Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aleksandra Bołdys | -- | 6530 | 2023-11-09 09:46:15 | | | |
| 2 | Lindsay Dong | Meta information modification | 6530 | 2023-11-12 13:52:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bołdys, A.; Bułdak, �.; Maligłówka, M.; Surma, S.; Okopień, B. Potential Therapeutic Strategies of Metabolic-Associated Fatty Liver Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/51341 (accessed on 08 February 2026).
Bołdys A, Bułdak �, Maligłówka M, Surma S, Okopień B. Potential Therapeutic Strategies of Metabolic-Associated Fatty Liver Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/51341. Accessed February 08, 2026.
Bołdys, Aleksandra, Łukasz Bułdak, Mateusz Maligłówka, Stanisław Surma, Bogusław Okopień. "Potential Therapeutic Strategies of Metabolic-Associated Fatty Liver Disease" Encyclopedia, https://encyclopedia.pub/entry/51341 (accessed February 08, 2026).
Bołdys, A., Bułdak, �., Maligłówka, M., Surma, S., & Okopień, B. (2023, November 09). Potential Therapeutic Strategies of Metabolic-Associated Fatty Liver Disease. In Encyclopedia. https://encyclopedia.pub/entry/51341
Bołdys, Aleksandra, et al. "Potential Therapeutic Strategies of Metabolic-Associated Fatty Liver Disease." Encyclopedia. Web. 09 November, 2023.
Copy Citation
Metabolic-associated Fatty Liver Disease is one of the outstanding challenges in gastroenterology. The increasing incidence of the disease is undoubtedly connected with the ongoing obesity pandemic. The lack of specific symptoms in the early phases and the grave complications of the disease require an active approach to prompt diagnosis and treatment. Therapeutic lifestyle changes should be introduced in a great majority of patients; but, in many cases, the adherence is not satisfactory. There is a great need for an effective pharmacological therapy for Metabolic-Associated Fatty Liver Disease, especially before the onset of steatohepatitis.
metabolic-associated fatty liver disease
incretins
diabetes
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a disease with a complex etiology. Several years ago, the terminology was changed to Metabolic-Associated Fatty Liver Disease MAFLD because it better indicated the necessity for treating the risk factors of this disease. But in June 2023, during the EASL (European Association for the Study of the Liver) Congress in Vienna led by three major pan-national liver associations (ALEH—the Latin American Association for the Study of the Liver, AASLD—the American Association for the Study of Liver Diseases, and EASL) and the NAFLD Nomenclature Initiative, another transition was proposed. Steatotic liver disease (SLD) was selected as a comprehensive term to cover the diverse causes of steatosis. The proposed replacement for NAFLD is metabolic dysfunction-associated steatotic liver disease—MASLD. The modified definition includes at least one of the five cardiometabolic risk factors. Individuals without metabolic disease and no other known cause should be diagnosed with cryptogenic SLD. Outside the MASLD, a new clinical entity—MetALD (Metabolic Alcohol-Related Liver Disease)—was established for individuals consuming higher weekly alcohol amounts (140–350 g per week for females and 210–420 g per week for males). Metabolic dysfunction-associated steatohepatitis (MASH) is the replacement term for nonalcoholic steatohepatitis (NASH) [1].
The development of MAFLD is treacherous. In the past, it has been considered as a benign disorder resulting from lipid accumulation in hepatocytes [2]. The first stage in the development of MAFLD is liver steatosis, which results from the accumulation of lipids inside hepatocytes. At some point, owing to excess fat, leading to lipotoxicity and accompanied by unfavorable genetic factors, inflammation ensues. MASH, which is a following stage in the development of MAFLD, is characterized by the inflammatory response activation of various cells, including the macrophageal lineage and stellate cells, which contribute to the excessive intercellular matrix synthesis that is responsible for liver fibrosis [3]. In some patients, the advanced stage of liver fibrosis leads to cirrhosis and its complications, including hepatocellular carcinoma (HCC). Owing to widespread occurrence (around 25%), MAFLD might become a leading cause of liver fibrosis and HCC (Figure 1) [4].
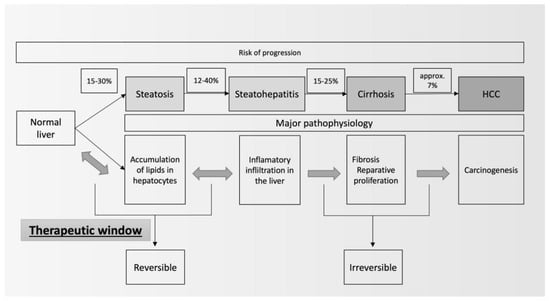
Figure 1. Natural history of MAFLD (modified from [5]).
There is an urgency to introduce effective therapies against MAFLD, especially at the early phase of its development. Currently, vitamin E and pioglitazone are recommended by international scientific organizations [6], but those drugs are used in more advanced stages of MAFLD (i.e., MASH), and the extent of the improvements in simple steatosis seems to be underwhelming. Therefore, there is significant interest in other therapeutic options that might be used to treat MAFLD, especially regarding the necessary pharmacological approach to other concomitant conditions in patients with features of metabolic syndrome (e.g., diabetes and hyperlipidemia). In this context, both the treatment and directions of development of MAFLD pharmacotherapy should be focused on seeking drugs that improve the courses of obesity, diabetes, insulin resistance, lipid disorders, hypertension, and hyperuricemia. Nevertheless, other potential therapeutic areas should also be explored (the endocrine system, microbiota, and functional foods).
One of the challenges in the introduction of new therapies is the proper assessment of their clinical efficacy. Despite having the best diagnostic yield, liver biopsies in an early stage of MAFLD (e.g., steatosis) are not recommended. The most accurate method of the noninvasive evaluation of liver steatosis is the MRI-proton density fat fraction (MRI-PDFF). However, the cost of the procedure and its limited availability mean that it is reserved nearly exclusively for clinical trials. There are other solutions to quantify steatosis. Ultrasound techniques (e.g., Fibroscan) are more accessible but less accurate than MRI. There are also several blood markers that can be used to estimate the extent or the prognosis of MAFLD (Fib-4, etc.).
2. Therapeutic Lifestyle Changes
The MAFLD incidence rises parallelly with the worldwide epidemic of obesity. The pathophysiological connection seems to be clear—excess fat intake, obesity, insulin resistance, and dyslipidemia [7]. Significant improvements in MAFLD may be achieved by implementing therapeutic lifestyle changes. Recent data suggest a positive correlation between the reduction in subcutaneous fat and the extent of liver steatosis [r = 0.42 (CI: 0.29–0.54)] [8]. This translates to improvements in LFTs. Dietary approaches have led to a decline in ALT activity [MD: (−4.48 IU/L)], and the effect was even more pronounced in patients who had also undertaken physical activity ALT [(−13.27) IU/L; 95% CI: (−21.39)—(−5.16)]. In those patients, an improvement in AST was also noted [(−7.02) IU/L; 95%: (−11.26)–(−2.78)] [4].
Recommendations for increased physical activity have been incorporated into the guidelines of most scientific societies dealing with MAFLD. AASLD [9] recommends that patients with NAFLD should be encouraged to increase their activity level to the maximum possible extent. Individualized prescriptive exercise recommendations may enhance sustainability and offer benefits independent of weight loss. EASL also recommends a progressive increase in aerobic exercise and resistance training [7].
3. Drugs Affecting Carbohydrate Metabolism
Type 2 diabetes mellitus (DMT2) is one of the metabolic diseases that increases the risk for the development of MAFLD [10]. Crucial pathophysiological factors in DMT2, including hyperglycemia and insulin resistance, play a key role in the progression of MAFLD [11]. Drugs that affect carbohydrate metabolism have been considered to play a significant role in the pathogenesis of MAFLD. The development of MAFLD, as a consequence of the obesity and diabetes pandemics, has led to parallel advancements in the development of novel antidiabetic drugs that simultaneously exhibit weight-reducing effects, contributing to the reduction in MAFLD progression [12]. These drugs include SGLT-2 inhibitors (Sodium-Glucose Cotransporter-2)—leading to glucose excretion in the urine and lowering blood glucose levels; incretin analogs, such as GLP-1 (Glucagon-like Peptide-1)—stimulating insulin secretion, suppressing glucose release from the liver, and slowing down gastric emptying—and GLP-1 + GIP (Glucose-Dependent Insulinotropic Peptide)—having effects similar to those of GLP-1 and influencing blood glucose control; GLP-1 + glucagon (GCG)—potentially affecting glucose metabolism and aiding in diabetes management; as well as the most recent triple-incretin agonist, GLP-1/GIP/GCGR. The impacts of other antidiabetic medications have also been explored in the context of MAFLD: metformin—reducing glucose production in the liver and increasing tissue sensitivity to insulin, DPP4i (Dipeptidyl Peptidase-4 inhibitors)—increasing GLP-1 and GIP levels, ketohexokinase inhibitors—a promising new area of research that may influence glucose metabolism, and insulin sensitizers—glitazones. These medications have the potential to improve the condition of patients with carbohydrate disorders, including MAFLD. However, understanding the full extent of their impact and effectiveness in MAFLD therapy requires further clinical research.
