
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raquel García-Álvarez | -- | 2425 | 2023-11-06 17:02:40 | | | |
| 2 | Peter Tang | Meta information modification | 2425 | 2023-11-07 06:17:01 | | |
Video Upload Options
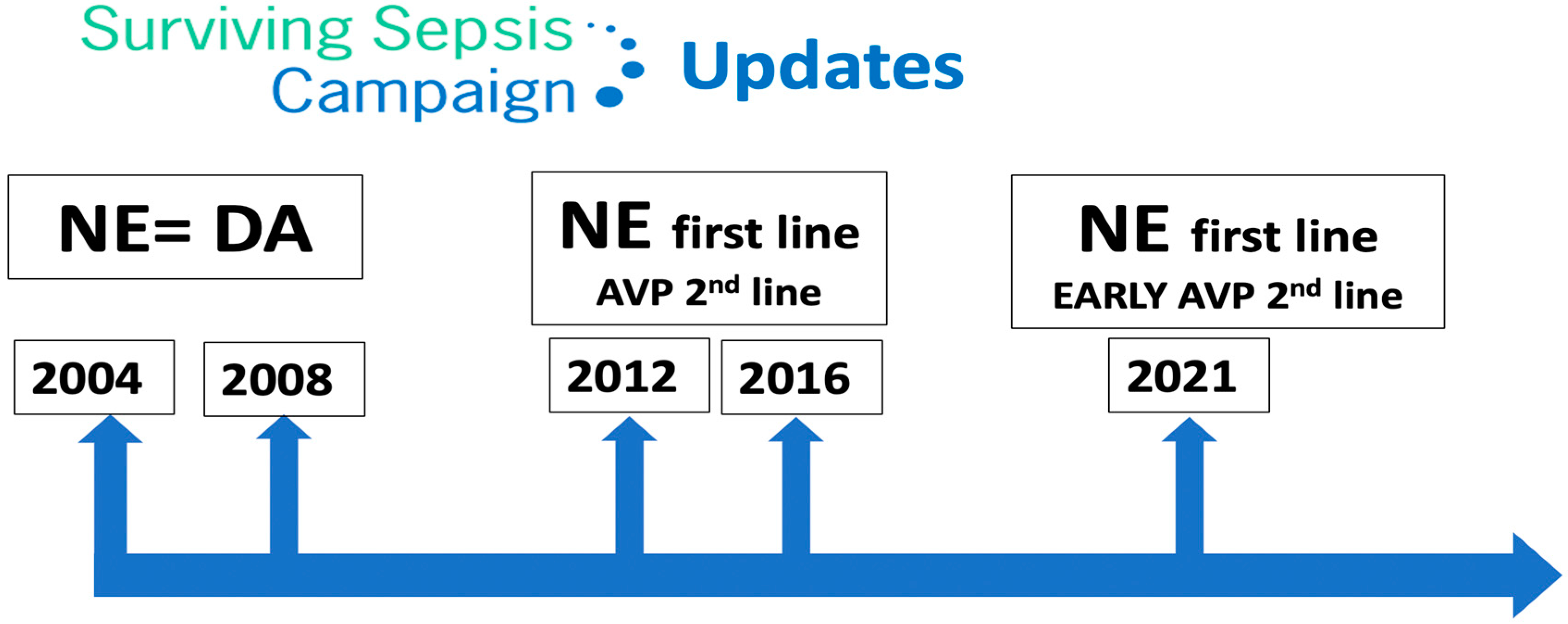
Septic shock is defined by the need for vasopressors to correct hypotension and lactic acidosis secondary to infection, with a high mortality rate. The Surviving Sepsis Campaign guidelines recommend vasopressin as a second-line vasopressor, added to norepinephrine.
