakur, A., Swamy, S.L., & Anderson, J.T. (2023, November 17). Contaminant Removal in Different Constructed Wetland Types. In Encyclopedia. https://encyclopedia.pub/entry/51757
Thakur, Tarun Kumar, et al. "Contaminant Removal in Different Constructed Wetland Types." Encyclopedia. Web. 17 November, 2023.
Copy Citation
Constructed wetlands (CWs) are artificially engineered treatment systems that utilize natural cycles or processes involving soils, wetland vegetation, and plant and soil-associated microbial assemblages to remediate contaminated water and improve its quality. CW treatment systems are typically categorized as free-water surface(FWSCWs), surface-flow constructed wetlands (SFCWs), subsurface-flow constructed wetlands (SSFCWs), and hybrid constructed wetlands (HCWs). Depending on the flow direction, Subsurface-flow CWs (SSFCWs) can be further classified into horizontal subsurface-flow (HSSF) and vertical subsurface-flow (VSSF) systems.
circular bioeconomy (CBE)
constructed wetlands (CWs)
ecorestoration
macrophytes
wastewater treatment
1. Role of Macrophytes or Vegetation
Planted vegetation is one of the essential components of CW technology, which helps remove pollutants from domestic wastewater. The presence of plants in CW systems is the only reason they are called “green technologies”. Plant (macrophyte) species used in CWs are usually the same species that live in the NWs. Plants suitable for CW use in CWs must meet the following criteria listed below [1].
-
Plants must adapt to local environmental conditions.
-
Plants must be practicable under local climatic conditions and may tolerate/resist potential pests, insects, and diseases.
-
Plants should tolerate various contaminants (e.g., N, OM, P, etc.) in the wastewater.
-
Plants should be easily adjusted in local CW environments to show relatively fast growth and spreading.
-
Plants should have a high pollutant elimination capacity.
The wetland vegetation’s roots, stems, and leaves act as substrates for the growth of microbes as they decompose OM [2]. This microbial community, called the “periphyton”, comprises “a complex mixture of algae, cyanobacteria, heterotrophic microbes, and detritus attached to submerged surfaces in most aquatic ecosystems”. It can absorb pollutants, eliminate them from the water column, and prevent further spreading of such contaminants. This periphyton and natural chemical processes accomplish about 90% of contaminant removal and waste breakdown. The wetland plants eliminate around 7–10% of the contaminants and serve as a C source for the microorganisms once they die and decay. Notably, the choice of vegetation for a CW should consider the varied rates at which different aquatic plant species absorb/uptake HMs/TEs from polluted ambient media.
2. Role of Substrate Materials
The filter bed of CWs plays an equally significant part in the overall performance of an artificial secondary wastewater treatment system. Selecting substrate materials for the filter bed requires a crucial design parameter that can significantly impact the bed’s performance. Growth media provide a physical basis for vegetation growth, additional sites for biofilm growth and nutrient absorption, and promote sedimentation and filtration of contaminants [3]. At present, most media have gravel layers of various types of origin in the filter media, mainly with a sand layer at the top. The media play several functions, as highlighted below.
-
The media supports the growth of planted vegetation.
-
It stabilizes the bed (contact effect with the roots of developed plants).
-
It provides a media filtration effect.
-
It ensures high permeability and reduces possible clogging problems.
-
It provides an attractive attachment area for many microbes (biofilm formation) that are involved in pollutant removal processes.
-
It supports many transformation and elimination processes.
3. Role of (Plant Root-Associated) Microbes
As a significant component of CWs, microbes are crucial in decontamination processes, including nutrient transformation and organic pollutant degradation [4]. Microbes can also remove HMs (that cannot be biodegraded) from domestic sewage through their bioaccumulation, biosorption, and biotransformation (also called speciation, i.e., transformation of species/valence states) [5]. Microbes can even utilize antimicrobial compounds (antibiotics) as their sole C source [6][7][8]. Additionally, microbes in CWs can ameliorate abiotic and biotic stress tolerance and improve pollutant-removal efficiency by augmenting phytoremediation processes [9].
For comprehending CWs’ performance patterns and exploring optimized strategies, a thorough examination of the microbial community structure and their diversity, particularly for active/functional microbes in CWs, is necessary. With the recent developments in molecular biotechnology techniques, it is now feasible to monitor/study and analyze “microbial communities and species composition” in intricate ecosystems or environments [10], in their systematic review, summarized the primary functional microbes of CW systems engaged in the elimination of antibiotics, emerging pollutants, HMs, N, and P and investigated the impacts of these contaminants on microbial diversity. Their findings indicated that Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria are the chief phyla of the functional microbes in CWs. These active microbes help eliminate contaminants from CWs through diverse processes, including biodegradation, biosorption, catalyzing chemical reactions, and stimulating plant growth and development. HMs and high N and P concentrations in wastewater substantially impact microbial richness and diversity, while antibiotics result in considerable fluctuations in microbial alpha (α) diversity.
Plants harbor a vast repertoire of beneficial microbes (actinomycetes, bacteria, fungi, etc.) in their endosphere (internal plant tissues), rhizosphere (region of the soil/water surrounding the plant roots), and phyllosphere (area surrounding the plant leaves). These free-living microbes or microbial root symbionts secrete various PGP secondary metabolites, including biocontrol agents, biosurfactants, chelating agents, exopolysaccharides, nitrogenous compounds, organic acids, phytoenzymes, phytohormones, and volatile compounds, all of which directly or indirectly promote plant health and nutrition and alleviate abiotic (pollutant) stress in soil and water. These microbes with plant growth-promoting (PGP) traits are usually called plant growth-promoting microbes (PGPM). When found associated with plant roots (including internal root tissues), they are called plant growth-promoting rhizomicrobes (PGPRM) or endophytes. The PGPM/PGPRM, in turn, derive their nutrients from the plant root exudates and photosynthates, which are rich sources of sugars and amino acids [11][12][13]. The antimicrobial products secreted by PGPM inhibit or kill a broad spectrum of disease-causing phytopathogens, such as pathogenic bacteria, fungi, nematodes, and viruses [14]. Therefore, the indigenous PGPM associated with the planted vegetation in CWs could indirectly facilitate (phyto)pathogen removal and phytoremediation by promoting plant growth.
4. Role of Influent-Feeding Mode
Another critical design parameter of CW is the influent-feeding mode [15]. The method of influent feeding in the CWs (for example, continuous, batch, and intermittent) significantly affects the contaminant’s removal efficiency. As usual, batch-feeding modes (alternating filler and drain cycles) can achieve better performance by promoting more oxidizing conditions than continuous operation. In particular, N and P removal efficiency can improve in this wetland [15]. Effect of physico-chemical pretreatment on the removal efficiency of horizontal subsurface-flow constructed wetlands was studied by Caselles-Osorio et al. [16].
5. Role of Constructed Wetland (CW) as a Catalyst in Phytoremediation
In CW systems, physicochemical and biological processes remove toxic substances, including inorganic and organic contaminants. A thorough knowledge of these processes is essential for designing different CW systems and comprehending pollutants’ fate once they reach the wetlands. Theoretically, wastewater treatment in CWs occurs as it moves through the wetland substrates and the rhizosphere of the plants. Notably, owing to the loss and release of O2 from the plant’s root systems (rhizomes, roots, and rootlets), a thin oxic layer surrounds each root hair [17]. Both aerobic and anaerobic microbes aid in OM decomposition. Gaseous nitrogen (N2) is released into the atmosphere due to microbial nitrification and subsequent denitrification processes. P is coprecipitated with compounds of aluminum (Al), calcium (Ca), and Fe present in the root-filter bed media [17]. In SFCWS, SSs get filtered out while settling down in the water column, whereas in SSFCWS, they are physically filtered out by the wetland media. In SSF and VF systems, bacterial and viral pathogens are minimized through adsorption and filtration by the microbial biofilms present on the gravel or sand layers.
Plant growth, death, and microbial degradation contribute to the biogeochemical cycle occurring within a CW ecosystem. Overall, CWs provide a safe and beneficial environment for plants to remove pollutants from wastewater without endangering their health. During plant growth, aquatic macrophytes remove most pollutants while also providing a suitable environment for the proliferation of microbes. Therefore, this integrated CW technology treats wastewater contaminants more effectively than conventional treatment technology. The mechanism of removal of various pollutants through the CWs is described below.
5.1. Removal of Total Dissolved Solids (TDS)
Total Dissolved Solids (TDS) are the number of materials dissolved in water and wastewater like carbonate (CO32−), HCO3−, NO3−, PO42−, SO42−, chloride (Cl−), sodium (Na+), Ca2+, magnesium (Mg2+), organic ions, and other ions. These materials may not be regarded as pollutants contributing to dissolved solids. A few ions in water are necessary for sustaining aquatic life, and they are biologically utilized or chemically reactive in CWs. Aquatic macrophytes show a coordinated action to increase the durability/treatment of high TDS stress, and halophytes can reduce wastewater TDS content through their accumulation in plant tissues [18].
5.2. Removal of Biological Oxygen Demand (BOD)
Biological oxygen demand (BOD) indicates the DO amount aerobic macro/microorganisms require to decompose dissolved organic materials in water at a specific temperature over a particular time. BOD is the most critical parameter for measuring O2 demand by microorganisms to degrade OM present in domestic wastewater. Its value is typically expressed in milligrams (mg) of O2 consumed per liter (l) of water sample during incubation at 20 °C for 5 days. BOD is frequently employed as a proxy for the level of organic contamination in domestic wastewater [19]. BOD removal followed the “first order plug flow approach” described by Kadlec and Knight (1996) [20]. This approach is designed for certain pollutants extracted mainly through biological processes [21] (Akinbile and Yusoff 2012). In CWs, aerobic and anaerobic degradations of soluble organic compounds are equally suitable for removing BOD. However, during the sequential “fill and drain” process, the performance of BOD elimination was significantly better than during the previous traditional operating period [22].
5.3. Removal of Total Nitrogen (TN)
Excessive discharge of N into the waterbodies makes them prone to eutrophication and “black-odorous”, which not only deteriorates the overall water quality but eventually poses severe health risks to aquatic flora and fauna as well as humans [4][23]. In wastewater, TN refers to the amount of all N sources, including nitrite (NO2−), NO3−, NH3, and organic N (org-N), and is usually expressed in mg/L. Biological processes are the main N removal mechanisms in CWs. N elimination in CWs occurs via ammonification, nitrification, denitrification, volatilization, and plant uptake [10][24][25]. N removal through CWs with the help of partial nitrification and denitrification processes involves converting the nitrification process, which oxidizes ammonium (NH4+) to NO2− and then NO3−, and the denitrification process, which converts NO3− to N2 (Figure 1). Ref. [26] has reported that microbes can eliminate around 90% of the N. Wetland plants can also convert inorganic N to org-N through their metabolism.