3.1. Sodium/Glucose Cotransporter-2 Inhibitors
Sodium/glucose cotransporter-2 inhibitors (SGLT2is), also known as flozins, are drugs that are capable of limiting glucose reabsorption in renal proximal tubules. By inducing glucosuria, they subsequently lower serum glucose levels [13]. The first drug from the group of SGLT2is (canagliflozin) was accepted by the US Food and Drug Administration for the therapy of DMT2 in 2013. During the last 10 years, other effective flozins have been implemented for the management of DMT2 e.g., dapagliflozin, empagliflozin, and ertugliflozin [14].
Since the introduction of SGLT2is, their range of indications has vastly expanded. Drugs that originally were developed as antidiabetic medications appeared to have cardio- and nephroprotective effects. The results of clinical trials, including DAPA-HF, DAPA-CKD, EMPEROR-Reduced, EMPEROR-Preserved, and CREDENCE, allowed the application of selected SGLT2is in patients with heart failure (HF) or chronic kidney disease (CKD) even without concomitant DMT2 [15]. The beneficial effects of SGLT2is in cardiovascular and renal systems, for which no sufficiently effective drugs were available, led to the investigation of their potential for improving hepatic cell functioning.
Data derived from studies both on rodents and humans show the positive effects of therapy with SGLT2is on MAFLD. The cellular mechanisms responsible for the amelioration of liver functions are still not fully elucidated but are thought to be connected with the activation of autophagy, endoplasmic reticulum stress reduction, and anti-apoptotic, anti-inflammatory, and antioxidant effects [16][17][18][19].
3.2. Agonists of Glucagon-like Peptide-1 Receptors
GLP-1 receptor agonists (GLP-1Ras) have shown substantial advantages in managing diabetes and obesity. Those properties are reflected by their action on pancreatic islets, beta cells, and the central nervous system [20][21][22]. Despite these benefits, the response rates to GLP-1Ras, as well as to other pharmaceutical treatments for liver steatosis in NAFLD, have not surpassed 50%. Research data suggest that GLP-1Ras may have beneficial effects on MAFLD by improving insulin sensitivity, reducing liver fat accumulation, and potentially exerting anti-inflammatory effects. These drugs have shown promise in reducing LFTs. They also could have an influence on the liver histology. Liraglutide is the most extensively investigated GLP-1 receptor agonist (GLP-1 RA) in the context of NAFLD treatment. Several trials have evaluated its effectiveness in these individuals, yielding positive results. The multicenter, double-blinded, randomized, placebo-controlled phase 2 trial showed the benefits of a GLP-1 analog (liraglutide) [23] in patients with MASH. A 48-week treatment with liraglutide at a dose of 1.8 mg (26 patients) resulted in histological improvement, without exacerbating fibrosis, when compared to the placebo group (also 26 patients) (RR = 4.3; 95% CI: 1.0−17.7, p = 0.0190). The primary histological outcome endpoint was an improvement in liver histology, which was defined as the resolution of steatohepatitis characterized by the disappearance of hepatocyte ballooning. The secondary endpoint included changes in the total NAFLD activity score (steatosis, hepatocyte ballooning, lobular inflammation, and fibrosis stages) as well as the serum liver enzyme concentration. The differences in the lobular inflammation and overall NAFLD activity scores were not statistically significant between the two groups.
Semaglutide is being assessed for reducing liver steatosis in patients with NAFLD who are undergoing antiretroviral therapy [24]. Additionally, baseline parameters predicting the clinical response in NAFLD during semaglutide treatment [25] and the applicability of semaglutide (oral or subcutaneous form) as an effective measure in improving NAFLD in patients with obesity and/or type 2 diabetes mellitus [26] are also being investigated. Orforglipron (also known as LY3502970 or OWL833), a new oral GLP-1 analog, is currently under investigation in phase 1 and 2 clinical trials [27][28] in patients with diabetes or obesity and who are overweight and have weight-related comorbidities (including MAFLD) [29].
3.3. Dual Agonists of Incretin Receptors (Glucagon-like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide)
Although GLP-1 is thought to affect the liver by reducing body weight, the mechanism of action of the dual GLP-1 and GIP receptor agonists is still not fully understood. It could result from GLP-1 agonism enhanced by the GIP action, but the exact mechanism of action is still unknown as there is still a lack of human data. One of the explanations suggests a potential impact on liver cells through an as-yet-unknown mechanism. As presented in their recent study on tirzepatide, Muller et al. indicated that signaling via the GIP receptor in human pancreatic islets is crucial for stimulating insulin secretion, unlike in mice where the action of tirzepatide is primarily associated with activity through GLP-1 [30]. The tirzepatide—dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor—agonist demonstrated substantially greater glucose control and weight loss compared with the selective GLP-1RA—dulaglutide [31]. Tirzepatide is currently under evaluation in the SYNERGY-NASH trial [32] in MASH patients without cirrhosis. In a post hoc analysis, patients who received tirzepatide experienced improvements in MASH-related biomarkers, such as ALT, CK-18 (cytokeratin-18), and Pro-C3 (N-terminal type III collagen propeptide), as well as an increase in the adiponectin level [33].
3.4. Dual Agonists of Incretin Receptors (Glucagon-like Peptide-1 and Glucagon)
The simultaneous activation of GLP-1 and glucagon receptors prevents the hyperglycemic response commonly associated with glucagon while also enhancing its catabolic effects and significantly amplifying hepatic glycolysis, glycogenolysis, and lipolysis. GLP-1 activation has been correlated with weight reduction, anorexigenic properties, and hypoglycemic effects, while the activation of GCGR is believed to mainly contribute to a reduction in hepatic steatosis and enhancement in mitochondrial respiration; the dual agonist of GLP1/GCG receptors are believed to improve the course of NAFLD and are currently being investigated for this indication.
The recently published results of a phase 2a active-comparator-controlled study [34] on efinopegdutide (NCT: 04944992) demonstrated that in 145 randomized patients with NAFLD, 24 weeks of treatment with a weekly dose of efinopegdutide at 10 mg (72 patients) resulted in a substantial reduction (p < 0.001) in liver fat content (LFC), as measured using magnetic resonance imaging (LFC reduction of 72.7% [90% CI: 66.8–78.7]) when compared to a weekly dose of semaglutide at 1 mg (73 patients, LFC reduction of 42.3% [90% CI: 36.5–48.1]). In a phase 2b study involving more than 800 overweight diabetic subjects with inadequate blood glucose control, another GLP1R/GCGR agonist, cotadutide, administered at dosages of 100–300 μg/d, yielded significant improvements in liver enzyme levels and indicators of liver fibrosis. In this study, cotadutide achieved noteworthy reductions in HbA1c and body weight at both 14 and 54 weeks compared to the placebo group (all p < 0.001). Additionally, 300 μg of cotadutide demonstrated improvements in the lipid profile, AST and ALT levels, propeptide of the type III collagen level, fibrosis-4 index, and nonalcoholic fatty liver disease fibrosis score compared to the placebo group, but this effect was not observed with liraglutide. [35].
Apparently, most studies related to this group of drugs in MAFLD treatment conclude at preclinical investigations or early stages of clinical trials. The future application of these drugs in the treatment of MAFLD without concurrent diabetes currently appears unlikely. Furthermore, the question of the extent to which the activation of the glucagon receptor influences the liver fat reduction and whether actions at the mitochondrial level may have a significant impact on this reduction remains to be settled.
3.5. Triple Incretin Receptor Agonists
The triple GLP1R/GCGR/GIPR agonist, efocipegtrutide (HM15211), distinguished by its prolonged duration of action, which is attributed to a non-peptidyl flexible linker, is being investigated in a phase 2 clinical trial for MASH (217 participants, aged 18–70 years, across multiple US sites with an expected completion date in November 2025) [36], and a phase 1 trial to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of HM15211 in obese subjects with NAFLD has been reported (66 participants, aged 18–65 years) [37].
Retatrutide (LY3437943), another triple agonist, is presently in development for the treatment of DMT2, obesity, and nonalcoholic fatty liver disease. Data emerging from the phase 2 study [38], specifically within the NAFLD subgroup, indicate the potential for reversing initial liver disease stages and mitigating obesity-related coexisting conditions. The results revealed that within the NAFLD population, liver fat normalization was achieved in 90% of cases subsequent to treatment with the two most elevated doses. [39].
3.6. Dipeptidyl Peptidase-4 Inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors, which increase levels of endogenous incretins, have also been investigated for their potential application in MAFLD treatment. They might help to improve insulin resistance and glucose metabolism, which are central factors in MAFLD development. A surface under the cumulative ranking curve analysis (SUCRA) revealed that DPP-4 inhibitors had the second highest (following GLP-1 agonists) probability (SUCRA 69.6%) of reducing intrahepatic steatosis, followed by pioglitazone (SUCRA 62.2%) [40]. However, further studies seem to be necessary to determine the full extent of their effects on MAFLD progression.
DPP-4 inhibitors constitute a category of indirect incretin mimetics as they impede the enzymatic breakdown of GLP-1, GIP, and oxyntomodulin; but, in the available guidelines or recommendations [41][42], there are still few studies focusing on hard end-points (inflammatory markers or fibrosis) regarding DPP-4 inhibitors in the treatment of NAFLD. That was demonstrated by dos Santos et al. [43], who indicated the overall poor quality of the studies and heterogeneity of the analyzed population.