1. Introduction
2. Physiology
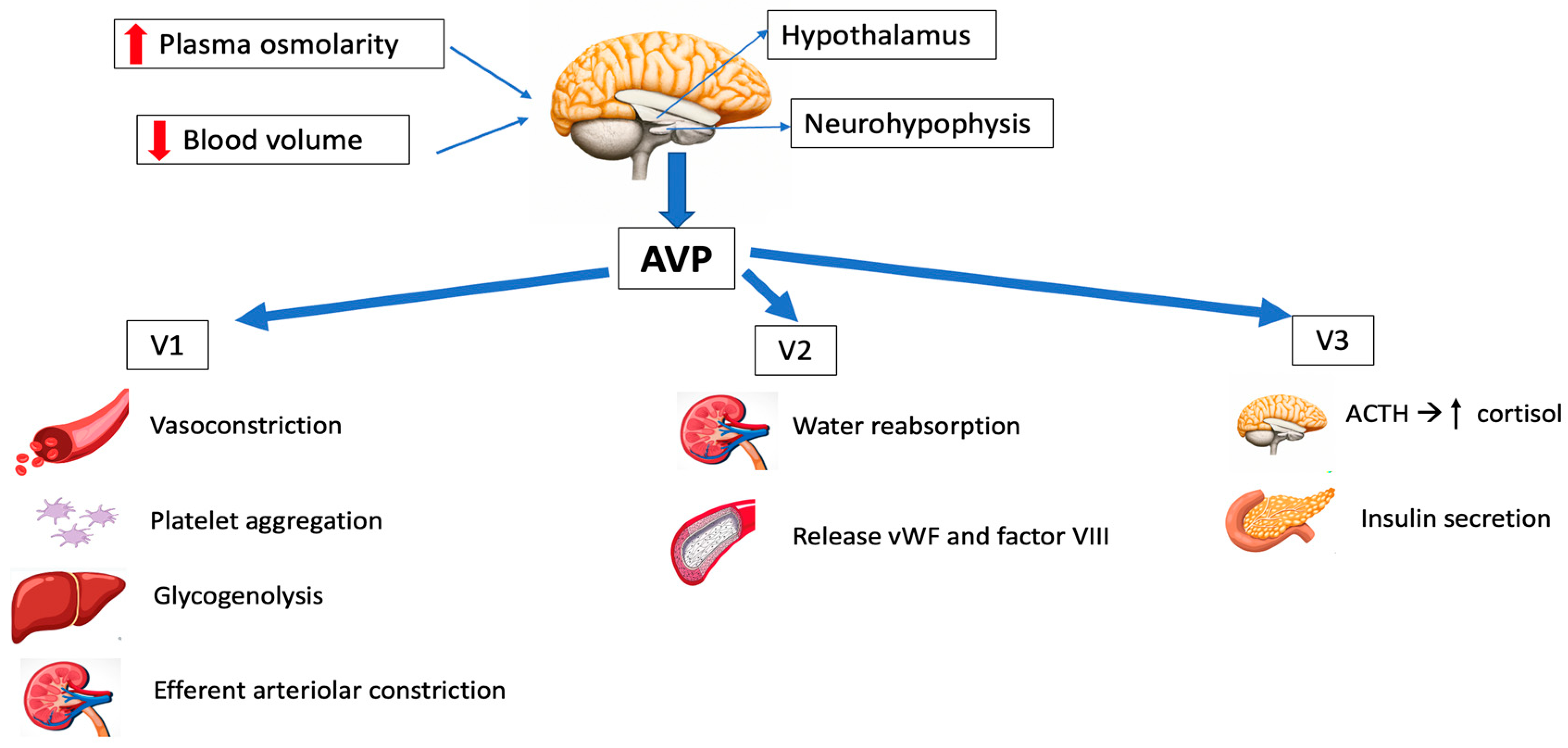
2.1. Synthesis and Release
2.2. AVP Receptors and Signal Transduction
|
VASOPRESSIN AGONISTS |
STRUCTURE |
RECEPTOR AFFINITY |
CLINICAL APPLICATION |
HALF-LIFE (min) |
|---|---|---|---|---|
|
ARGININE VASOPRESSIN (AVP) |
8-Arginine vasopressin |
V1, V2, V3 |
Sepsis, vasodilatory shock, cardiac arrest |
5–15 |
|
DESMOPRESSIN ACETATE (DDAVP) |
Deamino-Cys-D-Arg vasopressin |
V2 |
Central diabetes insipidus, bleeding disorders |
90–190 |
|
TERLIPRESSIN (TP) |
N3-triglycyl-8-lysin vasopressin |
V1 |
Portal hypertension, bleeding gastric and esophageal varices, septic shock |
240–360 |
|
SELEPRESSIN |
Phe-2-Ile-3-Hgn-4-Orn-8 vasopressin |
V1 |
Septic shock Not approved for clinical use |
10–30 |
|
ORNIPRESSIN |
8-L-Ornithine vasopressin acetate |
V1 |
Vasoconstricting agent during myomectomy in cirrhosis, as hepatorenal treatment |
60–120 |
2.3. Physiological Functions
- -
-
Osmoregulation
- -
-
Cardiovascular control
- -
-
Corticotropin secretion
- -
-
Hemostasis
3. Pharmacology
-
Arginine vasopressin (AVP) acts on the V1, V2 and V3 receptors, and has been employed in the management of refractory vasodilatory hypotension, cardiac arrest and septic shock. It has the great advantage of having a short half-life, so the dose can be easily titrated.
-
Desmopressin acetate (DDAVP) is a synthetic agonist with V2 receptor specificity and was first used in management of central diabetes insipidus. By directly affecting the endothelial V2 receptors, DDAVP also raises the plasma factor VIII and vWF concentrations in healthy subjects.
-
Terlipressin (TP) has a greater selectivity for the V1 receptor than AVP. It is a prodrug of AVP and undergoes metabolism by exopeptidases to yield the active metabolite lysine vasopressin in the circulation, producing a “slow release” effect and affording a longer biological half-life (6 h). Terlipressin has been used as treatment for bleeding gastric second to esophageal varices, portal hypertension and septic shock. The drug increases blood pressure