Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sumit Jangra | -- | 1350 | 2023-11-11 15:05:24 | | | |
| 2 | Catherine Yang | Meta information modification | 1350 | 2023-11-14 02:30:35 | | | | |
| 3 | Catherine Yang | -2 word(s) | 1348 | 2023-11-14 04:32:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Potts, J.; Jangra, S.; Michael, V.N.; Wu, X. Speed Breeding for Crop Improvement and Food Security. Encyclopedia. Available online: https://encyclopedia.pub/entry/51447 (accessed on 08 February 2026).
Potts J, Jangra S, Michael VN, Wu X. Speed Breeding for Crop Improvement and Food Security. Encyclopedia. Available at: https://encyclopedia.pub/entry/51447. Accessed February 08, 2026.
Potts, Jesse, Sumit Jangra, Vincent N. Michael, Xingbo Wu. "Speed Breeding for Crop Improvement and Food Security" Encyclopedia, https://encyclopedia.pub/entry/51447 (accessed February 08, 2026).
Potts, J., Jangra, S., Michael, V.N., & Wu, X. (2023, November 11). Speed Breeding for Crop Improvement and Food Security. In Encyclopedia. https://encyclopedia.pub/entry/51447
Potts, Jesse, et al. "Speed Breeding for Crop Improvement and Food Security." Encyclopedia. Web. 11 November, 2023.
Copy Citation
Amid a rapidly growing global population and increasing threats to crop yields, Speed Breeding (SB) in crop genetics is focused. It traces SB’s development from carbon arc lamp experiments 150 years ago to its modern use with LED technology which significantly accelerates breeding cycles. SB has applications in genetic mapping, genetic modification, and trait stacking, enhancing crop resilience by leveraging allelic diversity. It aligns well with breeding methods like single plant selection and single seed descent. The integration of SB with gene editing, genotyping, and genomic selection holds great promise.
genetic gain
controlled environment
shuttle breeding
1. The History and Development of SB in Enhancing Crop Genetics
Research dating back to 150 years ago, using carbon arc lamps, resulted in the discovery of plants being able to grow and procreate under conditions with artificial lights, speeding up the flowering cycle when subjected to continuous light [1][2]. Later, Utah State University collaborated with the National Aeronautics and Space Administration (NASA) to develop a dwarf wheat line selected for fast growth and development in a perpetual light environment. Additionally, space mirror utilization was proposed by Russian scientists to improve agricultural productivity [3][4].
Thereafter, the effects of light-emitting diode (LED) lights on plant development were evaluated in the 1990s by the University of Wisconsin, and improvements in LED technology have made indoor plant propagation systems more affordable, leading to increased crop productivity [5]. As a result of NASA’s research efforts, researchers at the University of Queensland named the technology SB (Figure 1), which they utilized in 2003 to hasten wheat breeding without specialized labs [6]. Specific protocols for inducing flowering in crops with environmental cues can be used to reduce the space and cost associated with inbred line development. Seed chipping and barcoding can assist marker-assisted selection (MAS), crossing, mapping population development, adult plant phenotyping, trait stacking, and development of Genetic modification (GM) pipelines [7][8]. This approach holds the potential to substantially accelerate the exploration and utilization of allelic diversity inherent in traditional landraces and the wild progenitors of cultivated crops. Through the application of this method, researchers can uncover and harness previously undiscovered reservoirs of resistance, ultimately contributing to the diversification and enhancement of crop resilience [9].