Among these trials, sitagliptin (100 mg/d) displayed effectiveness against hepatic steatosis and the hepatic collagen content regardless of DMT2, as demonstrated in a 1-year open-label randomized controlled trial [44]. Vildagliptin in DMT2 patients [45] and non-diabetic patients [46], omarigliptin [47], and teneligliptin (only in MASH) [48] exhibited improvements in liver function and certain noninvasive markers of NAFLD. The preliminary data for saxagliptin patients with DMT2 and concomitant NAFLD demonstrated that saxagliptin could attenuate insulin resistance and inflammatory injury by the downregulation of the hepatic and soluble form of DPP-4 and, as a result, reduce the degree of steatosis [49][50].
3.7. Glitazones
The AACE (American Association of Clinical Endocrinology) [41] and AASLD [9] clinical practice guidelines endorse the utilization of pioglitazone, a selective PPARγ (Peroxisome proliferator-activated receptor gamma) agonist, in cases of diagnosed MASH. This recommendation stems from the studies in which, irrespective of the presence of DMT2, pioglitazone reduced the degree of liver steatosis and improved disease activity indicators [51][52][53][54][55]. In the field of NAFLD, a one-year treatment with pioglitazone (compared to sulfonylureas) at a low dosage significantly (p < 0.05) improved liver steatosis and inflammation and systemic and adipose-tissue insulin resistance in patients with DMT2 [56]. Surrogate markers of NAFLD were improved: the liver fat equation decreased by (−1.76) ± 3.84 (p < 0.05); the hepatic steatosis index by (−1.35) ± 2.78 (p < 0.05); and the index of MASH by (−9.75) ± 43 (p < 0.05). Other thiazolidinediones considered in the treatment of NAFLD–rosiglitazone [57] and lobeglitazone (available only in Republic of Korea)—do not have a substantial number of studies confirming their effectiveness.
3.8. Ketohexokinase Inhibitors
KHK inhibitors (KHKis) are novel, promising compounds in the treatment of NAFLD. Fructose (C6H12O6) is a keto-hexose (ketose-hexose) isomer of glucose, and it plays a significant role in the development of NAFLD. KHK is an enzyme that is responsible for the initial and critical step in fructose metabolism, which is believed to increase the intrahepatic lipid (IHL) content. The pharmacological inhibition of KHK has led to a reduction in the IHL content among individuals with NAFLD; however, the full scope of KHK inhibition remains to be elucidated. The safety and efficacy of PF-06835919 (a KHKi developed by Pfizer) have been assessed through several studies. KHK inhibition has been previously investigated in a small phase 2 trial involving individuals with NAFLD. PF-06835919 exhibited a significant reduction in liver LFC after 6 weeks of treatment compared to the placebo group. There were data indicating that participants receiving PF-06835919 at the 300 mg dose demonstrated significant reductions in LFC compared to the placebo group, with a difference of −18.73% (p = 0.04) [58].
3.9. Metformin
Metformin, one of the basic drugs for treating DMT2, which enhances insulin sensitivity in the liver and muscles, has also been broadly studied for NAFLD treatment. According to AASLD recommendations [9] metformin (as well as acarbose—an alpha glucosidase inhibitor) should not be used for the treatment of steatohepatitis (as there is no observed benefit for hepatocyte necrosis or inflammation). However, it may be continued if needed for the treatment of hyperglycemia in people with DMT2 and NAFLD or MASH [59]. As several meta-analyses of paired-biopsy studies involving individuals with MASH have revealed, there has been limited clinical evidence indicating benefits in terms of disease activity or liver fibrosis [60][61] Preliminary studies indicated that a moderate effect was observed, mainly targeting hepatic steatosis and linked to weight loss [62][63]. Nevertheless, a meta-analysis of metformin trials revealed that the aggregated liver histologic scores for steatosis, ballooning, and fibrosis did not exhibit significant improvements.
4. Drugs Affecting Lipid Metabolism
4.1. Core Cholesterol-Lowering Therapy (Statins or/and Ezetimibe)
Hypercholesterolemia may lead to the progression of liver damage in MAFLD [64]. Statins play key roles in the therapy of hyperlipidemias [65]. However, many hepatotoxic properties have been attributed to their action. The cautious approach is reflected in the recommendations on the surveillance of statin therapy, which include routine checkups of ALT during the treatment of hyperlipidemia [66]. These drugs should not be used or should be withdrawn when the ALT level exceeds 3 times the upper limit of the reference range. Liver steatosis is generally associated with only a mild elevation in ALT levels. Interestingly, there have been reports on the reduction in LFTs during statin therapy [67]. This might stem from a direct reduction in intracellular cholesterol load as well as the pleiotropic actions of statins [68].
One of the first clinical trials focusing on the impact of a 20 mg dose of atorvastatin LFTs in patients with MAFLD was performed by Athyros et al. [69]. After 54 weeks of treatment, there were no statistically significant impacts of atorvastatin on ALT, AST, and ALP (alkaline phosphate) activities, which might be attributed to a relatively small sample size (63 patients). In a larger trial (GRAECE) subjects with mildly elevated aminotransferases (up to 3× above the reference range) were also treated with the statin. During the study a gradual reductions in the ALT, AST, and GGT activities were observed. At the end of observation (3 years), the ALT activity dropped from 57 ± 8 to 37 ± 6 IU/L (p < 0.0001); the AST and GGT activities were affected to a similar extent. No significant liver toxicity was reported for the statins. The study showed that surrogate markers of MAFLD might be improved during statin treatment [70]
According to recently aggregated data, the impact of statin in patients with NAFLD seems to significantly reduce the ALT (by 35.41%), AST (by 31.78%), and GGT (by 25.57%) [71]. The impact on the LFT is simultaneous with lipid profile improvements. But it is conceivable that the pleiotropic effects of statins (e.g., anti-inflammatory and antioxidant) may have an additional important contribution. Such an impact might be, to some extent, responsible for the reduced propensity of hepatic cell carcinoma in NAFLD patients who are on statin therapy (OR = 0.59; 95% CI: 0.39−0.89) [72]. The available data support the notion that patients with MAFLD should not be excluded from statin therapy owing to a slightly elevated LFT prior to the initiation of the therapy. In fact, improvements in LFTs may be expected during prolonged treatments. Both atorvastatin and rosuvastatin seem to be favorable in NAFLD treatment.
4.2. PCSK9 Inhibitors
Although statins and ezetimibe have been used for several decades, PCSK9 inhibitors (PCSK9i) are relatively new in the therapy of hypercholesterolemia. No direct link has been shown between the circulating PCSK9 level and markers of NAFLD [73]. However, there are specific mutations in the PCSK9 gene that were connected to liver steatosis (e.g., c.946 G.T and p. Gly316Cys) [74], but there has been no connection with the advancement toward liver fibrosis [75].
4.3. Peroxisome Proliferator-Activated Receptors Alpha Agonists
Fibrates belong to a family of PPAR alpha-receptor agonists. The main indication for these drugs is the treatment of hypertriglyceridemia. Their use since the introduction of the therapy has necessitated the periodical evaluation of aminotransferases owing to potential liver toxicity. However, studies in patients with MAFLD, who often have slightly elevated ALT levels, provide interesting input. It seems that fenofibrate at a dose of 300 mg per day for 24 weeks in an RCT reduced LFTs, including ALT (114.6 ± 8.5 vs. 57.3 ± 7.8 IU/L, p < 0.05). Additionally, indices of potential prevention in the progression toward more advanced stages of NAFLD were represented by a reduced TGF (transforming growth factor) beta level (15.2 ± 5.1 vs. 8.1 ± 3.2 ng/mL; p < 0.05) and reduced liver stiffness (12.95 ± 5.1 vs. 9.8 ± 3.7 kPa; p < 0.05) [76].
4.4. Selective Peroxisome Proliferator-Activated Receptor Modulators
Fibrates, which are described above and indicated in the therapy of hypertriglyceridemia, seem to have some beneficial impact on the course of NAFLD. A similar mechanism of action, which is based on selective PPAR alpha modulation (SPPARM), is used by a novel compound—pemafibrate. The drug, itself, possesses triglyceride-lowering properties [77], but it excels at improving NAFLD. Early small-scale studies showed a remarkable improvement in the reduction of ALT from 57.5 ± 8.8 to 30.3 ±5.8 IU/L (p < 0.01) and GGT from 63.9 ± 10.3 to 32.8 ± 6.6 IU/L (p < 0.01) during a 6-month treatment [78]. Even shorter studies with pemafibrate show substantial improvements in LFT, as shown by Seko et al. [79]. The ALT level was reduced from 75.1 IU/L to 43.6 IU/L (p = 0.001) at week 12; but, even in the intermittent analysis at 4 weeks of treatment, ALT showed a significant reduction in LFTs compared to the baseline values. However, there was no significant change in the more-solid NAFLD endpoints (FIB-4 index: 1.89 ± 1.17 vs. 1.95 ± 1.24; p = 0.351, CAP: 329.1 ± 35.6 vs. 314.6 ± 51.6 dB/m; p = 0.329, LSM [liver stiffness measurements]: 10.5 ± 8.8 vs. 9.2 ± 7.6 kPa p = 0.080). Those data underline the necessity of prolonged observations to assess drug efficacy. Similar results were obtained by Shinozaki et al. [80]. A 3-month therapy with pemafibrate for 38 NAFLD patients resulted in significant reduction in ALT (63.9 ± 3.6 vs. 41.6 ± 3.6; p < 0.001), which was accompanied by improvements in the NAFLD fibrosis score [(−2.27 ± 0.18) vs. (−2.38 ± 0.18); p = 0.009], but no change in the FIB-4 index was noted (1.51 ± 0.16 vs. 1.47 ± 0.12; p = 0.500). A longer, 6-month, observation also led to improvements in aminotransferases, which were accompanied by a reduction in the FIB-4 score [2.26 (1.07–3.12) vs. 2.08 (0.97–2.67); p = 0.041].