and improves the outcomes of hepatorenal syndrome (contracting the mesenteric arteries, resulting in decreased portal venous inflow and subsequently lowering portal pressure) [5]. The latest Surviving Sepsis Campaign (SSC) recommendations [21] do not advise its use in patients with septic shock, due to greater undesirable effects (more serious adverse events than NE, especially digital ischemia) [22][23].
-
Selepressin, another synthetic vasopressin analog, is a short-acting selective V1 receptor agonist. It may present benefits compared to AVP due its ability to induce pure vasoconstriction; it also has reduced antidiuretic effects, a lower risk of thrombotic complications (because of reduced release of vWF) and affords superior protection from increased permeability. However, recently, a trial was stopped due to futility criteria, because no difference was observed in vasopressor- and ventilator-free days [24]. The drug is currently not approved for clinical use.
4. Vasopressin in Septic Shock
|
TRIAL |
Intervention |
Control |
Intervention 28 Day Mortality |
Control 28 Day Mortality |
Absolute Difference (95%CI) p-Value |
|---|---|---|---|---|---|
|
VASST [32] |
Norepinephrine |
Vasopressin |
35.4% |
39.3% |
3.9 (−2.9–10.7) 0.26 |
|
VANISH [33] |
Norepinephrine |
Vasopressin |
30.9% |
27.5% |
3.4 (−5.4–12.3) |
|
SOAP II [34] |
Norepinephrine |
Dopamine |
48.5% |
52.5% |
1.17 (0.97–1.42) 0.10 |
|
ATHOS-3 [35] |
Angiotensin II |
Placebo |
46% |
54% |
Hazard ratio 0.78 (0.57–1.07) 0.12 |
|
CAT [36] |
Epinephrine |
Norepinephrine |
23% |
27% |
Hazard ratio 0.87 (0.48–1.58) 0.65 |
|
CATS [37] |
Epinephrine |
Norepinephrine + dobutamine |
40% |
34% |
Relative risk 0.86 (0.65–1.14) 0.31 |
5. AVP in Vasodilatory Shock in Heart Surgery
|
Predominant and independent risk factors |
|
|
Other risk factors |
|
OHT: orthotopic heart transplant; LVAD: left ventricular assist device; CHD: congenital heart disease; VAD: ventricular assist device; ACEIs: angiotensin-converting enzyme inhibitors; CPB: cardiopulmonary bypass; AVP: arginine vasopressin [41].
