Amid a rapidly growing global population and increasing threats to crop yields, Speed Breeding (SB) in crop genetics is focused. It traces SB’s development from carbon arc lamp experiments 150 years ago to its modern use with LED technology which significantly accelerates breeding cycles. SB has applications in genetic mapping, genetic modification, and trait stacking, enhancing crop resilience by leveraging allelic diversity. It aligns well with breeding methods like single plant selection and single seed descent. The integration of SB with gene editing, genotyping, and genomic selection holds great promise.
- genetic gain
- controlled environment
- shuttle breeding
1. The History and Development of SB in Enhancing Crop Genetics
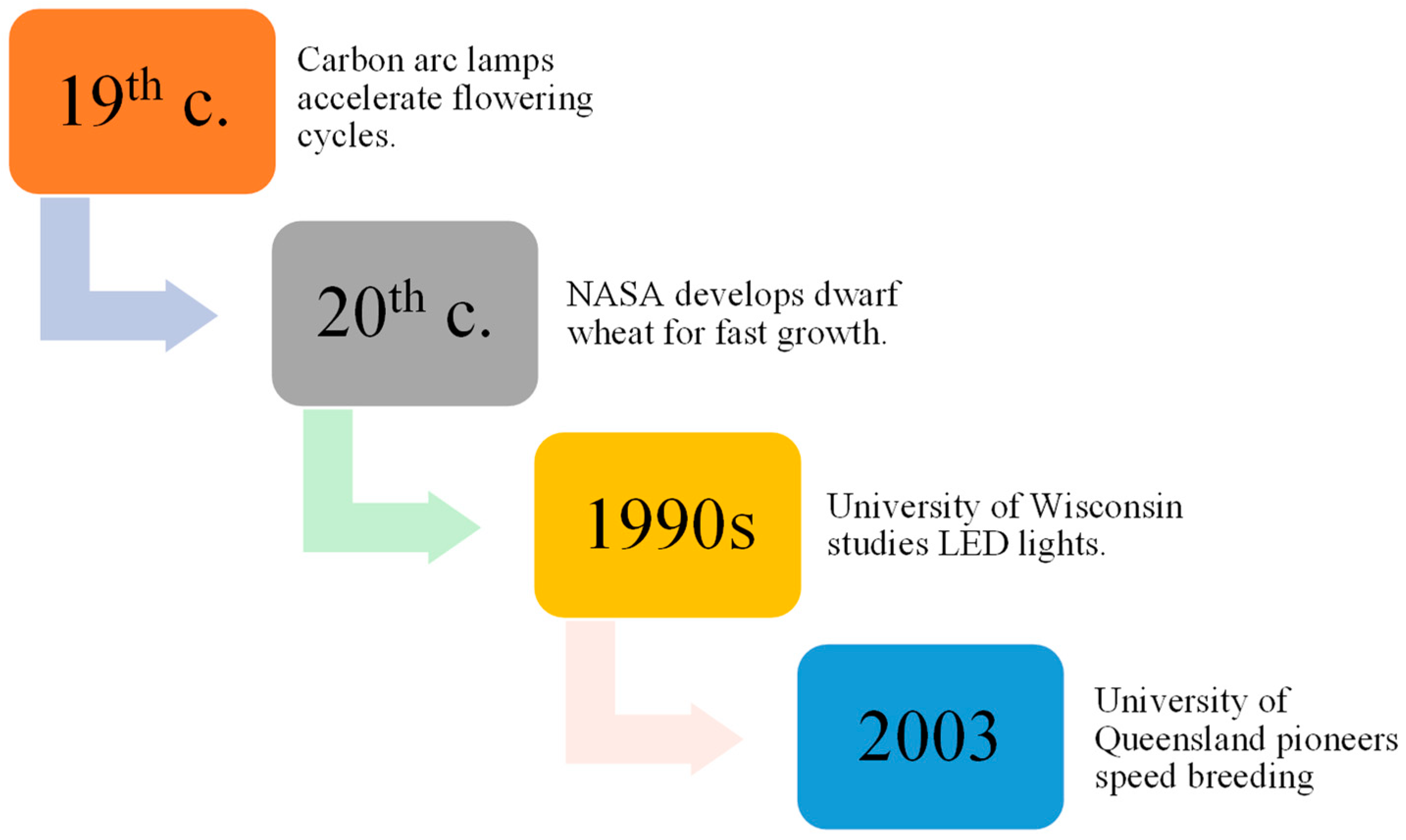
2. SB Applications and Selection Methods in Plant Breeding
This entry is adapted from the peer-reviewed paper 10.3390/crops3040025
References
- Gilland, B. World population and food supply. Food Policy 2002, 27, 47–63.