4.5. Multiple Peroxisome Proliferator-Activated Receptor Agonists
Saroglitazar is used for the treatment of diabetic dyslipidemia. Owing to its mechanism of action, it improves both the lipid profile (PPAR alpha agonist) and glucose metabolism (PPAR gamma agonist). Additionally, since the beginning of its use, it has been noted that saroglitazar might improve the course of comorbid MAFLD. A case series of 10 patients treated with saroglitazar at 4 mg per day and followed-up for nine months resulted in significant reductions in ALT (64.7 ± 15.56 vs. 46.2 ± 12.6 IU/l; p = 0.0058) and AST (43.4 ± 10.48 vs. 35.4 ± 6.59 IU/l; p = 0.0321) activities. Those findings were accompanied by a significant reduction in SWV—Shear-Wave Velocity (1.837 ± 0.0691 vs. 1.645 ± 0.0844; p = 0.0004), which might be considered as a surrogate marker of fibrosis [81]. The limitation of this observation was its retrospective nature without a control group. In a larger cohort of patients with NAFLD, although still without control group, saroglitazar also improved LFT, including ALT (56.47 ± 15.17 vs. 42.3 ± 11.26 IU/l; p < 0.0001), AST (48.57 ± 13.15 vs. 36.63 ± 8.14 IU/l; p < 0.0001), and GGT (54.97 ± 9.52 vs. 45.33 ± 5.94 IU/l; p < 0.0001) [82].
4.6. Acetyl-CoA Carboxylase Inhibitors
Cellular lipid overload is a consequence of both exogenous and endogenous lipid excesses [83]. Experimental data strongly suggest that patients with MAFLD experience elevated (over 3-fold) de novo fatty-acid synthesis [84]. Therefore, endogenous synthesis seems to be an important target for the pharmacological approach to MAFLD treatment. One of the options is the inhibition of hepatocyte-specific Acetyl-CoA carboxylases (ACC). Initial experiments on MK-4074, an ACC1 and ACC2 inhibitor, led to a remarkable reduction in the liver’s fatty-acid content, which reached 36% (8.6% in placebo group) after a 4-week therapy [85]. Unfortunately, probably owing to the concurrent inhibition of the PUFA-3 elongation, a rise in the TG level was noted (170 vs. 325 mg/dL). Further, phase II clinical trials on another ACC inhibitor (firsocostat) showed dose-dependent reductions in liver steatosis, as assessed based on MRI-PDFF, reaching 28.9% (vs. 8.4%; p = 0.002 in the placebo group) [86].
4.7. Fatty-acid Synthase Inhibitors
Fatty-acid synthase (FASN) is responsible for endogenous lipogenesis. Owing to its action, cells are exposed to an abundance of palmitate. This leads not only to the accumulation of lipid droplets in hepatocytes but also to the activation of stellate cells, leading to fibrosis. Therefore, the pharmacological inhibition of FASN seems to be a promising therapeutic target [87]. As a result, both lipid load and fibrosis might be inhibited, which are essential pathological pathways in the development of MASH. In a phase I clinical trial, the FASN inhibitor (TVB-2640) effectively reduced de novo lipogenesis in the liver, which was accompanied by a marginally significant ALT reduction (15.8 ± 8.4%; p = 0.05) [88].
4.8. Diacylglycerol Acyltransferase Inhibitors
A reduction in endogenous lipid synthesis is a promising therapeutic strategy in MAFLD treatment. The final stage in the synthesis of triglycerides is mediated by diacylglycerol acyltransferases (DGATs). The inhibition of these enzymes, especially DGAT2, shows promising results. Ervogastat (PF-06865571) has been studied in phase I and phase II clinical trials. In patients with MAFLD, a 6-week treatment resulted in a −35.4% (−47.4, −20.7; p = 0.0007) reduction in liver fat, as determined using MRI-PDFF [89]. Additionally, a trend toward improvement in LFT tests was observed. These results, though preliminary, are promising.
4.9. Fibroblast Growth Factor 21
The connection between the lipid overload and progression of MAFLD is also reflected in the experimental usage of fibroblast growth factor 21 (FGF21). FGF21 participates substantially in lipid and glucose metabolism, and it has been suggested that FGF21 may improve the course of MAFLD [90]. A pegylated FGF21 (pegozafermin), in a phase I/IIa clinical trial, showed an acceptable safety profile and efficacy [91], and its efficacy was confirmed in a larger 24-week phase II clinical trial (ENLIVEN) [92]. The study was primarily focused on the fibrosis assessed in liver biopsy specimens and showed significant improvements in MASH. Additionally, it greatly reduced the liver fat content up to 41.9 + 5.6% (vs. 5.0 + 5.2% in the placebo group), as assessed using MRI-PDFF, and a concomitant ALT reduction by up to 31.8 + 5.4%. These promising results warrant further long-term exploration in a phase III clinical trial in this indication. Currently, pegozafermin is being evaluated in a phase III clinical trial but in patients with severe hypertriglyceridemia [93].
5. The Role of Ursodeoxycholic Acid in Metabolic-Associated Fatty Liver Disease
UDCA, an agonist of the farnesoid X receptor, has been explored as a promising treatment option for MAFLD. It is a hydrophilic bile acid occurring naturally in the body, but its role still remains uncertain, as was shown in a meta-analysis conducted by Zhang et al. [94]. The meta-analysis demonstrated that UDCA treatment resulted in a significant reduction in ALT levels (p = 0.007), especially in the European population (p = 0.003), aged over 50 years old (p = 0.001). The effectiveness of UDCA in the treatment of NAFLD was more pronounced at longer treatment durations (p = 0.008).
Although the undeniable effect of UDCA is the normalization of the liver function parameters (ALT and GGT), its impact on hard endpoints, such as liver fibrosis and liver histology, has not been fully confirmed. Further research is needed to thoroughly assess its potential effects in these areas.
6. The Role of the Microbiota in the Pathogenesis and Management of Metabolic-Associated Fatty Liver Disease
The role of the gut microbiota in the pathogenesis and management of many disorders (including MALFD) has been a subject of growing interest. The alterations in the composition and function of the gut microbiota (described as dysbiosis) may contribute to the development and progression of MAFLD. Dysbiosis may lead to increased intestinal permeability, resulting in the translocation of microbial products, such as lipopolysaccharides (LPS), into the bloodstream. This process triggers inflammation and oxidative stress in the liver by, among other pathways, increasing the level of circulating TNF-α (Tumor Necrosing Factor alpha), IL(interleukin)-1, and IL-6, thereby promoting the development of hepatic steatosis and progression of MAFLD [95][96] The involvement of the microbiota in the development of MAFLD has led to the concept of using probiotics, prebiotics, and synbiotics (a combination of probiotics and prebiotics) in the treatment of MAFLD. The modulation of the gut composition through the use of specific strains of probiotic bacteria may potentially help to restore balance and improve liver function in MAFLD [97][98]. Prebiotics (such as fructans, galacto-oligosaccharides, starches and glucose-derived oligosaccharides, and peptic and non-carbohydrate oligosaccharides) are the substances that selectively stimulate the growth and activity of beneficial gut bacteria and promote their beneficial effects [99].
6.1. The Role of Helicobacter pylori in the Pathogenesis and Management of Metabolic-Associated Fatty Liver Disease
It has been postulated that H. pylori infection might be involved one of the many factors in the complex pathogenesis of MAFLD. H. pylori infection can impact the composition and function of the gut microbiota, insulin resistance, and systemic inflammation. [100] Regarding the gut microbiota, H. pylori infection can affect its composition and diversity, potentially influencing the development of MAFLD. However, the precise mechanisms underlying this relationship are not yet fully understood. Moreover, the systemic inflammatory state that contributes to the pathogenesis of MAFLD is associated with an H. pylori infection, during which the concentration of pro-inflammatory markers increases, potentially influencing the development of MAFLD in a yet-unclear manner [100].
