Septic shock is defined by the need for vasopressors to correct hypotension and lactic acidosis secondary to infection, with a high mortality rate. The Surviving Sepsis Campaign guidelines recommend vasopressin as a second-line vasopressor, added to norepinephrine.
- vasopressin
- septic shock
- vasodilatory shock
- vasoplegia
1. Introduction
2. Physiology
2.1. Synthesis and Release
Arginine vasopressin is a little peptide composed of nine amino acids, with arginine occupying the eighth position [8][7]. It is produced by magnocellular neurosecretory neurons located in the anterior hypothalamus that directly function as osmoreceptors. AVP subsequently migrates (as a prohormone) along the supraoptic-hypophyseal tract to the posterior pituitary gland (neurohypophysis), where it is stored in vesicles and is released into the circulation in response to appropriate stimuli. The most important stimuli producing AVP release are mainly an increase in plasma osmolarity and/or decreased blood volume [9][8]. Its half-life is 5–15 min [10][9].2.2. AVP Receptors and Signal Transduction
Three different types of AVP receptors have been identified (Figure 1): V1 (previously known as V1a, mainly vascular), V2 (mainly renal) and V3 (previously V1b, mainly central). Its location and function are summarized in Table 1.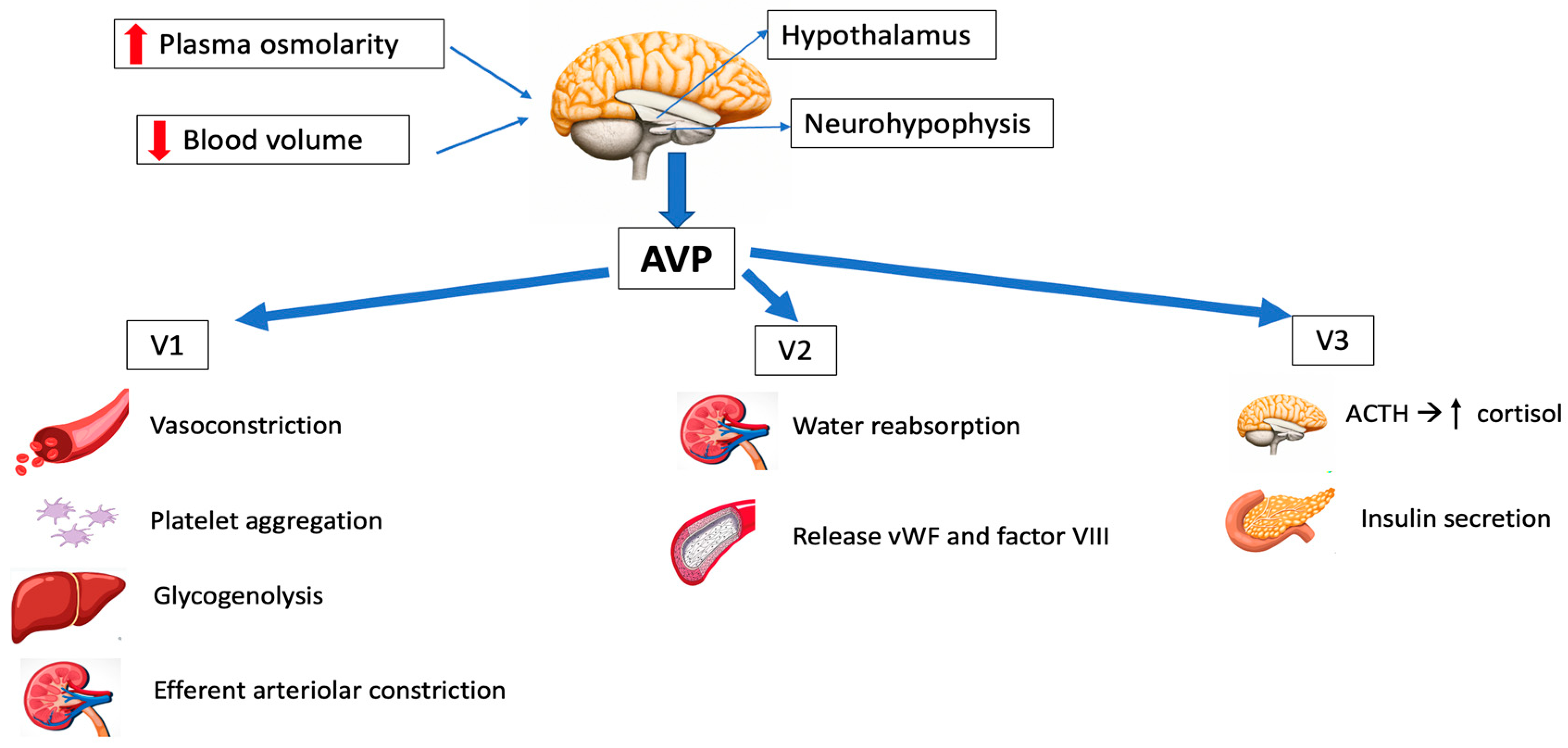
|
VASOPRESSIN AGONISTS |
STRUCTURE |
|||
|---|---|---|---|---|
RECEPTOR AFFINITY |
CLINICAL APPLICATION |
HALF-LIFE (min) |
||
|
|
|||
|
Other risk factors |
| |||
|
ARGININE VASOPRESSIN (AVP) |
8-Arginine vasopressin |
V1, V2, V3 |
Sepsis, vasodilatory shock, cardiac arrest |
5–15 |
|
DESMOPRESSIN ACETATE (DDAVP) |
Deamino-Cys-D-Arg vasopressin |
V2 |
Central diabetes insipidus, bleeding disorders |
90–190 |
|
TERLIPRESSIN (TP) |
N3-triglycyl-8-lysin vasopressin |
V1 |
Portal hypertension, bleeding gastric and esophageal varices, septic shock |
240–360 |
|
SELEPRESSIN |
Phe-2-Ile-3-Hgn-4-Orn-8 vasopressin |
V1 |
Septic shock Not approved for clinical use |
10–30 |
|
ORNIPRESSIN |
8-L-Ornithine vasopressin acetate |
V1 |
Vasoconstricting agent during myomectomy in cirrhosis, as hepatorenal treatment |
60–120 |
2.3. Physiological Functions
AVP has a significant role in osmoregulation, cardiovascular stability and overall homeostasis. Additionally, it acts as a corticotropin secretagogue and has an impact on cognition, learning and memory. However, since AVP does not go through the blood–brain barrier, its central nervous system effects are not relevant in the case of intravenous administration of the drug.- -
-
Osmoregulation
- -
-
Cardiovascular control
- -
-
Corticotropin secretion
- -
-
Hemostasis
3. Pharmacology
AVP requires parenteral administration, since trypsin rapidly hydrolyzes the molecule. It is not protein-bound, and the plasma half-life is 5–15 min, so continuous infusion is necessary. The clearance of AVP is mainly mediated by renal and liver vasopressinases [7,13][12][19], and a little part is eliminated in urine without undergoing changes. Normal plasma concentrations are less than 4 pg/mL. Throughout the years, many efforts have been made to modify AVP and develop analogs with different pharmacological characteristics that could overcome its limitations [13,20][12][20]. These analogs involve the alteration of one or more amino acids in the sequence of the molecule, aiming for longer half-lives and better receptor selectivity (Table 1):-
Arginine vasopressin (AVP) acts on the V1, V2 and V3 receptors, and has been employed in the management of refractory vasodilatory hypotension, cardiac arrest and septic shock. It has the great advantage of having a short half-life, so the dose can be easily titrated.
-
Desmopressin acetate (DDAVP) is a synthetic agonist with V2 receptor specificity and was first used in management of central diabetes insipidus. By directly affecting the endothelial V2 receptors, DDAVP also raises the plasma factor VIII and vWF concentrations in healthy subjects.