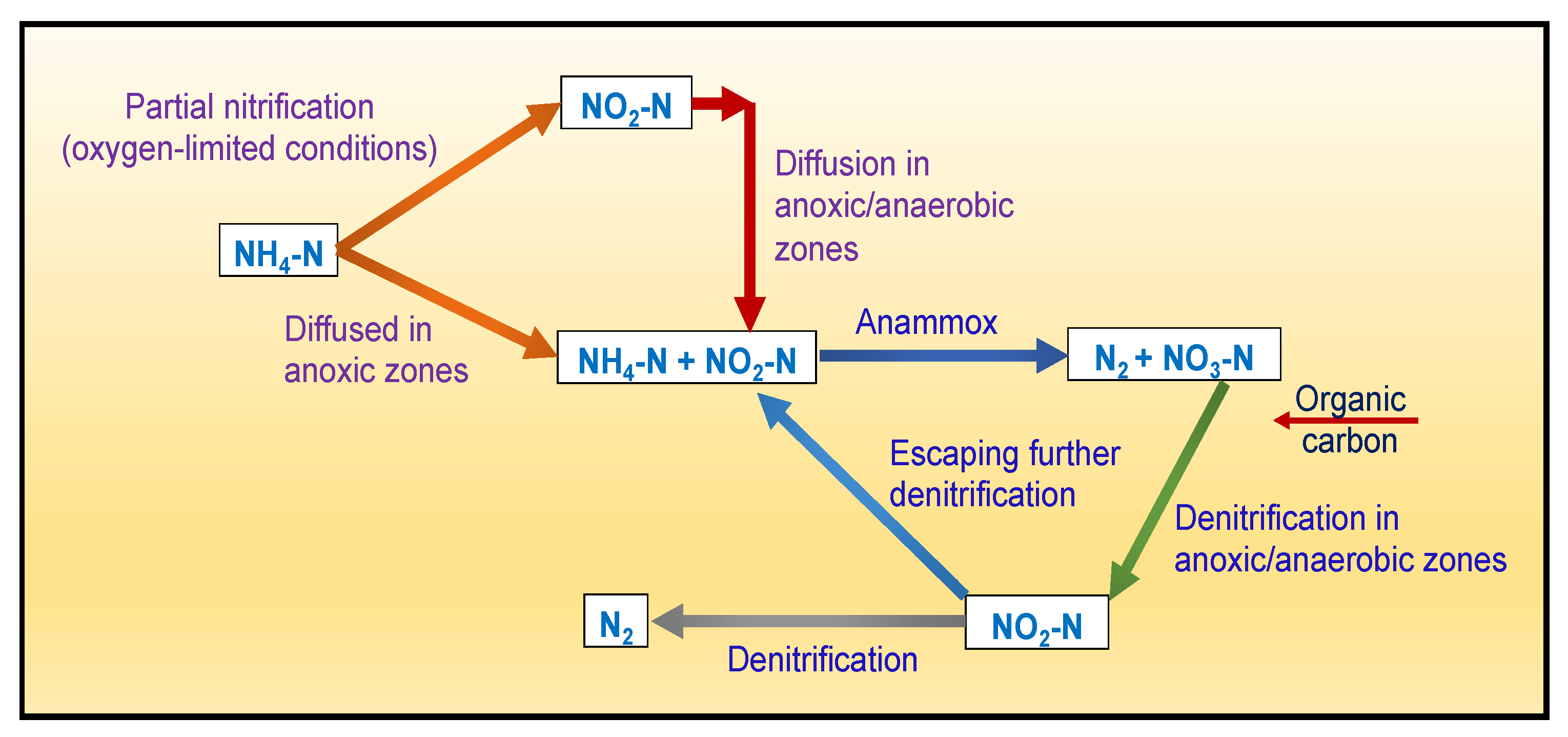
Figure 1. Conceptual diagram of nitrogen (N) removal via surface flow-constructed wetlands (SFCWs) (Source: [27] with modifications).
5.4. Removal of Nitrates (NO3−)
NO3− is an essential parameter for a specific state of decomposition of OM in domestic wastewater. NO3− uptake can cause a severe health condition in infants because of oxygen deprivation called “methemoglobinemia” or “blue baby syndrome” [28]. In CWs, microbial denitrification and vegetation uptake primarily achieve NO3− removal from domestic wastewater. This mechanism of NO3− elimination can occur biologically.
Biological Removal Mechanism(s)
This process of NO3− removal occurs in two stages: (1) nitrification (where NH4+ is converted to NO2−), and (2) denitrification (where NO2− is converted to NO3−). The first stage is done resolutely aerobically; the organisms depend on the oxidation of NH3 for cell growth and energy. The second stage is completed by the facultative chemolithotrophic bacteria that use organics for cellular growth and energy. The removal of NO3− is typically very high in the wetlands [29]. The central N removal mechanisms by functional microbes in CWs are depicted in Figure 2.
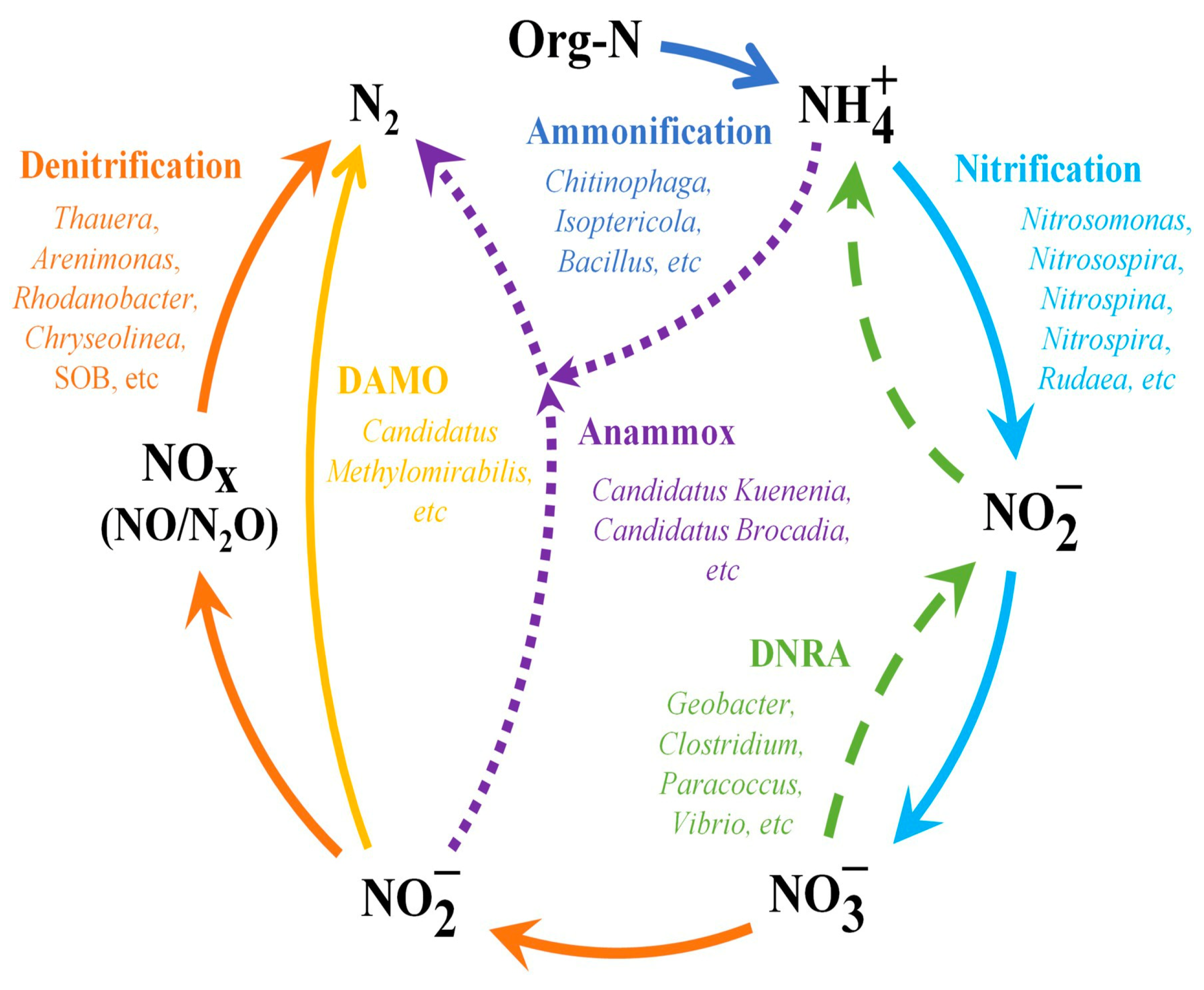
Figure 2. Flowchart showing the primary processes of microbe-assisted nitrogen (N) removal in constructed wetland (CW) systems (source: [10] with modifications).
According to most recent studies, microbes in CWs remove N primarily via ammonification, nitrification, and denitrification processes [29][30][31]. Ammonification in wastewater involves converting Org-N into NH4+; the latter is eliminated through other processes, including nitrification, plant uptake, and volatilization [30][32]. Notably, the most common genera of ammonifying bacteria are Bacillus, Chitinophaga, Isoptericola, and Sinorhizobium, as indicated in a review by Wang et al., 2022b [10]. As stated earlier, when it comes to nitrification and denitrification, microbes utilize NH4+ as an electron donor during the process of nitrification and oxidize NH4+ to NO2− and then to NO3− before using it as an electron acceptor during the denitrification process and finally reducing it to N2O or N2 [23][33]. Moreover, the microbes participating in nitrification are of two types: (1) “ammonia-oxidizing archaea” (AOA) and (2) “ammonia-oxidizing bacteria” (AOB), which convert NH4+ to NO2−, and “nitrite-oxidizing bacteria” (NOB) that transform NO2− to NO3− [23][34].
Mainly, AOA is more adaptable to low NH3 and high salt conditions than AOB [35][36]. As oxidation of NH3 is the initial and rate-limiting phase in the nitrification process, this may facilitate the AOA becoming the principal microbial group more rapidly and expedite the nitrification process [35]. The Nitrospinae, Nitrospirae, Proteobacteria, and Thaumarchaeota are well-known phyla that participate in nitrification. All the presently recognized AOA are found in the Thaumarchaeota phylum [35]. Regarding denitrification, the popular denitrifying bacterial phyla in CWs include Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria [37]. However, poor abundance and feeble competitiveness are common issues for nitrifying bacteria in CWs’ microbial population [26]. As a result, steady NH4+ oxidation will require a longer start-up time, making the nitrification process a limiting step in N removal [26]. Notably, recent reports have emphasized “heterotrophic nitrification and aerobic denitrification” (HN-AD) bacterial significance in this context [26][37][38]. In the start-up/initial stage of CWs, these bacteria may carry out the transformation of NH4+ and NO3−, converting the N present in the aqueous solution (i.e., dissolved N) to gaseous N (N2) for complete denitrification [26]. Additionally, they proliferate more quickly and can rapidly take over/dominate [26]. The old belief that only autotrophic bacteria are capable of carrying out nitrification and that denitrification can only occur in anaerobic environments has been disproved by the discovery of HN-AD bacteria, making it more advantageous for OM and N removal [37]. According to the reports by Wang et al. (2022b) [37], the HN-AD bacteria primarily belong to the genera Aeromonas, Dechloromonas, Ferribacterium, Hydrogenophaga and Zoogloea. Moreover, new N removal mechanisms like “sulfur autotrophic denitrification” (SAD) and “denitrifying anaerobic methane oxidation” (DAMO) have been identified in relation to the denitrification process [37][39]. “Sulfur-oxidizing bacteria” (SOB) use elemental S, sulfide (S2−), and thiosulfate (S2O32−) as electron donors and NO3− as an electron acceptor in anoxic environments for reducing NO3− to N2 during SAD [4][40]. Because of the readily accessible electron donors from S and related S compounds, this pathway may predominate in removing N from water/wastewater with a low C/N ratio [37]. The phylum Proteobacteria contains the majority of “sulfur-autotrophic denitrifying” bacteria, with Sulfurimonas and Thiobacillus as two of its well-known genera. In DAMO, CH4 is the sole C source and electron donor for reducing NO2− to N2 under anaerobic (O2-deficit) conditions [39][41]. More environmental advantages are made possible due to DAMO’s inherent ability to lessen the “greenhouse effect” and reduce N2O, the unneeded by-product, during N removal [39][41].