6.2. Modification of Microbiota
Antibiotics can eliminate unfavorable microbiota, and their efficacy has been confirmed in several liver diseases [101]. For treating cirrhosis and encephalopathy, as well as spontaneous bacterial peritonitis, fluoroquinolones (norfloxacin and ciprofloxacin), third-generation cephalosporins (ceftriaxone and cefotaxime), and trimethoprim–sulfamethoxazole are recommended; and neomycin, metronidazole, polymyxin B, and rifaximin have been used. β-Lactam/β-lactamase inhibitor combinations (BLBLIs) and carbapenems are recommended as the first choice in empirical treatments. Rifaximin, as a eubiotic, has a potential effect on the liver by modulating the bowels’ microbiota. Gangarapu et al. [102] found that the short-term administration of antibiotics improved clinical symptoms in NAFLD/MASH patients by lowering circulating endotoxin and IL-10 levels (0.9 ± 0.34 vs. 0.8 ± 0.13 EU/mL, p = 0.03 and 4.08 ± 0.9 vs. 3.73 ± 0.7 pg/mL, p = 0.006, respectively) as well as LFTs (AST: 50.4 ± 39 vs. 33 ± 14 IU/L, p = 0.01; ALT: 72 ± 48 vs. 45.2 ± 26.3 IU/L, p = 0.0001, GGT: 52 ± 33 vs. 41.2 ± 21.1 IU/L, p = 0.02). Similarly, a study by Abdal-Razik et al. [103] demonstrated a significant decrease in transaminases and transferase activity [ALT: 64.6 ± 34.2 vs. 38.2 ± 29.2 IU/L, p = 0.017; AST: 66.5 ± 42.5 vs. 40.1 ± 20.1 IU/L, p = 0.042; GGT: 56.7 ± 31.6 vs. 34.8 ± 28.6 IU/L, p = 0.046]; endotoxins; and IL-10, TNF-alpha, and IL-6 levels [0.82 ± 0.22 vs. 0.7 ± 0.09 EU/mL, p = 0.001; 40.6 ± 18.78 vs. 58 ± 23.16 pg/mL, p = 0.009; 19.18 ± 9.29 vs. 13.34 ± 8.31 pg/mL, p = 0.045; 8.34 ± 1.8 vs. 6.67 ± 1.1 pg/mL, p = 0.004, respectively]; and the NAFLD Liver Fat Score [from (−0.6) to (−0.2) vs. from (−0.7) to (−0.4) (p = 0.034)], following the administration of rifaximin for 6 months but not after 1 month of therapy.
7. Antioxidants
Vitamin E (Alpha-Tocopherol)
Current international guidelines for the management of liver diseases (e.g., AASLD, AACE, the European Association for the Study of Diabetes (EASD), and the European Association for the Study of Obesity (EASO)) indicate the use of vitamin E (alpha-tocopherol) in the therapy of MASH in patients without diabetes [6]. Lipid accumulation in hepatocytes leads to lipotoxicity and increases the level of oxidative stress, which results in liver injury and inflammation [104]. As a redox scavenger, vitamin E may prevent the damage caused by excessive oxidative stress [105]. The off-label use of vitamin E is connected with improvements in LFTs [106]. The current recommendations on the use of vitamin E in NAFLD treatment are based on the results presented in RCTs concerning patients suffering from MASH with or without type 2 diabetes mellitus (DMT2). In the PIVENS trial, the antioxidative properties of vitamin E appeared to have a positive effect on the liver histology both in reducing the NAFLD activity score (NAS) and resolving MASH [51][107].
8. Interactions between the Endocrine System and NAFLD
8.1. Thyroid Hormones
As mentioned earlier, hypothyroidism is an important risk factor for MAFLD [108]. The use of thyroid hormones to induce fat loss in the liver might be a promising therapeutic option, but the burden of side effects precludes their use in native form. However, it might be feasible to use specific agonists of thyroid receptor beta (TRb), which selectively affect liver metabolism while omitting most cardiac effects (e.g., tachycardia) resulting from TRa activation. Currently, there are several compounds under evaluation in the treatment of NAFLD. Resmetirom halved liver fat content, as assessed using MRI-PDFF, in patients with MASH during 36 weeks of treatment with an 80 mg daily dose of the drug [109]. This was accompanied by a significant reduction in ALT [11.0 ± 6.8 vs. (−15.4) ± 4.7 IU/L; p = 0.0019] and AST [3.6 ± 2.8 vs. (−7.4) ± 1.9 IU/L; p = 0.0016] activities at week 36. The 72-week open-label extension trial also showed resmetirom’s efficacy and sufficient tolerability.
8.2. Testosterone
Low testosterone levels (<346 ng/dL) in males have been linked with noninvasive markers of liver steatosis [110]. Long-term observations strongly suggest improvements in liver steatosis in hypogonadal men during testosterone replacement therapy. A 12-year follow-up showed that maintaining the optimal testosterone level with testosterone undecanoate resulted in a significant improvement in the Fatty Liver Index (from 83.6 ± 12.08 to 66.91 ± 19.38; p < 0.0001) and GGT (from 39.31 ± 11.62 to 28.95 ± 7.57 IU/L; p < 0.0005) but without a significant change in the ALT or AST activity [111]. Therefore, testosterone-replacement therapy is improving the metabolic status of males with hypogonadism. Conversely, testosterone or anabolic androgenic steroids may lead to liver damage in men with normal testosterone levels [112].
8.3. Estradiol
Estrogens play an important role in the metabolism of lipids [113]. Estradiol deficiency, which occurs after menopause, coincides with an increased propensity to develop NAFLD [114]. The loss of the protective effects of estrogens is also reflected by a reduction in the sex-hormone binding protein (SHBG), which leads to a relative “excess” in androgen levels [115]. Estradiol therapy might be beneficial for the course of MAFLD after menopause, but it is generally used with progestins, which may alleviate the benefits [116].
8.4. Growth Hormone
Growth hormone (GH) has a multidimensional influence on the human metabolism, including that of the liver. Both deficiency and excess may lead to several pathologies [117]. GH deficiency is commonly associated with liver steatosis, and the risk of NAFLD development in such patients is nearly two-fold OR = 1.85; 95% CI: [(1.05–3.28); p = 0.03] [118]. Patients with NAFLD tend to have a reduced peak GH secretory response (9.2 ± 6.4 vs. 15.4 ± 11.2 ng/mL; p = 0.001), and higher IGF-1 (Insulin-like Growth Factor-1) levels are associated with less-advanced FIB-4 scores [119].
9. Other Therapies
9.1. Xanthine Oxidase Inhibitors
A positive correlation between hyperuricemia and the incidence of MAFLD underlies the research concerning the probably beneficial effects of therapy with xanthine oxidase inhibitors (allopurinol and febuxostat) on liver functions [120].
In the mouse model of MASH, both xanthine oxidase inhibitors appeared to alleviate hepatic steatosis and fibrosis [121][122]. In a pilot interventional study with febuxostat in patients with MAFLD, serum levels of ALT [before: 73.0 (69.8–117.8); after: 70.5 (57.5–94.5) IU/L, p = 0.040] and AST [before: 50.5 (40.8–69.8); after: 44.5 (34.8–60.8) IU/L, p = 0.018] were significantly decreased, and hepatic steatosis, as confirmed by conducting a histopathological examination, was improved [121]. The results of an ongoing interventional randomized clinical trial comparing the influences of allopurinol and febuxostat on MAFLD (NCT05474560) may clarify the potential use of xanthine oxidase inhibitors for the therapy of liver diseases in the future [123].
9.2. Lubiprostone
A gut–liver axis dysfunction turned out to be one of the pathological mechanisms responsible for the progression of MAFLD. Thus, disturbances in the intestinal permeability and dysbiosis have become potential targets in the management of MAFLD [124].
Lubiprostone, an oral metabolite of prostaglandin E1, which increases intestinal fluid secretions by promoting the intraluminal chloride-anion efflux, was originally implemented for the therapy of idiopathic constipation and irritable bowel syndrome with constipation [125].
9.3. Pentoxifylline
Considering the multifactorial pathogenesis of MAFLD, which includes inflammation, drugs with potential anti-inflammatory effects, such as pentoxifylline, were evaluated for use in the aforementioned liver dysfunction. As a nonspecific phosphodiesterase 4 (PDE-4) inhibitor with the ability to decrease the transcription of TNFa, which is considered as one of the main proinflammatory agents responsible for the degradation of hepatocytes, the effects of therapy with pentoxifylline were examined both in rodents and humans [126][127].
9.4. Other Drugs
The data from selected meta-analyses might suggest that several drugs commonly used in patients with cardiovascular diseases with concomitant MAFLD (e.g., losartan and acetylsalicylic acid) have positive impacts on hepatic enzyme levels or protect against progression to advanced fibrosis [128][129]. Nevertheless, currently, the level of evidence is underwhelming.
10. Summary
The pathogenesis of MAFLD is multifactorial, and the pathogenic drivers of MAFLD can serve as therapeutic targets. Those can include: the modulation of food intake, increase in energy expenditure, improvement in adipocyte insulin sensitivity, inhibition of de novo lipogenesis, tapering of oxidative stress, and reduction in inflammation. MAFLD might be considered as a part of the clinical presentation of the metabolic syndrome. For several decades, there have been major improvements in the therapies of diabetes, hyperlipidemia, and hypertension. It seems that drugs affecting incretin receptors, PPAR, and TRb have significant impacts on the surrogate markers of liver steatosis. Based on the available research findings and recommendations from liver disease societies, it appears that some of the most significant factors are the achievement of a normal body weight and the effective treatment of metabolic disorders. Still, there is a lack of a sufficient number of studies indicating which of the cardiometabolic risk factors, included in the definition of MAFLD, most significantly lead(s) to disease progression. Thus, the need for further studies on therapies that will significantly and independently mitigate both the consequences and severity of liver steatosis seems essential. Therefore, alongside the established roles of pioglitazone and vitamin E in MASH treatment, it seems that drugs for weight reduction (including incretin-based medications that also have a glycemic-normalizing effect) and other drugs that affect the cardiometabolic risk will gradually gain importance; hence, the incessant necessity for exploring pathophysiological pathways that would allow a common mechanism of action to be found for steatosis resolution regardless of the type of factor that led to it.