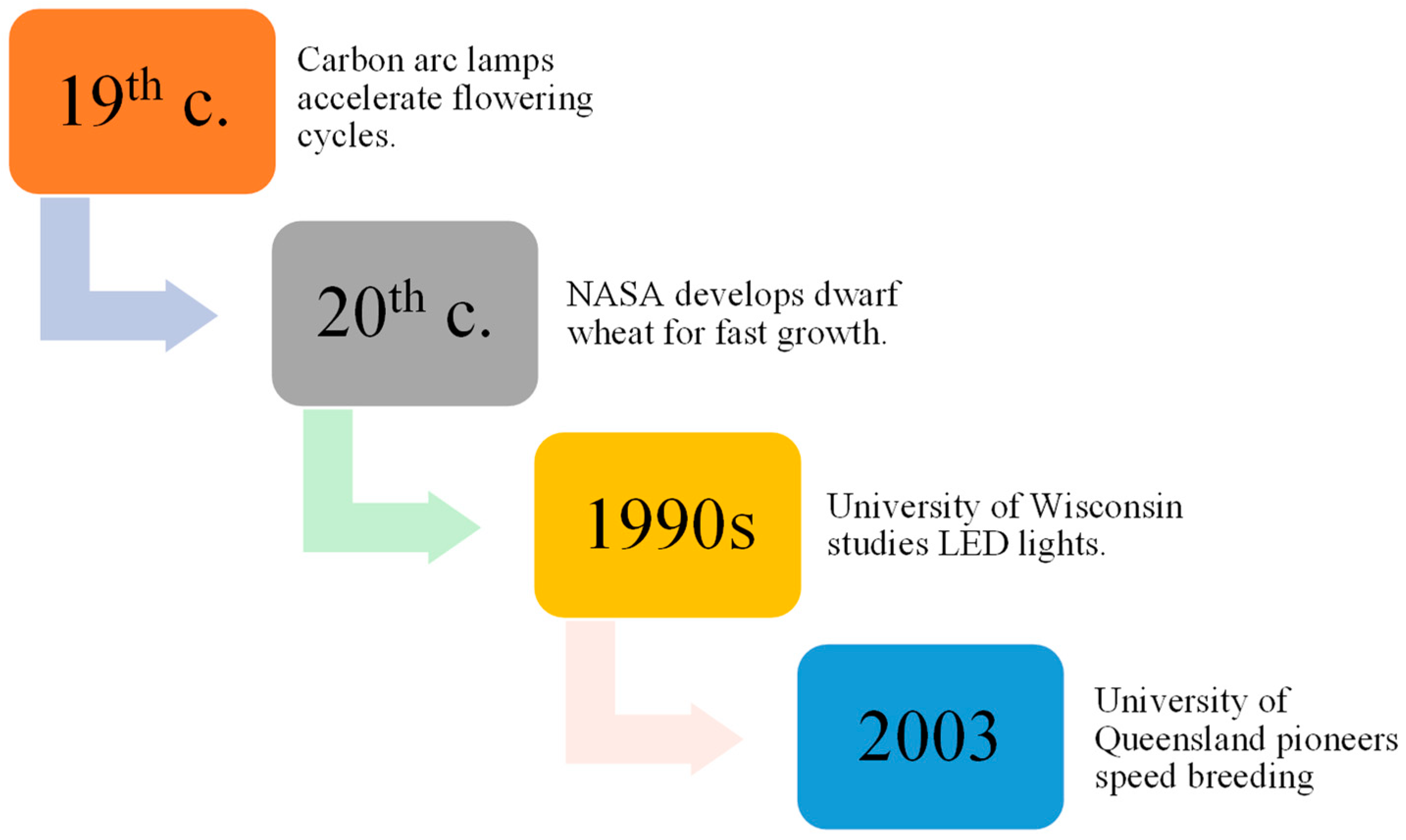
Figure 1. Evolution of SB techniques over time.
Early studies indicated that growing lettuce under red LEDs and blue fluorescent lamps resulted in growth rates comparable to cool-white fluorescent and incandescent lamps [10]. Further research showed that red LEDs promoted the elongation of hypocotyls and cotyledons, while blue LEDs prevented this elongation [11]. These findings spurred the development and use of LED illumination systems in plant growth chambers for various plant species, including wheat, Brassica rapa, potato leaf cuttings, Arabidopsis thaliana, and soybeans [12][13][14][15]. Comparison with conventional lighting sources revealed similar photosynthetic responses, showcasing the potential of LEDs and combinations of red, green, and blue light at different ratios for studying terrestrial plants [11]. Moreover, recent studies have demonstrated that the narrow blue + narrow amber LED light mixture outperforms white LEDs, HPS lamps, and narrow amber light treatments, resulting in the highest tomato mass (479 g). Dry mass and plant height showed only minor variations. Additionally, supplementing narrow amber light with 430 nm blue light increased chlorophyll content by 20%, emphasizing the importance of precise wavelength selection for tomato growth [16]. The study also suggests that winter wheat can be successfully grown indoors using various LED lighting configurations. Among these configurations, the treatment employing a 4:1 ratio of red and green LEDs yielded the highest crop production, assimilation rates, and flour quality. Elevated levels of blue light negatively impacted yield [17].