6. AVP in Cardiac Arrest
7. Authorized Indications and Dosages in Europe and the United States of America (USA)
8. Adverse Effects of AVP
References
- Oliver, G.; Schäfer, E.A. On the Physiological Action of Extracts of Pituitary Body and Certain Other Glandular Organs: Preliminary Communication. J. Physiol. 1895, 18, 277–279.
- Dünser, M.W.; Lindner, K.H.; Wenzel, V. A Century of Arginine Vasopressin Research Leading to New Therapeutic Strategies. Anesthesiology 2006, 105, 444–445.
- Czakó, L.; Mezei, G.; László, F.A. Treatment of Diabetes Insipidus with 1-Deamino-8-d-Arginine Vasopressin. Acta Med. Acad. Sci. Hung. 1975, 32, 75–84.
- Kehne, J.H.; Hughes, F.A.; Gompertz, M.L. The Use of Surgical Pituitrin in the Control of Esophageal Varix Bleeding; an Experimental Study and Report of Two Cases. Surgery 1956, 39, 917–925.
- Park, K.S.; Yoo, K.Y. Role of Vasopressin in Current Anesthetic Practice. Korean J. Anesthesiol. 2017, 70, 245.
- Sharshar, T.; Blanchard, A.; Paillard, M.; Raphael, J.C.; Gajdos, P.; Annane, D. Circulating Vasopressin Levels in Septic Shock. Crit. Care Med. 2003, 31, 1752–1758.
- Treschan, T.A.; Peters, J.; Warltier, D.C. The Vasopressin System. Anesthesiology 2006, 105, 599–612.
- Sklar, A.H.; Schrier, R.W. Central Nervous System Mediators of Vasopressin Release. Physiol. Rev. 1983, 63, 1243–1280.
- Sai, Y.; Okamura, T.; Amakata, Y.; Toda, N. Comparison of Responses of Canine Pulmonary Artery and Vein to Angiotensin II, Bradykinin and Vasopressin. Eur. J. Pharmacol. 1995, 282, 235–241.
- Evora, P.R.B.; Pearson, P.J.; Rodrigues, A.J.; Viaro, F.; Schaff, H.V. Effect of Arginine Vasopressin on the Canine Epicardial Coronary Artery: Experiments on V1-Receptor-Mediated Production of Nitric Oxide. Arq. Bras. Cardiol. 2003, 80, 483–494.
- Rotondo, F.; Butz, H.; Syro, L.V.; Yousef, G.M.; Di Ieva, A.; Restrepo, L.M.; Quintanar-Stephano, A.; Berczi, I.; Kovacs, K. Arginine Vasopressin (AVP): A Review of Its Historical Perspectives, Current Research and Multifunctional Role in the Hypothalamo-Hypophysial System. Pituitary 2016, 19, 345–355.
- Demiselle, J.; Fage, N.; Radermacher, P.; Asfar, P. Vasopressin and Its Analogues in Shock States: A Review. Ann. Intensive Care 2020, 10, 9.
- Birnbaumer, M. The V2 Vasopressin Receptor Mutations and Fluid Homeostasis. Cardiovasc. Res. 2001, 51, 409–415.
- Pivonello, R.; De Bellis, A.; Faggiano, A.; Di Salle, F.; Petretta, M.; Di Somma, C.; Perrino, S.; Altucci, P.; Bizzarro, A.; Bellastella, A.; et al. Central Diabetes Insipidus and Autoimmunity: Relationship between the Occurrence of Antibodies to Arginine Vasopressin-Secreting Cells and Clinical, Immunological, and Radiological Features in a Large Cohort of Patients with Central Diabetes Insipidus of Known and Unknown Etiology. J. Clin. Endocrinol. Metab. 2003, 88, 1629–1636.