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 2023, 101133.
- Lieverse, R.J.; Jansen, J.B.M.J.; Masclee, A.A.M.; Lamers, C.B.H.W. Gastrointestinal Disturbances with Obesity. Scand. J. Gastroenterol. 1993, 28, 53–58.
- Basha, A.; May, S.C.; Anderson, R.M.; Samala, N.; Mirmira, R.G. Non-Alcoholic Fatty Liver Disease: Translating Disease Mechanisms into Therapeutics Using Animal Models. Int. J. Mol. Sci. 2023, 24, 9996.
- Fernández, T.; Viñuela, M.; Vidal, C.; Barrera, F. Lifestyle changes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263931.
- Kim, H.; Lee, D.S.; An, T.H.; Park, H.-J.; Kim, W.K.; Bae, K.-H.; Oh, K.-J. Metabolic Spectrum of Liver Failure in Type 2 Diabetes and Obesity: From NAFLD to NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4495.
- Basu, R.; Noureddin, M.; Clark, J.M. Nonalcoholic Fatty Liver Disease: Review of Management for Primary Care Providers. Mayo Clin. Proc. 2022, 97, 1700–1716.
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402.
- Mátis, D.; Hegyi, P.; Teutsch, B.; Tornai, T.; Erőss, B.; Pár, G.; Váncsa, S. Improved body composition decreases the fat content in non-alcoholic fatty liver disease, a meta-analysis and systematic review of longitudinal studies. Front. Med. 2023, 10, 1114836.
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835.
- Gofton, C.; Upendran, Y.; Zheng, M.-H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31.
- Hazlehurst, J.M.; Tomlinson, J.W. Mechanisms in endocrinology: Non-alcoholic fatty liver disease in common endocrine disorders. Eur. J. Endocrinol. 2013, 169, R27–R37.
- Ranjbar, G.; Mikhailidis, D.P.; Sahebkar, A. Effects of newer antidiabetic drugs on nonalcoholic fatty liver and steatohepatitis: Think out of the box! Metabolism 2019, 101, 154001.
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086.
- Jasleen, B.; Vishal, G.K.; Sameera, M.; Fahad, M.; Brendan, O.; Deion, S.; Pemminati, S. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Benefits Versus Risk. Cureus 2023, 15, 1–11.
- Van Der Aart-van Der Beek, A.B.; De Boer, R.A.; Heerspink, H.J.L. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat. Rev. Nephrol. 2022, 18, 294–306.
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE(-/-) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818.
- Shaaban, H.H.; Alzaim, I.; El-Mallah, A.; Aly, R.G.; El-Yazbi, A.F.; Wahid, A. Metformin, pioglitazone, dapagliflozin and their combinations ameliorate manifestations associated with NAFLD in rats via anti-inflammatory, anti-fibrotic, anti-oxidant and anti-apoptotic mechanisms. Life Sci. 2022, 308, 120956.
- Sun, L.; Deng, C.; Gu, Y.; He, Y.; Yang, L.; Shi, J. Effects of dapagliflozin in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101876.
- Bellanti, F.; Buglio, A.L.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Aich, P.; Singh, S.P.; Serviddio, G.; Vendemiale, G. Impact of sodium glucose cotransporter-2 inhibitors on liver steatosis/fibrosis/inflammation and redox balance in non-alcoholic fatty liver disease. WJG 2022, 28, 3243–3257.
- Buldak, L.; Machnik, G.; Skudrzyk, E.; Boldys, A.; Maliglowka, M.; Kosowski, M.; Basiak, M.; Buldak, R.J.; Okopien, B. Exenatide prevents statin-related LDL receptor increase and improves insulin secretion in pancreatic beta cells (1.1E7) in a protein kinase A-dependent manner. J. Appl. Biomed. 2022, 20, 130–140.
- Bułdak, Ł.; Skudrzyk, E.; Machnik, G.; Bołdys, A.; Bułdak, R.J.; Okopień, B. Exenatide improves antioxidant capacity and reduces the expression of LDL receptors and PCSK9 in human insulin-secreting 1.1E7 cell line subjected to hyperglycemia and oxidative stress. Postępy Hig. I Med. Doświadczalnej 2022, 76, 16–23.
- Bułdak, Ł.; Machnik, G.; Skudrzyk, E.; Bołdys, A.; Okopień, B. The impact of exenatide (a GLP-1 agonist) on markers of inflammation and oxidative stress in normal human astrocytes subjected to various glycemic conditions. Exp. Ther. Med. 2019, 17, 2861–2869.
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690.
- Study of Semaglutide for Non-Alcoholic Fatty Liver Disease (NAFLD), a Metabolic Syndrome With Insulin Resistance, Increased Hepatic Lipids, and Increased Cardiovascular Disease Risk (The SLIM LIVER Study). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04216589 (accessed on 10 August 2023).
- Non-Alcoholic Fatty Liver Disease, the HEpatic Response to Oral Glucose, and the Effect of Semaglutide (NAFLD HEROES). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03884075 (accessed on 10 August 2023).
- Semaglutide in Nonalcoholic Fatty Liver Disease. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05813249 (accessed on 10 August 2023).
- A Multiple Dose Study of LY3502970 in Healthy Overweight and Obese Participants. Available online: https://ichgcp.net/clinical-trials-registry/NCT05841238 (accessed on 11 August 2023).
- A Study of LY3502970 in Participants With Obesity or Overweight With Weight-related Comorbidities. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05051579 (accessed on 11 August 2023).
- Pratt, E.; Ma, X.; Liu, R.; Robins, D.; Coskun, T.; Sloop, K.W.; Haupt, A.; Benson, C. Orforglipron (LY3502970), a novel, oral non-peptide glucagon-like peptide-1 receptor agonist: A Phase 1b, multicentre, blinded, placebo-controlled, randomized, multiple-ascending-dose study in people with type 2 diabetes. Diabetes Obes. Metab. 2023, 25, 2642–2649.
- El, K.; Douros, J.D.; Willard, F.S.; Novikoff, A.; Sargsyan, A.; Perez-Tilve, D.; Wainscott, D.B.; Yang, B.; Chen, A.; Wothe, D.; et al. The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets. Nat. Metab. 2023, 5, 945–954.
- Thomas, M.K.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D.A. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 388–396.
- A Study of Tirzepatide (LY3298176) in Participants With Nonalcoholic Steatohepatitis (NASH) (SYNERGY-NASH). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04166773 (accessed on 11 August 2023).
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355.
- Romero-Gómez, M.; Lawitz, E.; Shankar, R.R.; Chaudhri, E.; Liu, J.; Lam, R.L.H.; Kaufman, K.D.; Engel, S.S.; Bruzone, S.O.; Coronel, M.J.; et al. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J. Hepatol. 2023, 79, 888–897.
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021, 44, 1433–1442.
- Study to Evaluate Efficacy, Safety and Tolerability of HM15211 in Subjects. Available online: https://clinicaltrials.gov/study/NCT04505436 (accessed on 12 August 2023).
- A Study of Multiple Doses of HM15211 in Obese Subjects with NAFLD. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03744182 (accessed on 12 August 2023).
- A Study of LY3437943 in Participants Who Have Obesity or Are Overweight. Available online: https://clinicaltrials.gov/study/NCT04881760 (accessed on 12 August 2023).
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L. Triple–Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526.
- Kongmalai, T.; Srinonprasert, V.; Anothaisintawee, T.; Kongmalai, P.; McKay, G.; Attia, J.; Thakkinstian, A. New anti-diabetic agents for the treatment of non-alcoholic fatty liver disease: A systematic review and network meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1182037.
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings. Endocr. Pract. 2022, 28, 528–562.
- Valenzuela-Vallejo, L.; Guatibonza-García, V.; Mantzoros, C.S. Recent guidelines for Non-Alcoholic Fatty Liver disease (NAFLD)/ Fatty Liver Disease (FLD): Are they already outdated and in need of supplementation? Metabolism 2022, 136, 155248.
- Dos Santos, L.R.; Duarte, M.L.; Peccin, M.S.; Gagliardi, A.R.D.T.; Melnik, T. Dipeptidyl Peptidase IV Inhibitors for Nonalcoholic Fatty Liver Disease—Systematic Review and Metanalysis. CDR 2021, 17, e101120187811.
- Alam, S.; Ghosh, J.; Mustafa, G.; Kamal, M.; Ahmad, N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: A 1-year randomized control trial. HMER 2018, 10, 23–31.
- Aktaş, A.; Ozan, Z. The Efficacy and safety of vildagliptin treatment for nonalcoholic fatty liver disease in type 2 diabetes mellitus. Cumhur. Med. J. 2020, 42, 491–499.
- Jin, D.; Cui, Z.; Jin, S.; Zhou, T.; Guo, B.; Gao, P.; Li, G. Comparison of efficacy of anti-diabetics on non-diabetic NAFLD: A network meta-analysis. Front. Pharmacol. 2023, 13, 1096064.