2. SB Applications and Selection Methods in Plant Breeding
Due to the lengthy and extended cycles of selection and inbreeding inherent in traditional selection techniques, including pure line, recurrent, bulk, mass, and pedigree selection, they are considered unsuitable for application in SB. These conventional methods do not align with the accelerated breeding demands and precision required by SB in modern agricultural contexts [18]. In contrast, it is worth noting that certain breeding methods, such as single plant selection (SPS), and single seed descent (SSD), align exceptionally well with the principles of SB [19]. These techniques offer distinct benefits, as they require less cultivation space and labor during initial generations. This streamlined approach enables researchers to efficiently advance offspring under the conditions of HDP within controlled growth chambers and relatively compact nurseries, excluding greenhouse environments. This expedites breeding cycles while optimizing resource allocation for enhanced crop development. Furthermore, this method can be extended to field environments [20].
SB using SSD programs is highly efficient, especially for cereal crops like wheat and barley. Higher sowing densities in SB allowed rapid cycling of multiple generations annually. Under specific LED-supplemented glasshouse setups, up to six generations of wheat and barley per year were achieved at a density of 1000 plants/m2. Extending the photoperiod from 16 to 22 h significantly accelerated development [21]. Studies revealed that manipulating light quality, specifically the red to far-red ratio, significantly influences the flowering time of cool-season grain legumes such as peas, chickpeas, faba beans, lentils, and lupins. These findings are crucial for expediting SSD in legume breeding, offering practical applications in biotechnological tools for enhancing legume crops [22]. In another study, SSD was applied to create recombinant inbred lines in chickpeas, focusing on salinity tolerance. Researchers successfully identified multiple genetic loci (QTLs) associated with salinity tolerance, providing valuable insights for developing salt-tolerant chickpea varieties [23]. Similar approaches have been used in peanut breeding research, where controlled conditions, continuous light, optimal temperature, and SSD were combined in a greenhouse setting. These efforts consistently reduced the generation time for full-season maturity peanut cultivars from 145 to 89 days. This innovative speed breeding technique has the potential to significantly expedite peanut variety development, shortening the traditional breeding timeline from the first cross to commercial release to approximately six to seven years [24].
By utilization of various methods such as biparental population mapping, phenotyping, and genetic transformation experiments [21][25], SB stands out as a superior alternative to the doubled haploid (DH) technique, primarily due to its remarkable ability to overcome the hurdles of poor vigor and germination rates [26]. The speed breeding technique was applied in the cultivation of durum wheat. The study conducted a phenotypic analysis on a biparental population derived from Outrob 4 and Caoaroi, focusing on various traits such as leaf rust resistance, crown rot susceptibility, plant stature, root angle, and root number. Astonishingly, they managed to complete an entire generation of durum wheat in a mere 77 days [27]. In contrast to the DH method, which necessitates specific genotypes and well-equipped facilities, SB is more versatile and does not have these limitations. Nevertheless, the technology must address plant phenology and physiology to accelerate generation times [28].
Certain species encounter intricate challenges when employing the double haploid technique, involving factors like haploid production and chromosome doubling, as documented [29]. In these species, inadequate responses to tissue culture result in reduced haploid induction rates and significant associated costs [30][31]. Furthermore, there is a notable prevalence of plant mortality and abnormal development. For species with suboptimal tissue culture performance and no established chromosome doubling methods, these challenges persist. This scenario is exemplified by rye, watermelon, other secondary cucurbit species, tomato, and leguminous species [32][33][34][35].
In a harmonious synergy that expands the horizons of agricultural innovation and high-throughput phenotyping, genetic improvement, gene editing, and genotyping, as well as genomic selection can be integrated with emerging methodologies. This collaborative approach not only leverages the capabilities of each technique but also fosters a comprehensive framework for advancing the frontiers of modern plant breeding and crop enhancement. SB helps to reduce the cost and space requirements of HDP [36]. The use of recombinant inbred lines developed through self-fertilization can be better for genetic mapping than DH as they have a higher recombination frequency [37][38].
SSD methods hasten the progression and assessment of segregation generations within a condensed time frame under the accelerated conditions of SB. To increase the turnover rate of plant generations, various approaches include shuttle breeding, physiological stress, embryo rescue, and increasing CO2 concentration. Shuttle breeding involves growing two generations of wheat per year at different altitudes, latitudes, and climates, making it more accessible and less labor-intensive [24][39][40]. Overall, the techniques of SB hold the potential to substantially enhance genetic advancement by facilitating the creation and introduction of innovative cultivars.