A novel mechanism called “AOA” or anammox exists for N removal apart from the conventional nitrification-denitrification processes [29][42]. Under anaerobic conditions, NO2− is used as an electron acceptor in this pathway for directly transforming NH3 into N2 [31][42]. As a result, it provides an alternative denitrification mechanism when the O2 and C/N ratios are low [29]. Almost every anammox bacteria reported is a member of the phylum Planctomycetes [23][43]. Concerning NO3−, among the various nitrogenous contaminants, NO3-N is more prone to leaching and subsequently degenerate the quality of water than the others [43]. Thus, removing NO3− is crucial to safeguarding freshwater systems and subsurface water quality [43]. Besides denitrification, there is another pathway for reducing NO3, which is called “dissimilatory nitrate reduction to ammonium” (DNRA) [44]. The DNRA pathway converts NO3− to NH4+, thereby reducing it to available NH4+ suitable for utilization by other microbes, including ammonia-oxidizing archaea and AOB [23][34]. According to the published reports, it is more advantageous to the denitrification process in S2−-rich coastal and marine habitats where salinity levels are high [23]. Numerous investigations have revealed that a few denitrifying bacterial genera, including Clostridium, Desulfovibrio, and Vibrio, can carry out the DNRA process [23][45]. Nevertheless, it is currently challenging to discriminate between DNRA and denitrifying bacteria, necessitating future advancements in molecular biotechnology.
The phylum Proteobacteria has a sizable species number active in N transformation [10]. This species is prevalent in CWs and is the leading phylum in most systems, significantly removing N from various wastewater categories [31][46]. The three genera, viz., Nitrobacter, Nitrosomonas, and Nitrosospira, are related to the process of nitrification, while among denitrifying bacteria, the genera Arenimonass, Tauera, Thermomonas, and Thiobacillus are frequently found. The three prominent classes associated with N removal in CWs are Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria. They are rich in nitrifying bacteria, such as AOB and NOB, which are functionally crucial to CW ecology and are primarily responsible for removing N [47].
Additionally, an increasing body of research has linked the functional genes of N-removing microbes to their operational and quantitative analyses [33][34][48].
For instance, the plentitude of nirK- and nrfA-carrying microbes controls how well CWs performed at denitrification [48]; the prevalence of the functional genes for nitrification, viz., amoA-ammonia-oxidizing archaea, amoA-AOB, and nxrA, indicated the nitrifying-bacterial-growth status [49]. A summary of the active gene pools related to the various N removal processes, such as nitrification, denitrification, anammox, and DNRA, has been prepared [26][33][34]. With active genes, one may examine how microorganisms function in a particular habitat or ecosystem and offer a viable method for researchers to continue studying functional microbes in CW systems.
5.5. Removal of Phosphate (PO42−)
P is one of the primary elements contributing to the eutrophication process in waterbodies [50][51]. Excess P discharge into aquatic ecosystems from diverse sources encompassing agricultural, industrial, and residential sources can also adversely affect aquatic organisms by modifying water pH, reducing DO levels, and triggering algal/phytoplankton growth [50][51]. P occurs naturally in inorganic and organic forms/phosphates (PO42−). Soluble reactive P (SR-P) is the term used to describe the analytical measure of biologically available orthophosphates. In general, insoluble forms of P (inorganic and organic) and dissolved org-P are physiologically inaccessible until they are converted into soluble inorganic forms [52]. The basic phenomenon of removing P in CWs depends on its accumulation by the sediments, substrates, and plants. For removal, P can be processed physically, chemically, or biologically.
Physical Removal Mechanism(s)
In CWs, physical absorption through roots, leaves, and plant parts is usually deficient. Thus, macrophytes account for P removal at the beginning of their growing period, and precipitation of soluble and insoluble P in the influent, followed by sedimentation, is the physical form of removal occurring in the CW. Filtration through solid substances and fallen leaves in CWs reduces P from wastewater [20].
Chemical Removal Mechanism(s)
The main P removal by chemical mechanism in the CWs occurs through adsorption and precipitation (precipitation in the water column and adsorption by porous media) [25]. Adsorption and precipitation within substrates are well-established in performing the most critical roles in the PO42− removal process [53]. Other than this, sand, washed gravel, crushed rocks, and peats can also participate in the adsorption process. CW bed fills, on the other hand, have a short-term P sorption capability [25].
Biological Removal Mechanism(s)
The mechanism for removing P is through biological resources, but this process still does not allow much storage. Microbes are crucial for eliminating P from CWs and can regulate the transformation of P into different forms [51]. P uptake through microorganisms is relatively fast since bacteria, fungi, and algae can multiply rapidly. Maximal P transformation mediated by microbes involves the mineralization of organic PO42− to inorganic PO42− (a process also referred to as “decomposition”) or the conversion of insoluble, mobile, primary PO42− that are more readily utilized by organisms [25]. A comprehensive review on microorganism in constructed wetlands list the major functional microorganisms responsible for P removal in CWs [10].
“Phosphorus-accumulating organisms” (PAOs) are primarily responsible for biological P removal in CWs. PAOs can absorb wastewater PO42− and store it within their cells under oscillating oxic and anoxic environments [50][54][55]. Under anoxic conditions, PAOs degrade intracellular polyphosphates and uptake volatile fatty acids from the surrounding media. These fatty acids are subsequently stored as polyhydroxyalkanoates/poly-β-hydroxyalkanoates (PHAs) [56].
In contrast, PAOs utilize PHAs under oxic conditions to provide energy and absorb PO42, creating polyphosphate storage [55]. The process of microbial P removal in CWs is generally realized because the amount of P absorbed by PAOs will be higher than that of P discharged [50][54][55]. Pseudomonadota (formerly Proteobacteria), which plays a significant role in P elimination, is the major phylum [5][46][57]. Of these, the majority of the bacterial/microbial species linked to biological P removal are found in the classes Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria [54][57]. TP removal in CWs is facilitated by the Rhizobiaceae and Rhodobacteraceae of the class Alphaproteobacteria, which can uptake volatile fatty acids under oxic conditions and transform them into PHAs [56]. The genera Candidatus, Dechloromonas, and Rhodocyclus constitute the principal members of the class Betaproteobacteria. Among them, the bacterial group Candidatus Accumulibacter is a representative PAO dominant in large-scale wastewater treatment facilities and laboratory-scale reactors. Under anaerobic conditions, Dechloromonas can reduce perchlorate, assemble polyphosphate, and take up C [57]. Additionally, it has been demonstrated that Rhodocyclus significantly contributes to P elimination [58]. Pertinent research has identified three genera, viz., Acinetobacter, Klebsiella, and Pseudomonas, belonging to Gammaproteobacteria [55]. Pseudomonas is an efficient P-removal bacterium due to its considerable capacity to absorb wastewater P and store it as polyphosphates within its cell biomass [57]. According to [55], Pseudomonas can eliminate up to 80.6 percent of TP from household sewage. Notably, the first bacterial isolate from biomass belonged to the genus Acinetobacter, with a solid capacity to remove P [50]. Besides Proteobacteria, other taxa like Gemmatimonadacea can absorb surplus PO42− under oxic conditions [51].
The P-removal ability of PAOs primarily relies on their bioaccumulation and utilization of intracellular polyphosphates [55], which are directly correlated with exopolyphosphatase (ppx) and polyphosphate kinase (ppk) activities [50]. For achieving biological P removal, the enzymes ppk and ppx can catalyze aerobic P uptake and anaerobic P release, respectively [33]. Elevated temperatures, however, inhibit their functions; based on an earlier study, the ideal temperature ranges between 20.0 °C and 35.0 °C [50].
Moreover, besides PAOs, “phosphorus-solubilizing bacteria” (PSB) and “denitrifying phosphorus-accumulating organisms” (DNPAOs) have been identified in CW systems. Examples of PSB include the genera Corynebacterium and Enterobacter, which produce organic acids like citric and oxalic acids for converting the insoluble form of soil P into its soluble form for plant uptake [51]. DNPAOs may absorb polyphosphate in anoxic environments using NO3− or NO2− as electron acceptors [51]. There have been reports of DNPAOs in Alphaproteobacteria (including the genus Paracoccus) and Anaerolineae [56]. Interestingly, organophosphate hydrolases from the genera Brevundimonas and Chlorobaculum hydrolyze organophosphate esters, and Variovorax utilizes insoluble PO42− as a P source for its growth [59].
5.6. Removal of Heavy or Trace Metals
HMs are widely dispersed in aquatic ecosystems and regarded as toxic environmental contaminants as they are hard to break down and, if left untreated, can build up in the food web [60], resulting in biomagnification and causing health risks to humans. CWs have been extensively used to eliminate dissolved metal(loid)s or TEs. Although these pollutants are frequently found in mine drainage, treatment wetlands have been built for stormwater, landfill leachates, and other sources (such as leachates/FDG washwater at coal-fired power plants and domestic sewage), which also contain dissolved trace elements [61]. Through the exploitation of diverse processes like biomineralization, biosorption, and biotransformation (valence transformation), microbes in CW systems can efficiently remove HMs/TEs [5]. The principal HM-removal pathways/processes by functional microbes in CWs are shown in Figure 3. The phyla and genera of functional microbes are reviewed by Wang et al., 2022b [10].