- Hattori, S.; Nomoto, K.; Suzuki, T.; Hayashi, S. Beneficial effect of omarigliptin on diabetic patients with non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. Diabetol. Metab. Syndr. 2021, 13, 28.
- Gupta, V.K. 1029-P: Teneligliptin Significantly Reduces Liver Fat Content (LFC) and Delays Progression of NASH in Type 2 Diabetes Mellitus Patients. Diabetes 2019, 68 (Suppl. S1), 1029-P.
- Chen, L.; Zhang, X.; Zhang, L.; Zheng, D. Effect of Saxagliptin, a Dipeptidyl Peptidase 4 Inhibitor, on Non-Alcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3507–3518.
- Li, J.-J.; Zhang, P.; Fan, B.; Guo, X.-L.; Zheng, Z.-S. The efficacy of saxagliptin in T2DM patients with non-alcoholic fatty liver disease: Preliminary data. Rev. Assoc. Med. Bras. 2019, 65, 33–37.
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685.
- Aithal, G.P.; Thomas, J.A.; Kaye, P.V.; Lawson, A.; Ryder, S.D.; Spendlove, I.; Austin, A.S.; Freeman, J.G.; Morgan, L.; Webber, J. Randomized, Placebo-Controlled Trial of Pioglitazone in Nondiabetic Subjects with Nonalcoholic Steatohepatitis. Gastroenterology 2008, 135, 1176–1184.
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A Placebo-Controlled Trial of Pioglitazone in Subjects with Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307.
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern. Med. 2016, 165, 305.
- Bril, F.; Biernacki, D.M.; Kalavalapalli, S.; Lomonaco, R.; Subbarayan, S.K.; Lai, J.; Tio, F.; Suman, A.; Orsak, B.K.; Hecht, J.; et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2019, 42, 1481–1488.
- Della Pepa, G.; Russo, M.; Vitale, M.; Carli, F.; Vetrani, C.; Masulli, M.; Riccardi, G.; Vaccaro, O.; Gastaldelli, A.; Rivellese, A.A.; et al. Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: Clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial. Diabetes Res. Clin. Pract. 2021, 178, 108984.
- Ndakotsu, A.; Vivekanandan, G. The Role of Thiazolidinediones in the Amelioration of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, 1–9.
- Kazierad, D.J.; Chidsey, K.; Somayaji, V.R.; Bergman, A.J.; Birnbaum, M.J.; Calle, R.A. Inhibition of ketohexokinase in adults with NAFLD reduces liver fat and inflammatory markers: A randomized phase 2 trial. Med 2021, 2, 800–813.e3.
- Pinyopornpanish, K.; Leerapun, A.; Pinyopornpanish, K.; Chattipakorn, N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver 2021, 15, 827–840.
- Li, Y.; Liu, L.; Wang, B.; Wang, J.; Chen, D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Rep. 2013, 1, 57–64.
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904.
- Loomba, R.; Lutchman, G.; Kleiner, D.E.; Ricks, M.; Feld, J.J.; Borg, B.B.; Modi, A.; Nagabhyru, P.; Sumner, A.E.; Liang, T.J.; et al. Clinical trial: Pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2009, 29, 172–182.
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A Randomized Controlled Trial of Metformin versus Vitamin E or Prescriptive Diet in Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2005, 100, 1082–1090.
- Ioannou, G.N. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol. Metab. 2016, 27, 84–95.
- Bułdak, Ł. The treatment of heterozygous familial hypercholesterolemia—A local perspective. Endokrynol. Pol. 2021, 72, 189–190.
- Okopień, B.; Bułdak, Ł.; Bołdys, A. Current and future trends in the lipid lowering therapy. Pharmacol. Rep. 2016, 68, 737–747.
- Boutari, C.; Pappas, P.D.; Anastasilakis, D.; Mantzoros, C.S. Statins’ efficacy in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 2195–2206.
- Ahsan, F.; Oliveri, F.; Goud, H.K.; Mehkari, Z.; Mohammed, L.; Javed, M.; Althwanay, A.; Rutkofsky, I.H. Pleiotropic Effects of Statins in the Light of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Cureus 2020, 12, 1–12.
- Athyros, V.G.; Mikhailidis, D.P.; Didangelos, T.P.; Giouleme, O.I.; Liberopoulos, E.N.; Karagiannis, A.; Kakafika, A.I.; Tziomalos, K.; Burroughs, A.K.; Elisaf, M.S. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: A randomised study. Curr. Med. Res. Opin. 2006, 22, 873–883.
- Athyros, V.G.; Tziomalos, K.; Gossios, T.D.; Griva, T.; Anagnostis, P.; Kargiotis, K.; Pagourelias, E.D.; Theocharidou, E.; Karagiannis, A.; Mikhailidis, D.P. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post-hoc analysis. Lancet 2010, 376, 1916–1922.
- Pastori, D.; Pani, A.; Di Rocco, A.; Menichelli, D.; Gazzaniga, G.; Farcomeni, A.; D’Erasmo, L.; Angelico, F.; Del Ben, M.; Baratta, F. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Brit J. Clin. Pharma 2022, 88, 441–451.
- Zhang, J.; Fu, S.; Liu, D.; Wang, Y.; Tan, Y. Statin can reduce the risk of hepatocellular carcinoma among patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 353–358.
- Wargny, M.; Ducluzeau, P.-H.; Petit, J.-M.; Le May, C.; Smati, S.; Arnaud, L.; Pichelin, M.; Bouillet, B.; Lannes, A.; Blanchet, O.; et al. Circulating PCSK9 levels are not associated with the severity of hepatic steatosis and NASH in a high-risk population. Atherosclerosis 2018, 278, 82–90.
- Di Filippo, M.; Vokaer, B.; Seidah, N.G. A case of hypocholesterolemia and steatosis in a carrier of a PCSK9 loss-of-function mutation and polymorphisms predisposing to nonalcoholic fatty liver disease. J. Clin. Lipidol. 2017, 11, 1101–1105.
- Emma, M.R.; Giannitrapani, L.; Cabibi, D.; Porcasi, R.; Pantuso, G.; Augello, G.; Giglio, R.V.; Re, N.L.; Capitano, A.R.; Montalto, G.; et al. Hepatic and circulating levels of PCSK9 in morbidly obese patients: Relation with severity of liver steatosis. Biochim. Et. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158792.
- El-Haggar, S.M.; Mostafa, T.M. Comparative clinical study between the effect of fenofibrate alone and its combination with pentoxifylline on biochemical parameters and liver stiffness in patients with non-alcoholic fatty liver disease. Hepatol. Int. 2015, 9, 471–479.
- Araki, E.; Yamashita, S.; Arai, H.; Yokote, K.; Satoh, J.; Inoguchi, T.; Nakamura, J.; Maegawa, H.; Yoshioka, N.; Tanizawa, Y.; et al. Effects of Pemafibrate, a Novel Selective PPARα Modulator, on Lipid and Glucose Metabolism in Patients With Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care 2018, 41, 538–546.
- Ikeda, S.; Sugihara, T.; Hoshino, Y.; Matsuki, Y.; Nagahara, T.; Okano, J.; Kitao, S.; Fujioka, Y.; Yamamoto, K.; Isomoto, H. Pemafibrate Dramatically Ameliorated the Values of Liver Function Tests and Fibrosis Marker in Patients with Non-Alcoholic Fatty Liver Disease. Yonago Acta Med. 2020, 63, 188–197.
- Seko, Y.; Yamaguchi, K.; Umemura, A.; Yano, K.; Takahashi, A.; Okishio, S.; Kataoka, S.; Okuda, K.; Moriguchi, M.; Okanoue, T.; et al. Effect of pemafibrate on fatty acid levels and liver enzymes in non-alcoholic fatty liver disease patients with dyslipidemia: A single-arm, pilot study. Hepatol. Res. 2020, 50, 1328–1336.
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin. Exp. Hepatol. 2020, 6, 270–274.
- Roy, S. Clinical Case Series of Decrease in Shear Wave Elastography Values in Ten Diabetic Dyslipidemia Patients Having NAFLD with Saroglitazar 4 mg: An Indian Experience. Case Rep. Med. 2020, 2020, 4287075.
- Mitra, A. An Observational Study of Reduction in Glycemic Parameters and Liver Stiffness by Saroglitazar 4 mg in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Cureus 2020, 12, 1–7.
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689.
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735.
- Kim, C.-W.; Addy, C.; Kusunoki, J.; Anderson, N.N.; Deja, S.; Fu, X.; Burgess, S.C.; Li, C.; Ruddy, M.; Chakravarthy, M.; et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab. 2017, 26, 394–406.e6.
- Loomba, R.; Kayali, Z.; Noureddin, M.; Ruane, P.; Lawitz, E.J.; Bennett, M.; Wang, L.; Harting, E.; Tarrant, J.M.; McColgan, B.J.; et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1463–1473.e6.
- O’Farrell, M.; Duke, G.; Crowley, R.; Buckley, D.; Martins, E.B.; Bhattacharya, D.; Friedman, S.L.; Kemble, G. FASN inhibition targets multiple drivers of NASH by reducing steatosis, inflammation and fibrosis in preclinical models. Sci. Rep. 2022, 12, 15661.