- Christ-Crain, M.; Gaisl, O. Diabetes Insipidus. Presse Medicale Paris Fr. 2021, 50, 104093.
- Schurr, J.W.; Szumita, P.M.; DeGrado, J.R. Neuroendocrine Derangements in Early Septic Shock: Pharmacotherapy for Relative Adrenal and Vasopressin Insufficiency. Shock Augusta Ga 2017, 48, 284–293.
- Russell, J.A.; Walley, K.R. Vasopressin and Its Immune Effects in Septic Shock. J. Innate Immun. 2010, 2, 446–460.
- Cattaneo, M. Review of Clinical Experience of Desmopressin in Patients with Congenital and Acquired Bleeding Disorders. Eur. J. Anaesthesiol. Suppl. 1997, 14, 10–14; discussion 14–18.
- Holmes, C.L.; Patel, B.M.; Russell, J.A.; Walley, K.R. Physiology of Vasopressin Relevant to Management of Septic Shock. Chest 2001, 120, 989–1002.
- Glavaš, M.; Gitlin-Domagalska, A.; Dębowski, D.; Ptaszyńska, N.; Łęgowska, A.; Rolka, K. Vasopressin and Its Analogues: From Natural Hormones to Multitasking Peptides. Int. J. Mol. Sci. 2022, 23, 3068.
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247.
- Mårtensson, J.; Gordon, A.C. Terlipressin or Norepinephrine, or Both in Septic Shock? Intensive Care Med. 2018, 44, 1964–1966.
- Liu, Z.-M.; Chen, J.; Kou, Q.; Lin, Q.; Huang, X.; Tang, Z.; Kang, Y.; Li, K.; Zhou, L.; Song, Q.; et al. Terlipressin versus Norepinephrine as Infusion in Patients with Septic Shock: A Multicentre, Randomised, Double-Blinded Trial. Intensive Care Med. 2018, 44, 1816–1825.
- Laterre, P.-F.; Berry, S.M.; Blemings, A.; Carlsen, J.E.; François, B.; Graves, T.; Jacobsen, K.; Lewis, R.J.; Opal, S.M.; Perner, A.; et al. Effect of Selepressin vs Placebo on Ventilator- and Vasopressor-Free Days in Patients with Septic Shock: The SEPSIS-ACT Randomized Clinical Trial. JAMA 2019, 322, 1476–1485.
- Assaf, A. Adhesions after Laparoscopic Myomectomy: Effect of the Technique Used: ADHESIONS AFTER LAPAROSCOPIC MYOMECTOMY. Gynaecol. Endosc. 1999, 8, 225–229.
- Ojeda-Yuren, A.S.; Cerda-Reyes, E.; Herrero-Maceda, M.R.; Castro-Narro, G.; Piano, S. An Integrated Review of the Hepatorenal Syndrome. Ann. Hepatol. 2021, 22, 100236.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801.
- Cohen, J.; Vincent, J.-L.; Adhikari, N.K.J.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A Roadmap for Future Research. Lancet Infect. Dis. 2015, 15, 581–614.
- Vincent, J.-L.; Marshall, J.C.; Ñamendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the Worldwide Burden of Critical Illness: The Intensive Care Over Nations (ICON) Audit. Lancet Respir. Med. 2014, 2, 380–386.
- Gamper, G.; Havel, C.; Arrich, J.; Losert, H.; Pace, N.L.; Müllner, M.; Herkner, H. Vasopressors for Hypotensive Shock. Cochrane Database Syst. Rev. 2016.
- Annane, D.; Vignon, P.; Renault, A.; Bollaert, P.-E.; Charpentier, C.; Martin, C.; Troché, G.; Ricard, J.-D.; Nitenberg, G.; Papazian, L.; et al. Norepinephrine plus Dobutamine versus Epinephrine Alone for Management of Septic Shock: A Randomised Trial. Lancet Lond. Engl. 2007, 370, 676–684.