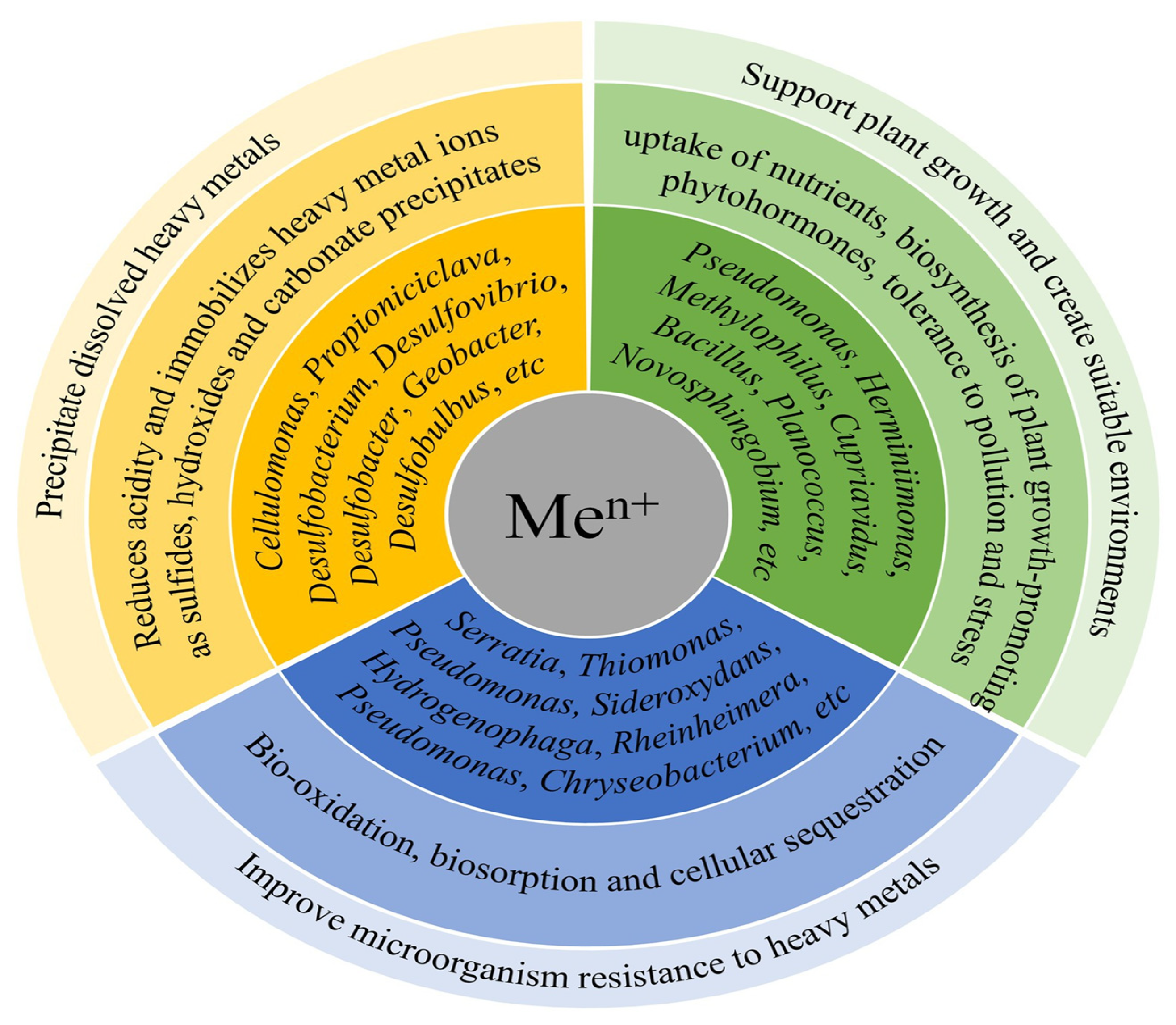
Figure 3. Illustration showing the principal mechanisms of removal of heavy metals (HMs) by microbes in constructed wetland (CW) systems (Source: [10] with modifications).
Ironically, HM ions usually harm microbes because they break cell membranes, damage DNA, impede enzyme activity, and interfere with cellular functions [60]. For this reason, HM tolerance is crucial for microbes in HM removal in CWs. The genera Sideroxydans and Thiomonas can oxidize Fe2+ to Fe3+, making its precipitation easy and rendering it less toxic [62]. Additionally, Yu et al. (2020) [60] discovered that the dominant genera Pseudomonas and Serratia displayed resistance to Cd2+ and Zn2+ when tested utilizing concentration gradients of these two HMs, leading to increases in their removal rates of 10.13 percent and 8.57 percent, respectively. Analysis of subcellular compartments further revealed the presence of bioaccumulated HMs primarily in the microbial cell membrane and cell wall. The exopolymers from Pseudomonas can bind to HMs and prevent their transport inside the biofilm through diffusion, attaining extracellular sequestration, which shields bacterial cells from HM stress [63].
Moreover, the presence of anionic functional groups on the cell surfaces of Pseudomonas and Serratia may further facilitate Cd2+ and Zn2+ adsorption [64]. These results suggest that culturing resistant bacteria/microbes is a feasible strategy for removing HMs from wastewater and call for further study. However, Serratia minimizes HM toxicity by secreting a variety of enzymes and proteins, including amino acids, histidine-binding proteins, HM-binding proteins, redox enzymes, and transporter proteins capable of effluxing HM ions [62]; this is unable to aid in the elimination of HMs by CWs. Hence, there is a difference between resistant and functional microbes, and further investigation into microbial HM removal mechanisms is necessary before a conclusion can be drawn. According to [60], functional microbes also evolved over a longer time in the control CW system that was not exposed to resistant microbes. The system’s microbial community structure most likely changed spontaneously, facilitating tolerance to HM stress. Contrarily, when exposed to HM-containing environments, systems supplemented with resistant microbial inoculants may display a less prominent microbial community evolution to achieve a dominant strain, saving biofilm stabilization time [44].
The elimination of HMs is also significantly influenced by plant-microbe interactions. Plants and microbes have coexisted for a long time, and microbes have formed intricate relationships with plants [9]. Particularly, endophytic and rhizospheric bacteria (PGPR) can facilitate plant growth and development through nutrient (Ca, Fe, Mg, N, and P) uptake, phytohormone production, and tolerance towards pollutant stress [9]. This, in turn, can reduce harmful metal-induced plant stress and help plant metal accumulation [9]. Conversely, the primary role of macrophytes in CW systems is to supply additional OM and O2 needed for the growth of microbes [65]. Therefore, healthy plant growth also offers a better habitat for microbial proliferation [66]. These symbiotic interactions enhance HM removal in CWs. For example, Syranidou et al. (2016) [67] showed that inoculating Juncus acutus L. with a particular endophytic bacterial consortium eliminated emerging contaminants and HMs more quickly and effectively than uninoculated plants. In another study by Vassallo et al. (2020) [9], it was shown that eight rhizobacterial isolates from P. australis roots belonging to the genera Bacillus, Planococcus, and Pseudomonas thrived in domestic sewage with high levels of HMs (45 mg/L and 0.09 mg/L of Fe and Se, respectively), and the more HM concentrations present, the more rapid they grew. In conclusion, due to their high levels of HM resistance and ability to improve phytoremediation effectiveness, PGPR has been demonstrated to be a trustworthy functional microbe for HM removal.
5.7. Removal of Pathogens
Pathogens commonly found in household/municipal sewage capable of inflicting different acute or chronic diseases in humans encompass varied microbial genera like Escherichia, Salmonella, and Vibrio among bacteria, Ascaris among intestinal nematodes, Enteroviruses and Rotaviruses among viruses, etc. Notably, the genera used as pollution indicators in domestic sewage include Clostridium, Citrobacter, Enterobacter, Escherichia, Klebsiella, and Streptococcus [68]. Severe disease conditions caused by various microbial pathogens and their toxins, along with other contaminants in wastewater, are amoebiasis (Entamoeba histolytica), botulism (Clostridium botulinum), campylobacteriosis (Campylobacter), cholera (Vibrio cholerae), cryptosporidiosis (Cryptosporidium parvum), giardiasis (Giardia lamblia), hemorrhagic diarrhea (Escherichia coli), hepatitis A (Hepatitis A virus), typhoid (Salmonella typhi), scabies, shigella infection (Shigella spp.), and other parasitic (helminthic and protozoan) infections (Endolimax nanus, Entamoeba coli, and whipworm) [68].
In a CW system, all kinds of pathogens, including bacteria, fungi, helminths, protozoa, viruses, etc., are anticipated to be somewhat eliminated; however, an SSFCW is expected to remove pathogens more thoroughly than SFCWs. Usually, a 1-2 log10 reduction in pathogen count can be expected in a FWSCW, but in systems with dense vegetation, the elimination of bacteria and viruses may be <1 log10 reduction. CWs frequently contain flora that helps eliminate other pollutants (nutrients) like N and P. As a result, the role of sunlight in killing bacteria and viruses is diminished in these systems since heavy vegetation prevents the exposure of pathogens to direct sun rays. According to published reports, in a well-designed and adequately operated/maintained FWSCW, pathogen removal is around <1-2 log10, 1-2 log10, 1-2 log10, and <1-2 log10 for bacteria, helminths, protozoa, and viruses, respectively. In contrast, the expected elimination of pathogenic bacteria, helminths, protozoa, and viruses in SSFCWs is relatively higher and is reported to be 1-3 log10, 2 log10, 2 log10, and 1-2 log10, respectively [69].
The removal efficiencies stated above as log10 can alternatively be understood in terms of how removal efficiencies are often/generally reported in terms of percentages (%); for instance, 1 log10 removal corresponds to 90% removal efficiency (RE), 2 log10 removal corresponds to 99% RE; 3 log10 removal corresponds to 99.9% RE; 4 log10 removal corresponds to 99.99% RE, and so on [70]. Various studies have been conducted in the last two decades on domestic wastewater treatment and developing alternative, low-cost, and sustainable strategies that can effectively reduce pollutant concentrations to acceptable/permissible environmental standards (Table 1).
Table 1. A detailed synopsis of the wastewater operational parameters and macrophyte- and microbe-assisted treatment efficiencies of FWSCWs, SSFCWs, HSSFCWs, VSSFCWs, and HFCWs using selected case studies from the last two decades (2003–2022).