- Syed-Abdul, M.M.; Parks, E.J.; Gaballah, A.H.; Bingham, K.; Hammoud, G.M.; Kemble, G.; Buckley, D.; McCulloch, W.; Manrique-Acevedo, C. Fatty Acid Synthase Inhibitor TVB-2640 Reduces Hepatic de Novo Lipogenesis in Males With Metabolic Abnormalities. Hepatology 2020, 72, 103–118.
- Calle, R.A.; Amin, N.B.; Carvajal-Gonzalez, S.; Ross, T.T.; Bergman, A.; Aggarwal, S.; Crowley, C.; Rinaldi, A.; Mancuso, J.; Aggarwal, N.; et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: Two parallel, placebo-controlled, randomized phase 2a trials. Nat. Med. 2021, 27, 1836–1848.
- Woo, Y.C.; Xu, A.; Wang, Y.; Lam, K.S.L. Fibroblast growth factor 21 as an emerging metabolic regulator: Clinical perspectives. Clin. Endocrinol. 2013, 78, 489–496.
- Loomba, R.; Lawitz, E.J.; Frias, J.P.; Ortiz-Lasanta, G.; Johansson, L.; Franey, B.B.; Morrow, L.; Rosenstock, M.; Hartsfield, C.L.; Chen, C.-Y.; et al. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 1b/2a multiple-ascending-dose study. Lancet Gastroenterol. Hepatol. 2023, 8, 120–132.
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008.
- To Evaluate the Efficacy and Safety of Pegozafermin in Subjects with Severe Hypertriglyceridemia (ENTRUST). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05852431 (accessed on 24 September 2023).
- Zhang, W.; Tang, Y.; Huang, J.; Hu, H. Efficacy of ursodeoxycholic acid in nonalcoholic fatty liver disease: An updated meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2020, 29, 696–705.
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762.
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683.
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805.
- Sharpton, S.R.; Maraj, B.; Harding-Theobald, E.; Vittinghoff, E.; Terrault, N.A. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Am. J. Clin. Nutr. 2019, 110, 139–149.
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92.
- Mavilia-Scranton, M.G.; Wu, G.Y.; Dharan, M. Impact of Helicobacter pylori Infection on the Pathogenesis and Management of Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2023, 11, 670.
- Dong, Y.; Sun, D.; Wang, Y.; Du, Q.; Zhang, Y.; Han, R.; Teng, M.; Zhang, T.; Shi, L.; Zheng, G.; et al. Evaluation of the current guidelines for antibacterial therapy strategies in patients with cirrhosis or liver failure. BMC Infect. Dis. 2022, 22, 23.
- Gangarapu, V.; Ince, A.T.; Baysal, B.; Kayar, Y.; Kılıç, U.; Gök, Ö.; Uysal, Ö.; Şenturk, H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 840–845.
- Abdel-Razik, A.; Mousa, N.; Shabana, W.; Refaey, M.; Elzehery, R.; Elhelaly, R.; Zalata, K.; Abdelsalam, M.; Eldeeb, A.A.; Awad, M.; et al. Rifaximin in nonalcoholic fatty liver disease: Hit multiple targets with a single shot. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1237–1246.
- Podszun, M.C.; Alawad, A.S.; Lingala, S.; Morris, N.; Huang, W.-C.A.; Yang, S.; Schoenfeld, M.; Rolt, A.; Ouwerkerk, R.; Valdez, K.; et al. Vitamin E treatment in NAFLD patients demonstrates that oxidative stress drives steatosis through upregulation of de-novo lipogenesis. Redox Biol. 2020, 37, 101710.
- Podszun, M.C.; Frank, J. Impact of vitamin E on redox biomarkers in non-alcoholic fatty liver disease. Redox Biol. 2021, 42, 101937.
- Luo, Q.; Wei, R.; Cai, Y.; Zhao, Q.; Liu, Y.; Liu, W.J. Efficacy of Off-Label Therapy for Non-alcoholic Fatty Liver Disease in Improving Non-invasive and Invasive Biomarkers: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front Med. 2022, 9, 793203.
- An, J.; Sohn, J.H. Pharmacological advances in the treatment of nonalcoholic fatty liver diseases: Focused on global results of randomized controlled trials. Clin. Mol. Hepatol. 2023, 29, S268–S275.
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, M. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int. J. Mol. Sci. 2020, 21, 5927.
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024.
- Polyzos, S.A.; Mousiolis, A.; Mintziori, G.; Goulis, D.G. Nonalcoholic fatty liver disease in males with low testosterone concentrations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1571–1577.
- Yassin, A.A.; Alwani, M.; Talib, R.; Almehmadi, Y.; Nettleship, J.E.; Alrumaihi, K.; Albaba, B.; Kelly, D.M.; Saad, F. Long-term testosterone therapy improves liver parameters and steatosis in hypogonadal men: A prospective controlled registry study. Aging Male 2020, 23, 1553–1563.
- Patil, V.; Jothimani, D.; Harika, K.; Hakeem, A.R.; Sachan, D.; Vij, M.; Rela, M. Versatility of Anabolic Androgenic Steroid-Induced Hepatotoxicity. J. Clin. Exp. Hepatol. 2022, 12, 216–221.
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256.
- Gutierrez-Grobe, Y.; Ponciano-Rodríguez, G.; Ramos, M.H.; Uribe, M.; Méndez-Sánchez, N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann. Hepatol. 2010, 9, 402–409.
- Cao, W.; Xu, Y.; Shen, Y.; Wang, Y.; Ma, X.; Bao, Y. Associations between sex hormones and metabolic-associated fatty liver disease in a middle-aged and elderly community. Endocr. J. 2022, 69, 1007–1014.
- Polyzos, S.A.; Lambrinoudaki, I.; Goulis, D.G. Menopausal hormone therapy in women with dyslipidemia and nonalcoholic fatty liver disease. Hormones 2022, 21, 375–381.
- Oxley, M.; Francis, H.; Sato, K. Growth Hormone Signaling in Liver Diseases: Therapeutic Potentials and Controversies. Semin. Liver Dis. 2023, 43, 024–030.
- Hwang, Y.; Lee, H.W.; Ahn, S.H.; Lee, E.J.; Ku, C.R.; Kim, S.U. Positive association between nonalcoholic fatty liver disease and growth hormone deficiency in patients with nonfunctioning pituitary adenoma. Front. Endocrinol. 2023, 13, 1057769.
- Dichtel, L.E.; Corey, K.E.; Haines, M.S.; Chicote, M.L.; Kimball, A.; Colling, C.; Simon, T.G.; Long, M.T.; Husseini, J.; Bredella, M.A.; et al. The GH/IGF-1 Axis Is Associated with Intrahepatic Lipid Content and Hepatocellular Damage in Overweight/Obesity. J. Clin. Endocrinol. Metab. 2022, 107, e3624–e3632.
- Liu, Z.; Que, S.; Zhou, L.; Zheng, S. Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: A Meta-analysis of Prospective Studies. Sci. Rep. 2015, 5, 14325.
- Nishikawa, T.; Nagata, N.; Shimakami, T.; Shirakura, T.; Matsui, C.; Ni, Y.; Zhuge, F.; Xu, L.; Chen, G.; Nagashimada, M.; et al. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice. Sci. Rep. 2020, 10, 815.
- Kakimoto, M.; Fujii, M.; Sato, I.; Honma, K.; Nakayama, H.; Kirihara, S.; Fukuoka, T.; Ran, S.; Hirohata, S.; Kitamori, K.; et al. Antioxidant action of xanthine oxidase inhibitor febuxostat protects the liver and blood vasculature in SHRSP5/Dmcr rats. J. Appl. Biomed. 2023, 21, 80–90.
- NCT05474560. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05474560 (accessed on 9 June 2023).
- Kessoku, T.; Kobayashi, T.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Iwaki, M.; Ozaki, A.; Kasai, Y.; Nogami, A.; Honda, Y.; et al. The Role of Leaky Gut in Nonalcoholic Fatty Liver Disease: A Novel Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 8161.
- Wilson, N.; Schey, R. Lubiprostone in constipation: Clinical evidence and place in therapy. Ther. Adv. Chronic Dis. 2015, 6, 40–50.
- El-Deen, R.M.; Heeba, G.H.; Abdel-latif, R.G.; Khalifa, M.M.A. Comparative effectiveness of phosphodiesterase 3, 4, and 5 inhibitors in amelioration of high-fat diet-induced nonalcoholic fatty liver in rats. Fundam. Clin. Pharmacol. 2020, 34, 353–364.
- Vachliotis, I.D.; Polyzos, S.A. The Role of Tumor Necrosis Factor-Alpha in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease. Curr. Obes. Rep. 2023, 12, 191–206.
- Malladi, N.; Alam, M.J.; Maulik, S.K.; Banerjee, S.K. The role of platelets in non-alcoholic fatty liver disease: From pathophysiology to therapeutics. Prostaglandins Other Lipid Mediat. 2023, 169, 106766.
- Meng, C.; Song, Z.; Zhang, L.; Geng, Y.; Sun, J.; Miao, G.; Liu, P. Effects of losartan in patients with NAFLD: A meta-analysis of randomized controlled trial. Open Life Sci. 2023, 18, 20220583.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
594
Revisions:
2 times
(View History)
Update Date:
12 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