- Russell, J.A.; Walley, K.R.; Singer, J.; Gordon, A.C.; Hébert, P.C.; Cooper, D.J.; Holmes, C.L.; Mehta, S.; Granton, J.T.; Storms, M.M.; et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock. N. Engl. J. Med. 2008, 358, 877–887.
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients with Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509.
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. N. Engl. J. Med. 2010, 362, 779–789.
- Khanna, A.; English, S.W.; Wang, X.S.; Ham, K.; Tumlin, J.; Szerlip, H.; Busse, L.W.; Altaweel, L.; Albertson, T.E.; Mackey, C.; et al. Angiotensin II for the Treatment of Vasodilatory Shock. N. Engl. J. Med. 2017, 377, 419–430.
- Myburgh, J.A.; Higgins, A.; Jovanovska, A.; Lipman, J.; Ramakrishnan, N.; Santamaria, J. CAT Study investigators A Comparison of Epinephrine and Norepinephrine in Critically Ill Patients. Intensive Care Med. 2008, 34, 2226–2234.
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and Septic Shock—Basics of Diagnosis, Pathophysiology and Clinical Decision Making. Med. Clin. N. Am. 2020, 104, 573–585.
- Levin, M.A.; Lin, H.-M.; Castillo, J.G.; Adams, D.H.; Reich, D.L.; Fischer, G.W. Early On-Cardiopulmonary Bypass Hypotension and Other Factors Associated with Vasoplegic Syndrome. Circulation 2009, 120, 1664–1671.
- Omar, S.; Zedan, A.; Nugent, K. Cardiac Vasoplegia Syndrome: Pathophysiology, Risk Factors and Treatment. Am. J. Med. Sci. 2015, 349, 80–88.
- Argenziano, M.; Chen, J.M.; Choudhri, A.F.; Cullinane, S.; Garfein, E.; Weinberg, A.D.; Smith, C.R.; Rose, E.A.; Landry, D.W.; Oz, M.C. Management of Vasodilatory Shock after Cardiac Surgery: Identification of Predisposing Factors and Use of a Novel Pressor Agent. J. Thorac. Cardiovasc. Surg. 1998, 116, 973–980.
- Nakada, T.-A.; Russell, J.A.; Wellman, H.; Boyd, J.H.; Nakada, E.; Thain, K.R.; Thair, S.A.; Hirasawa, H.; Oda, S.; Walley, K.R. Leucyl/Cystinyl Aminopeptidase Gene Variants in Septic Shock. Chest 2011, 139, 1042–1049.
- Soar, J.; Berg, K.M.; Andersen, L.W.; Böttiger, B.W.; Cacciola, S.; Callaway, C.W.; Couper, K.; Cronberg, T.; D’Arrigo, S.; Deakin, C.D.; et al. Adult Advanced Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2020, 156, A80–A119.
- Holmberg, M.J.; Issa, M.S.; Moskowitz, A.; Morley, P.; Welsford, M.; Neumar, R.W.; Paiva, E.F.; Coker, A.; Hansen, C.K.; Andersen, L.W.; et al. Vasopressors during Adult Cardiac Arrest: A Systematic Review and Meta-Analysis. Resuscitation 2019, 139, 106–121.
- Martikainen, T.J.; Tenhunen, J.J.; Uusaro, A.; Ruokonen, E. The Effects of Vasopressin on Systemic and Splanchnic Hemodynamics and Metabolism in Endotoxin Shock. Anesth. Analg. 2003, 97, 1756–1763.
- Russell, J.A. Bench-to-Bedside Review: Vasopressin in the Management of Septic Shock. Crit. Care Lond. Engl. 2011, 15, 226.
- Dünser, M.W.; Mayr, A.J.; Tür, A.; Pajk, W.; Barbara, F.; Knotzer, H.; Ulmer, H.; Hasibeder, W.R. Ischemic Skin Lesions as a Complication of Continuous Vasopressin Infusion in Catecholamine-Resistant Vasodilatory Shock: Incidence and Risk Factors. Crit. Care Med. 2003, 31, 1394–1398.