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77.
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10.
- Hickey, L.T.; Germán, S.E.; Pereyra, S.A.; Diaz, J.E.; Ziems, L.A.; Fowler, R.A.; Platz, G.J.; Franckowiak, J.D.; Dieters, M.J. Speed breeding for multiple disease resistance in barley. Euphytica 2017, 213, 64.
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754.
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M.; et al. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409.
- Cazzola, F.; Bermejo, C.J.; Guindon, M.F.; Cointry, E. Speed breeding in pea (Pisum sativum L.), an efficient and simple system to accelerate breeding programs. Euphytica 2020, 216, 178.
- Hussain, K.; Mahrukh; Nisa, R.T.; Zaid, A.; Mushtaq, M. The utilization of speed breeding and genome editing to achieve zero hunger. In Sustainable Agriculture in the Era of the OMICs Revolution; Prakash, C.S., Fiaz, S., Nadeem, M.A., Baloch, F.S., Qayyum, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–15. ISBN 978-3-031-15567-3.
- Jacquier, N.M.; Gilles, L.M.; Martinant, J.-P.; Rogowsky, P.M.; Widiez, T. Maize in Planta haploid inducer lines: A cornerstone for doubled haploid technology. In Doubled Haploid Technology; Volume 2: Hot Topics, Apiaceae, Brassicaceae, Solanaceae; Humana: New York, NY, USA, 2021; Volume 2288, pp. 25–48.
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.; Barta, D.; Ignatius, R.; Martin, T. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205.
- Hoenecke, M.; Bula, R.; Tibbitts, T. Importance of blue photon levels for lettuce seedlings grown under red-light-emitting diodes. HortScience 1992, 27, 427–430.
- Zhou, W. Advanced ASTROCULTURETM Plant Growth Unit: Capabilities and Performances; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2005.
- Morrow, R.C.; Duffie, N.A.; Tibbitts, T.W.; Bula, R.J.; Barta, D.J.; Ming, D.W.; Wheeler, R.M.; Porterfield, D.M. Plant Response in the ASTROCULTURETM Flight Experiment Unit; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1995.
- Link, B.; Durst, S.; Zhou, W.; Stankovic, B. Seed-to-seed growth of Arabidopsis thaliana on the International Space Station. Adv. Space Res. 2003, 31, 2237–2243.
- Croxdale, J.; Cook, M.; Tibbitts, T.W.; Brown, C.S.; Wheeler, R.M. Structure of Potato tubers formed during spaceflight. J. Exp. Bot. 1997, 48, 2037–2043.
- Wu, B.-S.; Mansoori, M.; Trumpler, K.; Addo, P.W.; MacPherson, S.; Lefsrud, M. Effect of amber (595 Nm) light supplemented with narrow blue (430 Nm) light on tomato biomass. Plants 2023, 12, 2457.
- Guo, X.; Xue, X.; Chen, L.; Li, J.; Wang, Z.; Zhang, Y. Effects of LEDs light spectra on the growth, yield, and quality of winter wheat (Triticum aestivum L.) cultured in plant factory. J. Plant Growth Regul. 2023, 42, 2530–2544.
- Jagadish, S.V.K.; Bahuguna, R.N.; Djanaguiraman, M.; Gamuyao, R.; Prasad, P.V.V.; Craufurd, P.Q. Implications of high temperature and elevated CO2 on flowering time in plants. Front. Plant Sci. 2016, 7, 913.
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342.
- Bugbee, B.; Koerner, G. Yield comparisons and unique characteristics of the dwarf wheat cultivar ‘USU-Apogee’. Adv. Space Res. 1997, 20, 1891–1894.
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963.
- Croser, J.S.; Pazos-Navarro, M.; Bennett, R.G.; Tschirren, S.; Edwards, K.; Erskine, W.; Creasy, R.; Ribalta, F.M. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tiss. Organ. Cult. 2016, 127, 591–599.
- Byerlee, D.; Fischer, K. Accessing modern science: Policy and institutional options for agricultural biotechnology in developing countries. World Dev. 2002, 30, 931–948.