References
- Gilland, B. World population and food supply. Food Policy 2002, 27, 47–63.
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77.
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10.
- Hickey, L.T.; Germán, S.E.; Pereyra, S.A.; Diaz, J.E.; Ziems, L.A.; Fowler, R.A.; Platz, G.J.; Franckowiak, J.D.; Dieters, M.J. Speed breeding for multiple disease resistance in barley. Euphytica 2017, 213, 64.
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754.
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M.; et al. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409.
- Cazzola, F.; Bermejo, C.J.; Guindon, M.F.; Cointry, E. Speed breeding in pea (Pisum sativum L.), an efficient and simple system to accelerate breeding programs. Euphytica 2020, 216, 178.
- Hussain, K.; Mahrukh; Nisa, R.T.; Zaid, A.; Mushtaq, M. The utilization of speed breeding and genome editing to achieve zero hunger. In Sustainable Agriculture in the Era of the OMICs Revolution; Prakash, C.S., Fiaz, S., Nadeem, M.A., Baloch, F.S., Qayyum, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–15. ISBN 978-3-031-15567-3.
- Jacquier, N.M.; Gilles, L.M.; Martinant, J.-P.; Rogowsky, P.M.; Widiez, T. Maize in Planta haploid inducer lines: A cornerstone for doubled haploid technology. In Doubled Haploid Technology; Volume 2: Hot Topics, Apiaceae, Brassicaceae, Solanaceae; Humana: New York, NY, USA, 2021; Volume 2288, pp. 25–48.
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.; Barta, D.; Ignatius, R.; Martin, T. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205.
- Hoenecke, M.; Bula, R.; Tibbitts, T. Importance of blue photon levels for lettuce seedlings grown under red-light-emitting diodes. HortScience 1992, 27, 427–430.
- Zhou, W. Advanced ASTROCULTURETM Plant Growth Unit: Capabilities and Performances; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2005.
- Morrow, R.C.; Duffie, N.A.; Tibbitts, T.W.; Bula, R.J.; Barta, D.J.; Ming, D.W.; Wheeler, R.M.; Porterfield, D.M. Plant Response in the ASTROCULTURETM Flight Experiment Unit; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1995.
- Link, B.; Durst, S.; Zhou, W.; Stankovic, B. Seed-to-seed growth of Arabidopsis thaliana on the International Space Station. Adv. Space Res. 2003, 31, 2237–2243.
- Croxdale, J.; Cook, M.; Tibbitts, T.W.; Brown, C.S.; Wheeler, R.M. Structure of Potato tubers formed during spaceflight. J. Exp. Bot. 1997, 48, 2037–2043.
- Wu, B.-S.; Mansoori, M.; Trumpler, K.; Addo, P.W.; MacPherson, S.; Lefsrud, M. Effect of amber (595 Nm) light supplemented with narrow blue (430 Nm) light on tomato biomass. Plants 2023, 12, 2457.
- Guo, X.; Xue, X.; Chen, L.; Li, J.; Wang, Z.; Zhang, Y. Effects of LEDs light spectra on the growth, yield, and quality of winter wheat (Triticum aestivum L.) cultured in plant factory. J. Plant Growth Regul. 2023, 42, 2530–2544.
- Jagadish, S.V.K.; Bahuguna, R.N.; Djanaguiraman, M.; Gamuyao, R.; Prasad, P.V.V.; Craufurd, P.Q. Implications of high temperature and elevated CO2 on flowering time in plants. Front. Plant Sci. 2016, 7, 913.
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342.
- Bugbee, B.; Koerner, G. Yield comparisons and unique characteristics of the dwarf wheat cultivar ‘USU-Apogee’. Adv. Space Res. 1997, 20, 1891–1894.
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963.
- Croser, J.S.; Pazos-Navarro, M.; Bennett, R.G.; Tschirren, S.; Edwards, K.; Erskine, W.; Creasy, R.; Ribalta, F.M. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tiss. Organ. Cult. 2016, 127, 591–599.
- Byerlee, D.; Fischer, K. Accessing modern science: Policy and institutional options for agricultural biotechnology in developing countries. World Dev. 2002, 30, 931–948.