| Sl. No. | Latin and Common Names of Plant (Macrophyte) Species | Percentage Removal of Inorganic and Organic Pollutant(s) (%) | Percentage Removal of Pollutant Indicator Organism(s)/Pathogen(s) (%) | Microbes Involved in the Removal of Nutrient(s)/Pollutant(s) | Constructed-Wetland-Based Phytoremediation Set-Up(s)/System(s) Used | Type of Treated Wastewater | Reference(s) |
|---|---|---|---|---|---|---|---|
| 1. | Salix atrocinerea Brot. (grey willow) and Typha latifolia L. (cattail) | BOD5 (87.5) COD (89.0) SS (66.5) |
Fecal bacteria (99.9) | - | Full–scale, pilot-plant constructed wetland | Domestic wastewater | [71] |
| 2. | Control unit (A): Phragmites mauritianus Kunth (reed grass) and T. latifolia | COD (33.6, 56.3, and 60.7) NH4+-N (11.2, 25.2, and 23.0) NO2-N (23.9, 38.5, and 23.1) NO3−-N (32.2, 40.3, and 44.3) |
TC and FC (43.0–72.0) | - | Horizontal subsurface-flow constructed wetland | Domestic wastewater | [72] |
| 3. | Control unit (A): Colocasia esculenta (L.) Schott (taro) and T. latifolia | COD (64.7, 74.8, and 79.4) NH3 (74.0–75.0) P4 (72.0–77.0) SO42− (74.0–75.0) |
- | - | Engineered wetland | Domestic wastewater | [73] |
| T. latifolia | NH3 (74.0) PO42− (69.0) SO42− (72.0) |
- | - | ||||
| 4. | Phragmites sp. | BOD5 (93.0) COD (88.0) TDS (93.0) TSS (93.0) |
FC (76.0–99.0) Fecal Streptococci (49.0–85.0) Note: These were removed in two phases in four distinct seasons | - | Subsurface-flow constructed wetland | Municipal wastewater | [74] |
| Typha sp. | BOD5 (63.0) COD (50) TDS (58.0) TSS (58.0) |
FC (50.0–99.0) Fecal Streptococci (33.0–85.0) Note: These were removed in two phases in four distinct seasons |
- | ||||
| 5. | Pontederia crassipes Mart. [formerly Eichhornia crassipes (Mart.) Solms] (common water hyacinth) and Phragmites australis (Cav.) Trin. ex Steud. (common reed) | BOD5 (72.1) COD (67.2) Org-N (59.3) SS (64.6) Settleable solids (91.8) TN (38.0) TP (43.0) |
- | - | Surface-flow constructed wetland | Secondary-treated domestic wastewater | [75] |
| 6. | Typha angustifolia L. and Scirpus grossus L.f. (club-rush or bulrush) | BOD5 (68.2) NH4+-N (74.4) NO3−-N (50.0) TP (19.0) TSS (71.9) |
- | - | Free-water surface wetland | Secondary-treated municipal wastewater |
[76] |
| 7. | Lemna minor L. (common duckweed), P. australis, Schoenoplectus tabernaemontani (C.C. Gmel.) Palla (syn. Scirpus validus Vahl) (softstem bulrush), and Typha orientalis C. Presl (cumbungi) |
BOD5 (70.4) NH3-N (40.6) SS (71.8) TP (29.6) |
TC and FC (99.7 and 99.6, respectively) | - | Constructed wetland | Sewage water | [77] |
| 8. | A Acorus gramineus Sol. ex Aiton (Japanese sweet flag), B Iris pseudacorus L. (yellow flag) | BOD5 (A 71.3, B 72.5) COD (A 61.71, B 61.5) TN (11.24–21.95) TP (33.15) |
- | - | Constructed wetland | Domestic wastewater | [78] |
| 9. | A Iris pseudacorus L. and B Acorus gramineus Soland | BOD5 (72.5, 71.3) COD (61.1, 61.7) TN (70.9, 70.7) TP (86.9, 84.8) A HMs (Cd, Cr, and Pb)—15.3, 21.3, and 24.5, respectively) |
- | - | Model wetlands | Rural or urban domestic wastewater | [78] |
| 10. | T. angustifolia | BOD5 (80.78) NH4+-N (95.75) TN (66.5) TP (58.59) |
- | - | Free-water surface wetland | Secondary-treated municipal wastewater | [79] |
| 11. | Canna and Heliconia | TSS (88.0) COD (42–83) |
- | - | Horizontal subsurface-flow constructed wetland |
Domestic wastewater | [80] |
| 12. | P. australis and T. latifolia | BOD5 (>86.0) COD (>86.0) |
- | - | Vertical-flow constructed wetland |
Domestic wastewater | [81] |
| 13. | Canna | BOD5 (94.0) TN (93.0) |
- | - | Vertical subsurface-flow constructed wetland and horizontal subsurface-flow constructed wetland | Sewage water | [82] |
| 14. | Cyperus alternifolius L. (umbrella papyrus) | COD (83.6) NH4+-N (71.4) TN (64.5) TP (68.1) TSS (99.0) |
- | - | Hybrid-flow constructed wetland | Municipal wastewater | [83] |
| 15. | Acorus calamus Linn. (sweet flag) | COD (73.0–93.0) TN (46.0–87.0) TOC (40.0–66.0) TP (75.0–90.0) |
- | - | Vertical-flow constructed wetland |
Domestic wastewater | [84] |
| 16. | Anthurium andraeanum Linden (flamingo flower), Strelitzia reginae Aiton (crane flower), Zantedeschia aethiopica (L.) Spreng. (calla lily), and Agapanthus africanus L. (African lily) |
BOD5 (81.94) TN (49.38) TP (50.14) TSS (61.56) |
TC (99.30) | - | Vertical subsurface-flow constructed wetland | Secondary-treated municipal wastewater | [85] |
| 17. | Canna, Cyperus papyrus L. (papyrus or Nile grass), and P. australis | BOD5 (90) COD (88.0) TSS (92) |
TC, FC, and E. coli (94.0–99.0) | - | Vertical-flow constructed wetland | Municipal wastewater | [86] |
| 18. | Canna indica L. (Indian shot) and T. orientalis | BOD5 (62.8) NH4+-N (80.72) NO3−-N (12.8) TN (51.1) |
- | - | Hybrid-flow constructed wetland | Municipal wastewater | [87] |
| 19. | Scirpus alternifolios (umbrella papyrus) | BOD5 (84.9) COD (89.8) NH4-N (82.2) TKN (82.7) TP (76.5) TSS (98.1) |
- | - | Vertical subsurface-flow constructed wetland | Wastewater | [88] |
| 20. | T. angustifolia | NH4+-N (95.2) TP (69.6) |
- | - | Subsurface-flow constructed wetland | Artificial wastewater | [16] |
| 21. | Canna and P. australis | BOD5 (92.8, 93.6) COD (91.5) NH3 (62.3, 57.1) TSS (92.3, 94.0) |
- | - | Vertical-flow and horizontal-flow constructed wetlands | Municipal wastewater | [89] |
| 22. | Alternanthera sessilis (L.) R.Br. ex DC. (Brazilian spinach), C. esculenta, P. australis, Pistia stratiotes L. (water lettuce), Persicaria hydropiper (L.) Delarbre (syn. Polygonum hydropiper L.) (water pepper), and T. latifolia |
BOD5 (90.0) NH4-N (86.0) NO3-N (84.0) TDS (78.0) TE (As, Co, Cr, Cu, Mn, Ni, Pb, and Zn—85.0, 49.0, 35.0, 95.0, 87.0, 39.0, 92.0, and 55.0, respectively) TSS (65.0) |
- | - | Subsurface-flow constructed wetland | Sewage wastewater | [90] |
| 23. | T. latifolia | COD (53.0–70.0) NH3 (12.0–15.0) P (18.0–25.0) |
- | - | Surface, up-flow constructed wetland | Sewage wastewater | [91] |
| 24. | Phragmites | CODCr (75.7) NH3-N (96.8) TN (96.7) TP (90.4) |
- | N was removed by Paenibacillus sp., Pseudomonas oleovorans, Pseudomonas pseudoalcaligenes, Pseudomonas stutzeri (LZ-4), Pseudomonas stutzeri (LZ-14), Pseudomonas stutzeri (XP-2), and Pseudomonas pseudoalcaligenes (Note: These microbes remove N from wetlands with processes like adsorption, filtration, precipitation, sedimentation, and volatization); Pseudomonas mendocina LR contributed to the maximal N removal (97.35%) |
Laboratory-scale constructed wetland microcosm | River water and domestic wastewater | [92] |
| 25. | Canna and Phragmites | NH4+-N, NO3−-N and TKN (52.99) | - | - | Vertical-flow constructed wetland | Secondary-treated sewage wastewater | [93] |
| 26. | I. pseudacorus, P. australis, and T. latifolia |
BOD5 (41.0) Dissolved P (59.0) HM (Pb—98.0) NH4+-N (66.0) TP (46.0) SS (66) |
- | - | Constructed wetland | Mine water and Sewage | [94] |
| 27. | Agapanthus africanus (L.) Hoffman. (African lily), Canna ffuses, C. indica, Watsonia borbonica (Pourr.) Goldblatt (Cape bugle lily), and Z. aethiopica |
BOD5 (90.0) NH4+ (84.0) PO42− (92.0) |
- | - | Horizontal subsurface-flow constructed wetland | Sewage water | [95] |
| 28. | Aquatic plants | BOD5 (87.9) CODCr (90.6) NH3-N (66.7) TN (63.4) TP (92.6) |
- | - | New-type, multi-layer artificial wetland | Domestic wastewater | [96] |
| 29. | Canna × generalis | NO3− (51.9) P (8.9) Phenolic compounds (1.0) |
- | - | Constructed wetland | Domestic wastewater | [28] |
| 30. | A. calamus, C. indica, Iris japonica Thunb. (butterfly flower), P. australis, T. angustifolia, and Zizania caduciflora (Trin.) Hand.-Mazz. (wild rice) |
TP (79.6, 87.9, 90.3, and 93.2) |
- | - | Integrated vertical-flow constructed wetland | Synthetic domestic wastewater | [97] |
| 31. | P. australis | BOD5 (84.0) COD (75.0) NH4+ (32.0) TP (22.0) TSS (75.0) |
- | - | Vertical subsurface-flow constructed wetland | Sewage water | [98] |
| 32. | C. indica | BOD5 (88.11, 80.51, and 89.78) NH4+-N (94.81, 39.39) TN (56.17, 50.0, and 55.06) TP (94.82, 93.04, and 93.31) |
- | N was removed by denitrifying bacteria | Hybrid vertical down-flow constructed wetland |
Domestic wastewater | [99] |
| 33. | A. calamus and P. australis | TN (45.2) | - | N was removed by a large number of rhizospheric bacteria (out of that, 17.9–26.8% non-rhizospheric bacteria removed N from the soil) | Horizontal subsurface-flow constructed wetland | Domestic wastewater | [100] |
| 34. | Centella asiatica (L.) Urb. (Indian pennywort), E. crassipes, P. australis, T. latifolia, and Chrysopogon zizanioides (L.) Roberty (vetiver grass) |
BOD5 (81.0 and 82.0) TKN (63.0 and 69.0) TSS (79.0 and 89.0) |
- | - | Hybrid constructed wetland | Domestic wastewater | [101] |
| 35. | Typha domingensis Pers. (southern cattail) | BOD5 (56.0) TKN (41.0) TP (37.0) TSS (78.0) |
- | - | Constructed floating wetland | Domestic sewage | [102] |
| 36. | P. australis | BOD5 (93.0) COD (91.0) TN (67.0) TP (62.0) TSS (95.0) |
TC, FC, and fecal Streptococci (64.0, 63.0, and 61.0, respectively) | - | Hybrid constructed wetland | Wastewater | [103] |
| 37. | Typha and Commelina benghalensis L. (Benghal dayflower) | NO3− (84.0) PO43− (77.0) |
TC and FC, E. coli, Enterococcus, Clostridium, and Salmonella (65.0–70.0) | - | Horizontal-flow constructed wetland | Primary and secondary-treated sewage | [104] |
| 38. | Pennisetum purpureum Schumach. (Napier grass) and T. latifolia | BOD5 (up to 87) (inlet BOD5 of 748–1642 mg L−1) COD (up to 81) (inlet COD of 835–2602 mg L−1) |
- | - | Horizontal subsurface-flow constructed wetlands |
Industrial (brewery) wastewater | [105] |