- O’Connor, D.J.; Wright, G.C.; Dieters, M.J.; George, D.L.; Hunter, M.N.; Tatnell, J.R.; Fleischfresser, D.B. Development and application of speed breeding technologies in a commercial peanut breeding program. Peanut Sci. 2013, 40, 107–114.
- Kumar, V.; Singh, K.; Shah, M.P.; Singh, A.K.; Kumar, A.; Kumar, Y. Application of omics technologies for microbial community structure and function analysis in contaminated environment. In Wastewater Treatment: Cutting-Edge Molecular Tools, Techniques and Applied Aspects; Shah, M.P., Sarkar, A., Mandal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–40.
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both multi-segment light intensity and extended photoperiod lighting strategies, with the same daily light integral, promoted Lactuca sativa L. growth and photosynthesis. Agronomy 2019, 9, 857.
- Alahmad, S.; Dinglasan, E.; Leung, K.M.; Riaz, A.; Derbal, N.; Voss-Fels, K.P.; Able, J.A.; Bassi, F.M.; Christopher, J.; Hickey, L.T. Speed breeding for multiple quantitative traits in durum wheat. Plant Methods 2018, 14, 36.
- Gudi, S.; Kumar, P.; Singh, S.; Tanin, M.J.; Sharma, A. Strategies for accelerating genetic gains in crop plants: Special focus on speed breeding. Physiol. Mol. Biol. Plants 2022, 28, 1921–1938.
- Shariatpanahi, M.E.; Niazian, M.; Ahmadi, B. Methods for chromosome doubling. Methods Mol. Biol. 2021, 2287, 127–148.
- Melchinger, A.E.; Molenaar, W.S.; Mirdita, V.; Schipprack, W. Colchicine alternatives for chromosome doubling in maize haploids for doubled-haploid production. Crop Sci. 2016, 56, 559–569.
- Watts, A.; Sankaranarayanan, S.; Raipuria, R.K.; Watts, A. Production and application of doubled haploid in brassica improvement. In Brassica Improvement; Wani, S., Thakur, A., Jeshima Khan, Y., Eds.; Springer: Cham, Switzerland, 2020; pp. 67–84.
- Croser, J.S.; Lülsdorf, M.M.; Davies, P.A.; Clarke, H.J.; Bayliss, K.L.; Mallikarjuna, N.; Siddique, K.H.M. Toward doubled haploid production in the Fabaceae: Progress, constraints, and opportunities. Crit. Rev. Plant Sci. 2006, 25, 139–157.
- Forster, B.P.; Heberle-Bors, E.; Kasha, K.J.; Touraev, A. The resurgence of haploids in higher plants. Trends Plant Sci. 2007, 12, 368–375.
- Seguí-Simarro, J.M.; Corral-Martínez, P.; Parra-Vega, V.; González-García, B. Androgenesis in recalcitrant Solanaceous crops. Plant Cell Rep. 2011, 30, 765–778.
- Dong, Y.-Q.; Zhao, W.-X.; Li, X.-H.; Liu, X.-C.; Gao, N.-N.; Huang, J.-H.; Wang, W.-Y.; Xu, X.-L.; Tang, Z.-H. Androgenesis, gynogenesis, and parthenogenesis haploids in cucurbit species. Plant Cell Rep. 2016, 35, 1991–2019.
- Massel, K.; Lam, Y.; Wong, A.C.; Hickey, L.T.; Borrell, A.K.; Godwin, I.D. Hotter, Drier, CRISPR: The latest edit on climate change. Theor. Appl. Genet. 2021, 134, 1691–1709.
- Mobini, S.H.; Warkentin, T.D. A simple and efficient method of in vivo rapid generation technology in pea (Pisum sativum L.). In Vitro Cell Dev. Biol.-Plant 2016, 52, 530–536.
- Naqvi, R.Z.; Siddiqui, H.A.; Mahmood, M.A.; Najeebullah, S.; Ehsan, A.; Azhar, M.; Farooq, M.; Amin, I.; Asad, S.; Mukhtar, Z.; et al. Smart breeding approaches in post-genomics era for developing climate-resilient food crops. Front. Plant Sci. 2022, 13, 972164.
- Bonea, D. Speed breeding and its importance for the improvement of agricultural crops. AAMC 2022, 52, 59–66.
- Pandey, S.; Singh, A.; Parida, S.K.; Prasad, M. Combining speed breeding with traditional and genomics-assisted breeding for crop improvement. Plant Breed. 2022, 141, 301–313.