- O’Connor, D.J.; Wright, G.C.; Dieters, M.J.; George, D.L.; Hunter, M.N.; Tatnell, J.R.; Fleischfresser, D.B. Development and application of speed breeding technologies in a commercial peanut breeding program. Peanut Sci. 2013, 40, 107–114.
- Kumar, V.; Singh, K.; Shah, M.P.; Singh, A.K.; Kumar, A.; Kumar, Y. Application of omics technologies for microbial community structure and function analysis in contaminated environment. In Wastewater Treatment: Cutting-Edge Molecular Tools, Techniques and Applied Aspects; Shah, M.P., Sarkar, A., Mandal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–40.
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both multi-segment light intensity and extended photoperiod lighting strategies, with the same daily light integral, promoted Lactuca sativa L. growth and photosynthesis. Agronomy 2019, 9, 857.
- Alahmad, S.; Dinglasan, E.; Leung, K.M.; Riaz, A.; Derbal, N.; Voss-Fels, K.P.; Able, J.A.; Bassi, F.M.; Christopher, J.; Hickey, L.T. Speed breeding for multiple quantitative traits in durum wheat. Plant Methods 2018, 14, 36.
- Gudi, S.; Kumar, P.; Singh, S.; Tanin, M.J.; Sharma, A. Strategies for accelerating genetic gains in crop plants: Special focus on speed breeding. Physiol. Mol. Biol. Plants 2022, 28, 1921–1938.
- Shariatpanahi, M.E.; Niazian, M.; Ahmadi, B. Methods for chromosome doubling. Methods Mol. Biol. 2021, 2287, 127–148.
- Melchinger, A.E.; Molenaar, W.S.; Mirdita, V.; Schipprack, W. Colchicine alternatives for chromosome doubling in maize haploids for doubled-haploid production. Crop Sci. 2016, 56, 559–569.
- Watts, A.; Sankaranarayanan, S.; Raipuria, R.K.; Watts, A. Production and application of doubled haploid in brassica improvement. In Brassica Improvement; Wani, S., Thakur, A., Jeshima Khan, Y., Eds.; Springer: Cham, Switzerland, 2020; pp. 67–84.
- Croser, J.S.; Lülsdorf, M.M.; Davies, P.A.; Clarke, H.J.; Bayliss, K.L.; Mallikarjuna, N.; Siddique, K.H.M. Toward doubled haploid production in the Fabaceae: Progress, constraints, and opportunities. Crit. Rev. Plant Sci. 2006, 25, 139–157.
- Forster, B.P.; Heberle-Bors, E.; Kasha, K.J.; Touraev, A. The resurgence of haploids in higher plants. Trends Plant Sci. 2007, 12, 368–375.
- Seguí-Simarro, J.M.; Corral-Martínez, P.; Parra-Vega, V.; González-García, B. Androgenesis in recalcitrant Solanaceous crops. Plant Cell Rep. 2011, 30, 765–778.
- Dong, Y.-Q.; Zhao, W.-X.; Li, X.-H.; Liu, X.-C.; Gao, N.-N.; Huang, J.-H.; Wang, W.-Y.; Xu, X.-L.; Tang, Z.-H. Androgenesis, gynogenesis, and parthenogenesis haploids in cucurbit species. Plant Cell Rep. 2016, 35, 1991–2019.
- Massel, K.; Lam, Y.; Wong, A.C.; Hickey, L.T.; Borrell, A.K.; Godwin, I.D. Hotter, Drier, CRISPR: The latest edit on climate change. Theor. Appl. Genet. 2021, 134, 1691–1709.
- Mobini, S.H.; Warkentin, T.D. A simple and efficient method of in vivo rapid generation technology in pea (Pisum sativum L.). In Vitro Cell Dev. Biol.-Plant 2016, 52, 530–536.
- Naqvi, R.Z.; Siddiqui, H.A.; Mahmood, M.A.; Najeebullah, S.; Ehsan, A.; Azhar, M.; Farooq, M.; Amin, I.; Asad, S.; Mukhtar, Z.; et al. Smart breeding approaches in post-genomics era for developing climate-resilient food crops. Front. Plant Sci. 2022, 13, 972164.
- Bonea, D. Speed breeding and its importance for the improvement of agricultural crops. AAMC 2022, 52, 59–66.
- Pandey, S.; Singh, A.; Parida, S.K.; Prasad, M. Combining speed breeding with traditional and genomics-assisted breeding for crop improvement. Plant Breed. 2022, 141, 301–313.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
14 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