| 39. | C. indica and Typha angustata Bory & Chaub. (accepted name: Typha domingensis Pers.) | BOD, COD, NH3-N, TDS, TKN, TP, and TVS (A 65.0–B 62.0, A 64.0–B 61.0, A 21.0–B 58.0, A 34.0–B 33.0, A 15.0–B 35.0, and A 54.0– B 40.0) [at first stage] and (A 88.0–B 84.0, A 90.0–B 90.0, A 52.0–B 82.0, A 58.0–B 61.0, A 50.0–B 47.0, and A 71.0–B 64.0) [for the second stage reactor] Note: The nutrient removal was measured at two different hydraulic loadings at A 0.150 m day−1 and at B 0.225 m day−1 |
- | - | Two-stage vertical-flow constructed wetland | Domestic wastewater | [106] |
| 40. | C. papyrus and P. australis | BOD5 (80.69) COD (69.87) NH3-N (69.69) TP (50.0) |
TC and FC (98.08 and 95.61, respectively) | - | Vertical-flow subsurface constructed wetlands |
Municipal wastewater | [107] |
| 41. | A. calamus and C. indica | BOD5 (78.74 and 81.79) TDS (18.96 and 22.31) TN (56.33 and 60.37) PO42− (79.57 and 81.53) |
- | - | Pilot-scale vertical subsurface-flow constructed wetland | Primary-treated domestic sewage | [108] |
| 42. | Myriophyllum elatinoides Gaudich. (water milfoil) | NH4+ (91.35) NO3− (95.16) TN (90.36) TP (96.14) |
- | Bacteroides and Firmicutes carried out denitrification; N was removed by Pseudomonas, Dechloromonas, Desulfobacca, and Desulfomicrobium; PO42− was removed by Chlorobaculum, Methanobacterium, and Rhodoblastus |
Multi-stage, surface-flow constructed wetland | Domestic sewage | [40] |
| 43. | C. esculenta and Dracaena sanderiana Sander ex Mast. (Chinese water bamboo) |
BOD5/ (74.0) NH4-N (90.0) TSS (76.0) |
TC (59.0) | - | Novel vertical-flow and free-water surface constructed wetland | Dormitory sewage | [109] |
| 44. | A. calamus and reeds | TN (15.0) TP (18.0) |
- | Bioremediation and degradation of diesel, petroleum, and other alkanes could be achieved by Tistrella; N was removed by Achromobacter, Aeromicrobium, Aquicella, Azospirillum, Fluviicola, Halomonas, Limnohabitans, Methylophilacterium, Perlucidibaca, Pseudomonas, Rhodobacter, Rhodospirillaceae, and Variovorax; S compounds were removed by Desulfovibrio and Rhodocista |
Constructed wetland | Domestic sewage | [59] |
| 45. | C. indica, C. alternifolius, and Thalia dealbata Fraser ex Roscoe (powdery alligator-flag) |
COD (95.2) NH4-N (98.1) PO4−-P (85.3) TN (87.9) TP (86.1) |
- | - | Hybrid constructed wetland | Domestic sewage | [110] |
| 46. | Chrysopogon zizanioides L. (vetiver and khus) | BOD5 (83.36) COD (92.34) NH4-N (89.41) NO3-N (90.72) PO4−-P (92.81) TCr (95.20) TN (93.54) TSS (94.66) |
- | - | Horizontal subsurface-flow constructed wetland | Tannery wastewater | [111] |
| 47. | A Commelina benghalensis L. (Benghal dayflower) and B T. latifolia | A,B BOD (61.0 and 59.0) A,B COD (58.0 and 53.0) A,B NH4+ (60.3 and 51.5) A,B NO3-N (60.3 and 51.5) A,B PO42−(61.0 and 64.0) |
A,B TC (41.0 and 39.0) A,B FC (50.0 and 30.0) A,B E. coli (45.0 and 35.0) |
- | Horizontal-flow constructed wetland | Domestic wastewater | [111] |
| 48. | Oenanthe javanica DC. (water celery) | COD (38.65) NH4+-N (28.20) TN (18.82) TP (14.57) |
- | Purification of micro-polluted water was improved indirectly by inoculating low temperature-resistant Bacillus spp., via altering the community structure of the wetland microbes (i.e., through stimulating beneficial microbes for treating sewage while inhibiting microbial pathogens) | Vertical subsurface-flow constructed wetland | Micro-polluted lake water | [112] |
| 49. | A Canna indica L. (Indian shot) and B Iris sibrica L. (Siberian flag) | A,B COD (83.6 and 66.3) A,B NH4+-N (82.7 and 44.1) A,B TN (76.8 and 43.8) |
- | - | Horizontal subsurface-flow constructed wetland | Domestic wastewater | [62] |
| 50. | P. australis | TP (36.2– 87.5) | - | - | Microbial-enhanced constructed wetlands | P-rich Saline wastewater | [113] |
Notes: BOD5: biochemical oxygen demand measured in a 5-day test; COD: chemical oxygen demand; C: carbon; FC: fecal coliforms; HM: heavy metal(s); NH3: ammonia; NH4+: ammonium; NO3−: nitrate; Org-N: organic N; PO42−: phosphate; SO42−: sulphate; SS: suspended solids; TC: total coliforms; TCr: total chromium; TDS: total dissolved solids; TE: trace element(s); TN: total nitrogen; TP: total phosphorus; TSS: total suspended solids; TVS: total volatile solids.
References
- Tanner, C.C. Plants for constructed wetland treatment systems–A comparison of the growth and nutrient uptake of eight emergent species. Ecol. Eng. 1996, 7, 59–83.
- Gingerich, R.T.; Panaccione, D.G.; Anderson, J.T. The role of fungi and invertebrates in litter decomposition in mitigated and reference wetlands. Limnologica 2015, 54, 23–32.
- Sirianuntapiboon, S.; Kongchum, M.; Jitmaikasem, W. Effects of hydraulic retention time and media of constructed wetland for treatment of domestic wastewater. Afr. J. Agric. Res. 2006, 1, 027–037.
- Wang, Y.; Shen, L.; Wu, J.; Zhong, F.; Cheng, S. Step-feeding ratios affect nitrogen removal and related microbial communities in multi-stage vertical flow constructed wetlands. Sci. Total. Environ. 2020, 721, 137689.
- Si, Z.; Wang, Y.; Song, X.; Cao, X.; Zhang, X.; Sand, W. Mechanism and performance of trace metal removal by continuous-flow constructed wetlands coupled with a micro-electric field. Water Res. 2019, 164, 114937.
- Bijalwan, A.; Thakur, T. Effect of IBA and age of cuttings on rooting behaviour of Jatropha curcas L. in different seasons in Western Himalaya, India. Afr. J. Plant Sci. 2010, 4, 387–390.
- Bessa, V.; Moreira, I.; Tiritan, M.; Castro, P. Enrichment of bacterial strains for the biodegradation of diclofenac and carbamazepine from activated sludge. Int. Biodeterior. Biodegrad. 2017, 120, 135–142.
- Kumar, S.; Bijalwan, A.; Singh, B.; Rawat, D.; Yewale, A.G.; Riyal, M.K.; Thakur, T.K. Comparison of Carbon Sequestration Potential of Quercus leucotrichophora Based Ag-roforestry Systems and Natural Forest in Central Himalaya, India. Water Air Soil Pollut. 2021, 232, 350.
- Vassallo, A.; Miceli, E.; Fagorzi, C.; Castronovo, L.M.; Del Duca, S.; Chioccioli, S.; Venditto, S.; Coppini, E.; Fibbi, D.; Fani, R. Temporal Evolution of Bacterial Endophytes Associated to the Roots of Phragmites australis Exploited in Phytodepuration of Wastewater. Front. Microbiol. 2020, 11, 1652.
- Wang, J.; Long, Y.; Yu, G.; Wang, G.; Zhou, Z.; Li, P.; Zhang, Y.; Yang, K.; Wang, S. A Review on Microorganisms in Constructed Wetlands for Typical Pollutant Removal: Species, Function, and Diversity. Front. Microbiol. 2022, 13, 845725.
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas Rhizobacteria of Avicennia marina of Indian Sundarbans Promote Rice Growth Under Saline and Heavy Metal Stresses Through Exopolysaccharide Production. Front. Microbiol. 2019, 10, 1207.
- Agarwal, S.; Mukherjee, P.; Pramanick, P.; Mitra, A. Seasonal Variations in Bioaccumulation and Translocation of Toxic Heavy Metals in the Dominant Vegetables of East Kolkata Wetlands: A Case Study with Suggestive Ecorestorative Strategies. Appl. Biochem. Biotechnol. 2023, 195, 2332–2358.
- Roy, M.; Pandey, V.C. Role of microbes in grass-based phytoremediation. In Phytoremediation Potential of Perennial Grasses; Pandey, V.C., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–327.
- Mishra, P.; Mishra, J.; Dwivedi, S.K.; Arora, N.K. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries. Microorganisms for Sustainability; Arora, N., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 259–285.
- Zhang, D.Q.; Tan, S.K.; Gersberg, R.M.; Zhu, J.; Sadreddini, S.; Li, Y. Nutrient removal in tropical subsurface flow constructed wetlands under batch and continuous flow conditions. J. Environ. Manag. 2012, 96, 1–6.
- Caselles-Osorio, A.; Garcia, J. Effect of physico-chemical pretreatment on the removal efficiency of horizontal subsurface-flow constructed wetlands. Environ. Pollut. 2007, 146, 55–63, ISSN 0269-7491.
- Brix, H.; Schierup, H. Danish experience with sewage treatment in constructed wetlands. In Constructed Wetlands for Wastewater Treatment; Hammer, D.A., Ed.; Lewis Publishers: Chelsea, MI, USA, 1989; pp. 565–573.
- Shelef, O.; Gross, A.; Rachmilevitch, S. Role of Plants in a Constructed Wetland: Current and New Perspectives. Water 2013, 5, 405–419.
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Chemistry for Environmental Engineering and Science, 5th ed.; McGraw-Hill: New York, NY, USA, 2003.
- Kadlec, R.H.; Knight, R.L. Treatment Wetlands; IWA Publishing: London, UK, 1996.
- Akinbile, C.O.; Yusoff, M.S. Water Hyacinth (Eichhornia crassipes) and Lettuce (Pistia stratiotes) effectiveness in aquaculture wastewater treatment in Malaysia. Int. J. Phytoremediation 2012, 14, 201–211.
- US, EPA. Manual: Constructed Wetlands Treatment of Municipal Wastewaters; EPA/625/R-99/010; U.S. EPA Office of Research and Development: Cincinnati, OH, USA, 2000.
- Zhang, M.; Wang, Z.-J.; Huang, J.-C.; Sun, S.; Cui, X.; Zhou, W.; He, S. Salinity-driven nitrogen removal and its quantitative molecular mechanisms in artificial tidal wetlands. Water Res. 2021, 202, 117446.
- Ye, F.; Li, Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol. Eng. 2009, 35, 1043–1050.
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total. Environ. 2007, 380, 48–65.
- Tan, X.; Yang, Y.-L.; Liu, Y.-W.; Li, X.; Zhu, W.-B. Quantitative ecology associations between heterotrophic nitrification-aerobic denitrification, nitrogen-metabolism genes, and key bacteria in a tidal flow constructed wetland. Bioresour. Technol. 2021, 337, 125449.
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448.
- Ojoawo, S.O.; Udayakumar, G.; Naik, P. Phytoremediation of Phosphorus and Nitrogen with Canna × generalis Reeds in Domestic Wastewater through NMAMIT Constructed Wetland. Aquat. Procedia 2015, 4, 349–356.
- Hu, Y.; He, F.; Ma, L.; Zhang, Y.; Wu, Z. Microbial nitrogen removal pathways in integrated vertical-flow constructed wetland systems. Bioresour. Technol. 2016, 207, 339–345.
- Xie, E.; Ding, A.; Zheng, L.; Lu, C.; Wang, J.; Huang, B.; Xiu, H. Seasonal variation in populations of nitrogen-transforming bacteria and correlation with nitrogen removal in a full-scale horizontal flow constructed wetland treating Polluted River water. Geomicrobiol. J. 2016, 33, 338–346.
- Zhao, Y.; Cao, X.; Song, X.; Zhao, Z.; Wang, Y.; Si, Z.; Lin, F.; Chen, Y.; Zhang, Y. Montmorillonite supported nanoscale zero-valent iron immobilized in sodium alginate (SA/Mt-NZVI) enhanced the nitrogen removal in vertical flow constructed wetlands (VFCWs). Bioresour. Technol. 2018, 267, 608–617.
- Lee, S.; Maniquiz-Redillas, M.C.; Choi, J.; Kim, L.-H. Nitrogen mass balance in a constructed wetland treating piggery wastewater effluent. J. Environ. Sci. 2014, 26, 1260–1266.
- Tan, X.; Yang, Y.-L.; Liu, Y.-W.; Yin, W.-C.; Fan, X.-Y. The synergy of porous substrates and functional genera for efficient nutrients removal at low temperature in a pilot-scale two-stage tidal flow constructed wetland. Bioresour. Technol. 2021, 319, 124135.
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S.; Zhou, W. Dissimilatory nitrate reduction processes and corresponding nitrogen loss in tidal flow constructed wetlands. J. Clean. Prod. 2021, 295, 126429.
- Wang, W.; Su, Y.; Wang, B.; Wang, Y.; Zhuang, L.; Zhu, G. Spatiotemporal shifts of ammonia-oxidizing archaea abundance and structure during the restoration of a multiple pond and plant-bed/ditch wetland. Sci. Total. Environ. 2019, 684, 629–640.
- Zhao, L.; Fu, G.; Wu, J.; Pang, W.; Hu, Z. Bioaugmented constructed wetlands for efficient saline wastewater treatment with multiple denitrification pathways. Bioresour. Technol. 2021, 335, 125236.
- Wang, T.; Xiao, L.; Lu, H.; Lu, S.; Li, J.; Guo, X.; Zhao, X. Nitrogen removal from summer to winter in a field pilot-scale multistage constructed wetland-pond system. J. Environ. Sci. 2022, 111, 249–262.
- Liu, T.; Lu, S.; Wang, R.; Xu, S.; Qin, P.; Gao, Y. Behavior of selected organophosphate flame retardants (OPFRs) and their influence on rhizospheric microorganisms after short-term exposure in integrated vertical-flow constructed wetlands (IVCWs). Sci. Total. Environ. 2020, 710, 136403.
- Huang, T.; Liu, W.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. A stable simultaneous anammox, denitrifying anaerobic methane oxidation and denitrification process in integrated vertical constructed wetlands for slightly polluted wastewater. Environ. Pollut. 2020, 262, 114363.
- Li, X.; Li, Y.; Lv, D.; Li, Y.; Wu, J. Nitrogen and phosphorus removal performance and bacterial communities in a multi-stage surface flow constructed wetland treating rural domestic sewage. Sci. Total. Environ. 2020, 709, 136235.
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S. Depth-specific distribution and significance of nitrite-dependent anaerobic methane oxidation process in tidal flow constructed wetlands used for treating river water. Sci. Total. Environ. 2020, 716, 137054.
- Kraiem, K.; Kallali, H.; Wahab, M.A.; Fra-Vazquez, A.; Mosquera-Corral, A.; Jedidi, N. Comparative study on pilots between ANAMMOX favored conditions in a partially saturated vertical flow constructed wetland and a hybrid system for rural wastewater treatment. Sci. Total. Environ. 2019, 670, 644–653.
- Li, Q.; Bu, C.; Ahmad, H.A.; Guimbaud, C.; Gao, B.; Qiao, Z.; Ding, S.; Ni, S.-Q. The distribution of dissimilatory nitrate reduction to ammonium bacteria in multistage constructed wetland of Jining, Shandong, China. Environ. Sci. Pollut. Res. 2021, 28, 4749–4761.
- Rahman, M.M.; Roberts, K.L.; Grace, M.R.; Kessler, A.J.; Cook, P.L.M. Role of organic carbon, nitrate and ferrous iron on the partitioning between denitrification and DNRA in constructed stormwater urban wetlands. Sci. Total Environ. 2019, 666, 608–617.
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S.; Zhou, W. Nitrogen removal through collaborative microbial pathways in tidal flow constructed wetlands. Sci. Total. Environ. 2021, 758, 143594.
- Si, Z.; Song, X.; Wang, Y.; Cao, X.; Zhao, Y.; Wang, B.; Chen, Y.; Arefe, A. Intensified heterotrophic denitrification in constructed wetlands using four solid carbon sources: Denitrification efficiency and bacterial community structure. Bioresour. Technol. 2018, 267, 416–425.
- Ajibade, F.O.; Wang, H.-C.; Guadie, A.; Ajibade, T.F.; Fang, Y.-K.; Sharif, H.M.A.; Liu, W.-Z.; Wang, A.-J. Total nitrogen removal in biochar amended non-aerated vertical flow constructed wetlands for secondary wastewater effluent with low C/N ratio: Microbial community structure and dissolved organic carbon release conditions. Bioresour. Technol. 2021, 322, 124430.
- Zhao, Y.; Zhao, Z.; Song, X.; Jiang, X.; Wang, Y.; Cao, X.; Si, Z.; Pan, F. Effects of nZVI dosing on the improvement in the contaminant removal performance of constructed wetlands under the dye stress. Sci. Total. Environ. 2020, 703, 134789.
- Interstate Technology & Regulatory Council. Phytotechnology Technical and Regulatory Guidance and Decision Trees, Revised; ITRC: Washington, DC, USA, 2009. Available online: www.itrcweb.org (accessed on 17 September 2023).
- Du, L.; Chen, Q.; Liu, P.; Zhang, X.; Wang, H.; Zhou, Q.; Xu, D.; Wu, Z. Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by Integrated Vertical-flow Constructed Wetlands (IVCWs). Bioresour. Technol. 2017, 243, 204–211.
- Wang, Q.; Ding, J.; Xie, H.; Hao, D.; Du, Y.; Zhao, C.; Xu, F.; Kong, Q.; Wang, B. Phosphorus removal performance of microbial-enhanced constructed wetlands that treat saline wastewater. J. Clean. Prod. 2021, 288, 125119.
- Mitsch, J.W.; Gosselink, J.G. Wetlands; Van Nostrand Reinhold Company: New York, NY, USA, 1986.
- Babatunde, A.; Zhao, Y.; Burke, A.; Morris, M.; Hanrahan, J. Characterization of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environ. Pollut. 2009, 157, 2830–2836.
- Shi, X.; Fan, J.; Zhang, J.; Shen, Y. Enhanced phosphorus removal in intermittently aerated constructed wetlands filled with various construction wastes. Environ. Sci. Pollut. Res. 2017, 24, 22524–22534.
- Tian, J.; Yu, C.; Liu, J.; Ye, C.; Zhou, X.; Chen, L. Performance of an ultraviolet Mutagenetic polyphosphate-accumulating bacterium PZ2 and its application for wastewater treatment in a newly designed constructed wetland. Appl. Biochem. Biotechnol. 2017, 181, 735–747.
- Lv, R.; Wu, D.; Ding, J.; Yuan, X.; Zhou, G.; Zhang, Y.; Kong, Q.; Zhao, C.; Du, Y.; Xu, F.; et al. Long-term performance and microbial mechanism in intertidal wetland sediment introduced constructed wetlands treating saline wastewater. J. Clean. Prod. 2021, 310, 127409.
- Huang, J.; Xiao, J.; Guo, Y.; Guan, W.; Cao, C.; Yan, C.; Wang, M. Long-term effects of silver nanoparticles on performance of phosphorus removal in a laboratory-scale vertical flow constructed wetland. J. Environ. Sci. 2020, 87, 319–330.
- Li, X.; Zhang, M.; Liu, F.; Li, Y.; Li, Y.; Xiao, R.; Wu, J. Bacterial community dynamics in a Myriophyllum elatinoides purification system for swine wastewater in sediments. Appl. Soil Ecol. 2017, 119, 56–63.
- Wu, H.; Gao, X.; Wu, M.; Zhu, Y.; Xiong, R.; Ye, S. The efficiency and risk to groundwater of constructed wetland system for domestic sewage treatment-A case study in Xiantao, China. J. Clean. Prod. 2020, 277, 123384.
- Yu, G.; Wang, G.; Li, J.; Chi, T.; Wang, S.; Peng, H.; Chen, H.; Du, C.; Jiang, C.; Liu, Y.; et al. Enhanced Cd2+ and Zn2+ removal from heavy metal wastewater in constructed wetlands with resistant microorganisms. Bioresour. Technol. 2020, 316, 123898.
- Thakur, T.K.; Dutta, J.; Upadhyay, P.; Patel, D.K.; Thakur, A.; Kumar, M.; Kumar, A. Assessment of land degradation and restoration in coal mines of central India: A time series analysis. Ecol. Eng. 2021, 175, 106493.
- Chen, X.; Zhong, F.; Chen, Y.; Wu, J.; Cheng, S. The Interaction Effects of Aeration and Plant on the Purification Performance of Horizontal Subsurface Flow Constructed Wetland. Int. J. Environ. Res. Public Health 2022, 19, 1583.
- Teitzel, G.M.; Parsek, M.R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2003, 69, 2313–2320.
- Cristani, M.; Naccari, C.; Nostro, A.; Pizzimenti, A.; Trombetta, D.; Pizzimenti, F. Possible use of Serratia marcescens in toxic metal biosorption (removal). Environ. Sci. Pollut. Res. 2012, 19, 161–168.
- Zhou, Y.; Tigane, T.; Li, X.; Truu, M.; Truu, J.; Mander, Ü. Hexachlorobenzene dechlorination in constructed wetland mesocosms. Water Res. 2013, 47, 102–110.
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial Endophytes: Potential Role in Developing Sustainable Systems of Crop Production. Crit. Rev. Plant Sci. 2000, 19, 1–30.
- Syranidou, E.; Christofilopoulos, S.; Gkavrou, G.; Thijs, S.; Weyens, N.; Vangronsveld, J.; Kalogerakis, N. Exploitation of Endophytic Bacteria to Enhance the Phytoremediation Potential of the Wetland Helophyte Juncus acutus. Front. Microbiol. 2016, 07, 1016.
- Agarwal, S.; Darbar, S.; Saha, S. Challenges in management of domestic wastewater for sustainable development. In Current Directions in Water Scarcity Research; Elsevier: Amsterdam, The Netherlands, 2022; pp. 531–552.
- Maiga, Y.; von Sperling, M.; Mihelcic, J.R. Constructed Wetlands. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project). (Mihelcic JR, Verbyla ME (eds), Part 4: Management of Risk from Excreta and Wastewater-Section: Sanitation System Technologies, Pathogen Reduction in Sewered System Technologies); Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: East Lansing, MI, USA, 2017.
- Dotro, G.; Langergraber, G.; Molle, P.; Nivala, J.; Puigagut Juárez, J.; Stein, O.R.; von Sperling, M. Treatment Wetlands; Biological Wastewater Treatment Series (Volume 7); IWA Publishing: London, UK, 2017.
- Ansola, G.; González, J.M.; Cortijo, R.; de Luis, E. Experimental and full–scale pilot plant constructed wetlands for municipal wastewaters treatment. Ecol. Eng. 2003, 21, 43–52.
- Kaseva, M. Performance of a sub-surface flow constructed wetland in polishing pre-treated wastewater—A tropical case study. Water Res. 2004, 38, 681–687.
- Mbuligwe, S.E. Comparative effectiveness of engineered wetland systems in the treatment of anaerobically pre-treated domestic wastewater. Ecol. Eng. 2004, 23, 269–284.
- Solano, M.; Soriano, P.; Ciria, M. Constructed Wetlands as a Sustainable Solution for Wastewater Treatment in Small Villages. Biosyst. Eng. 2004, 87, 109–118.
- Slak, A.S.; Bulc, T.G.; Vrhovsek, D. Comparison of nutrient cycling in a surface-flow constructed wetland and in a facultative pond treating secondary effluent. Water Sci. Technol. 2005, 51, 291–298.
- Jinadasa, K.B.S.N.; Tanaka, N.; Mowjood, M.I.M.; Werellagama, D.R.I.B. Free water surface constructed wetlands for domestic wastewater treatment: A tropical case study. Chem. Ecol. 2006, 22, 181–191.
- Song, Z.; Zheng, Z.; Li, J.; Sun, X.; Han, X.; Wang, W.; Xu, M. Seasonal and annual performance of a full-scale constructed wetland system for sewage treatment in China. Ecol. Eng. 2006, 26, 272–282.
- Zhang, X.-B.; Liu, P.; Yang, Y.-S.; Chen, W.-R. Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes. J. Environ. Sci. 2007, 19, 902–909.
- Katsenovich, Y.P.; Hummel-Batista, A.; Ravinet, A.J.; Miller, J.F. Performance evaluation of constructed wetlands in a tropical region. Ecol. Eng. 2009, 35, 1529–1537.
- Konnerup, D.; Koottatep, T.; Brix, H. Treatment of domestic wastewater in tropical, subsurface flow constructed wetlands planted with Canna and Heliconia. Ecol. Eng. 2009, 35, 248–257.
- Morari, F.; Giardini, L. Municipal wastewater treatment with vertical flow constructed wetlands for irrigation reuse. Ecol. Eng. 2009, 35, 643–653.
- Xinshan, S.; Qin, L.; Denghua, Y. Nutrient Removal by Hybrid Subsurface Flow Constructed Wetlands for High Concentration Am-monia Nitrogen Wastewater. Procedia Environ. Sci. 2010, 2, 1461–1468.
- Zhai, J.; Xiao, H.W.; Kujawa-Roeleveld, K.; He, Q.; Kerstens, S.M. Experimental study of a novel hybrid constructed wetland for water reuse and its application in Southern China. Water Sci. Technol. 2011, 64, 2177–2184.
- Zhao, Y.J.; Hui, Z.; Chao, X.; Nie, E.; Li, H.J.; He, J.; Zheng, Z. Efficiency of two-stage combinations of subsurface vertical down-flow and up-flow constructed wetland systems for treating variation in influent C/N ratios of domestic wastewater. Ecol. Eng. 2011, 37, 1546–1554.
- Zurita, F.; Belmont, M.A.; De Anda, J.; White, J.R. Seeking a way to promote the use of constructed wetlands for domestic wastewater treatment in developing countries. Water Sci. Technol. 2011, 63, 654–659.
- Abou-Elela, S.I.; Hellal, M.S. Municipal wastewater treatment using vertical flow constructed wetlands planted with Canna, Phragmites and Cyprus. Ecol. Eng. 2012, 47, 209–213.
- Chang, J.-J.; Wu, S.-Q.; Dai, Y.-R.; Liang, W.; Wu, Z.-B. Treatment performance of integrated vertical-flow constructed wetland plots for domestic wastewater. Ecol. Eng. 2012, 44, 152–159.
- Villara, M.M.P.; Domínguez, E.R.; Tack, F.; Ruiz, J.M.H.; Morales, R.S.; Arteaga, L.E. Vertical subsurface wetlands for wastewater purification. Procedia Eng. 2012, 42, 1960–1968.
- Abou-Elela, S.I.; Golinielli, G.; Abou-Taleb, E.M.; Hellal, M.S. Municipal wastewater treatment in horizontal and vertical flows constructed wetlands. Ecol. Eng. 2013, 61, 460–468.
- Rai, U.; Tripathi, R.; Singh, N.; Upadhyay, A.; Dwivedi, S.; Shukla, M.; Mallick, S.; Singh, S.; Nautiyal, C. Constructed wetland as an ecotechnological tool for pollution treatment for conservation of Ganga river. Bioresour. Technol. 2013, 148, 535–541.
- Badhe, N.; Saha, S.; Biswas, R.; Nandy, T. Role of algal biofilm in improving the performance of free surface, up-flow constructed wetland. Bioresour. Technol. 2014, 169, 596–604.
- Shao, Y.; Pei, H.; Hu, W.; Chanway, C.P.; Meng, P.; Ji, Y.; Li, Z. Bioaugmentation in lab scale constructed wetland microcosms for treating polluted river water and domestic wastewater in northern China. Int. Biodeterior. Biodegrad. 2014, 95, 151–159.
- Sharma, G.; Priya; Brighu, U. Performance Analysis of Vertical up-flow Constructed Wetlands for Secondary Treated Effluent. APCBEE Procedia 2014, 10, 110–114.
- Younger, P.L.; Henderson, R. Synergistic wetland treatment of sewage and mine water: Pollutant removal performance of the first full-scale system. Water Res. 2014, 55, 74–82.
- Calheiros, C.S.C.; Bessa, V.S.; Mesquita, R.B.R.; Brix, H.; Rangel, A.O.S.S.; Castro, P.M.L. Constructed wetland with a polyculture of ornamental plants for wastewater treatment at a rural tourism facility. Ecol. Eng. 2015, 79, 1–7, ISSN 0925-8574.
- Lu, S.; Pei, L.; Bai, X. Study on method of domestic wastewater treatment through new-type multi-layer artificial wetland. Int. J. Hydrog. Energy 2015, 40, 11207–11214.
- Xu, D.; Wang, L.; Li, H.; Li, Y.; Howard, A.; Guan, Y.; Li, J.; Xu, H. The forms and bioavailability of phosphorus in integrated vertical flow constructed wetland with earthworms and different substrates. Chemosphere 2015, 134, 492–498.
- Abdelhakeem, S.G.; Aboulroos, S.A.; Kamel, M.M. Performance of a vertical subsurface flow constructed wetland under different operational conditions. J. Adv. Res. 2016, 7, 803–814.
- Huang, Z.; Zhang, X.; Cui, L.; Yu, G. Optimization of operating parameters of hybrid vertical down-flow constructed wetland systems for domestic sewerage treatment. Environ. Manag. 2016, 180, 384–389.
- Hua, Y.; Peng, L.; Zhang, S.; Heal, K.V.; Zhao, J.; Zhu, D. Effects of plants and temperature on nitrogen removal and microbiology in pilot-scale horizontal subsurface flow constructed wetlands treating domestic wastewater. Ecol. Eng. 2017, 108, 70–77.
- Ali, M.; Rousseau, D.P.; Ahmed, S. A full-scale comparison of two hybrid constructed wetlands treating domestic wastewater in Pakistan. J. Environ. Manag. 2018, 210, 349–358.
- Benvenuti, T.; Hamerski, F.; Giacobbo, A.; Bernardes, A.M.; Zoppas-Ferreira, J.; Rodrigues, M.A. Constructed floating wetland for the treatment of domestic sewage: A real-scale study. Environ. Chem. Eng. 2018, 6, 5706–5711.
- Elfanssi, S.; Ouazzani, N.; Latrach, L.; Hejjaj, A.; Mandi, L. Phytoremediation of domestic wastewater using a hybrid constructed wetland in mountainous rural area. Int. J. Phytoremediation 2018, 20, 75–87.
- Mishra, V.K.; Otter, P.; Shukla, R.; Goldmaier, A.; Alvarez, J.A.; Khalil, N.; Avila, C.; Arias, C.; Ameršek, I. Application of horizontal flow constructed wetland and solar driven disinfection technologies for wastewater treatment in India. Water Pract. Technol. 2018, 13, 469–480.
- Worku, A.; Tefera, N.; Kloos, H.; Benor, S. Constructed wetlands for phytoremediation of industrial wastewater in Addis Ababa, Ethiopia. Nanotechnol. Environ. Eng. 2018, 3, 9.
- Yadav, A.; Chazarenc, F.; Mutnuri, S. Development of the “French system” vertical flow constructed wetland to treat raw domestic wastewater in India. Ecol. Eng. 2018, 113, 88–93.
- García-Ávila, F.; Avilés-Añazco, A.; Sánchez-Cordero, E.; Valdiviezo-Gonzáles, L.; Ordoñez, M.D.T. The challenge of improving the efficiency of drinking water treatment systems in rural areas facing changes in the raw water quality. S. Afr. J. Chem. Eng. 2021, 37, 141–149.
- Barya, M.P.; Gupta, D.; Thakur, T.K.; Shukla, R.; Singh, G.; Mishra, V.K. Phytoremediation performance of Acorus calamus and Canna indica for the treatment of primary treated domestic sewage through vertical subsurface flow constructed wetlands: A field-scale study. Water Pract. Technol. 2020, 15, 528–539.
- Nguyen, X.C.; Nguyen, D.D.; Tran, Q.B.; Nguyen, T.T.H.; Tran, T.K.A.; Tran, T.C.P.; Nguyen, T.H.G.; Tran, T.N.T.; La, D.D.; Chang, S.W.; et al. Two-step system consisting of novel vertical flow and free water surface constructed wetland for effective sewage treatment and reuse. Bioresour. Technol. 2020, 306, 123095.
- Xiong, C.; Tam, N.F.; Dai, Y.; Zhang, X.; Li, R.; Zheng, Y.; Wang, L.; Yang, Y. Enhanced performance of pilot-scale hybrid constructed wetlands with A/O reactor in raw domestic sewage treatment. J. Environ. Manag. 2020, 258, 110026.
- Aregu, M.B.; Asfaw, S.L.; Khan, M.M. Developing horizontal subsurface flow constructed wetland using pumice and Chrysopogon zizanioides for tannery wastewater treatment. Environ. Syst. Res. 2021, 10, 33.
- Shukla, R.; Gupta, D.; Singh, G.; Mishra, V.K. Performance of horizontal flow constructed wetland for secondary treatment of domestic wastewater in a remote tribal area of Central India. Sustain. Environ. Res. 2021, 31, 13.
- Gao, J.; Li, Q.; Zhang, J.; Wang, S.; Song, B.; Huang, Z. Purification of Micro-Polluted Lake Water by Biofortification of Vertical Subsurface Flow Constructed Wetlands in Low-Temperature Season. Water 2022, 14, 896.
More
Information
Subjects:
Water Resources
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
20 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