-
Terlipressin (TP) has a greater selectivity for the V1 receptor than AVP. It is a prodrug of AVP and undergoes metabolism by exopeptidases to yield the active metabolite lysine vasopressin in the circulation, producing a “slow release” effect and affording a longer biological half-life (6 h). Terlipressin has been used as treatment for bleeding gastric second to esophageal varices, portal hypertension and septic shock. The drug increases blood pressure and improves the outcomes of hepatorenal syndrome (contracting the mesenteric arteries, resulting in decreased portal venous inflow and subsequently lowering portal pressure) [5]. The latest Surviving Sepsis Campaign (SSC) recommendations [21] do not advise its use in patients with septic shock, due to greater undesirable effects (more serious adverse events than NE, especially digital ischemia) [22,23][22][23].
-
Selepressin, another synthetic vasopressin analog, is a short-acting selective V1 receptor agonist. It may present benefits compared to AVP due its ability to induce pure vasoconstriction; it also has reduced antidiuretic effects, a lower risk of thrombotic complications (because of reduced release of vWF) and affords superior protection from increased permeability. However, recently, a trial was stopped due to futility criteria, because no difference was observed in vasopressor- and ventilator-free days [24]. The drug is currently not approved for clinical use.
4. Vasopressin in Septic Shock
Septic shock is the most frequent cause of vasodilatory shock. In 2016, the Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3) defined sepsis as life-threatening organ dysfunction resulting from dysregulated host responses to infection. Septic shock was defined as a subgroup of sepsis in which circulatory and cellular metabolic abnormalities are severe enough to significantly elevate the risk of mortality: despite adequate fluid resuscitation, patients experience hypotension requiring the use of vasopressors and have a raised serum lactate concentration of over 2 mmol/L [27]. Sepsis mortality remains higher (25–30%) [28], and even 40–50% when shock is present [29]. The Surviving Sepsis Campaign (SSC) [21] is a global initiative aimed at improving the management of sepsis. The campaign was launched in 2002 by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine. The SSC initially focused on creating guidelines for the treatment of sepsis, which were first published in 2004 and were updated in 2008, 2012, 2016 and 2021. These guidelines have become widely accepted as the standard of care for sepsis, to be undertaken as a medical emergency. The SSC guidelines provide evidence-based recommendations for the management of septic shock which include source control, antibiotic therapy, fluid resuscitation, vasopressor therapy, supportive care and monitoring and follow-up. Regarding vasopressor therapy, since 2012 the SSC recommended NE as the first-line vasopressor agent (it previously recommended either NE or dopamine) and suggested adding AVP. However, since 2021 the SSC suggests adding AVP early [21], rather than increasing the NE dose (Figure 2).The authors of the SSC recognize that certain evidence implies that AVP might be superior to NE in terms of clinical outcomes. However, due to its higher costs and lesser availability, they consider NE as a first-line agent instead of AVP. A recent Cochrane review found that there was insufficient mortality-related evidence to consider any vasopressor as being superior to others [30]. NE is the first-line vasopressor in septic shock because it has been found to be superior to dopamine and equivalent to AVP and epinephrine in randomized controlled trials (RCTs) [31,32,33,34,35,36][31][32][33][34][35][36] (Table 2).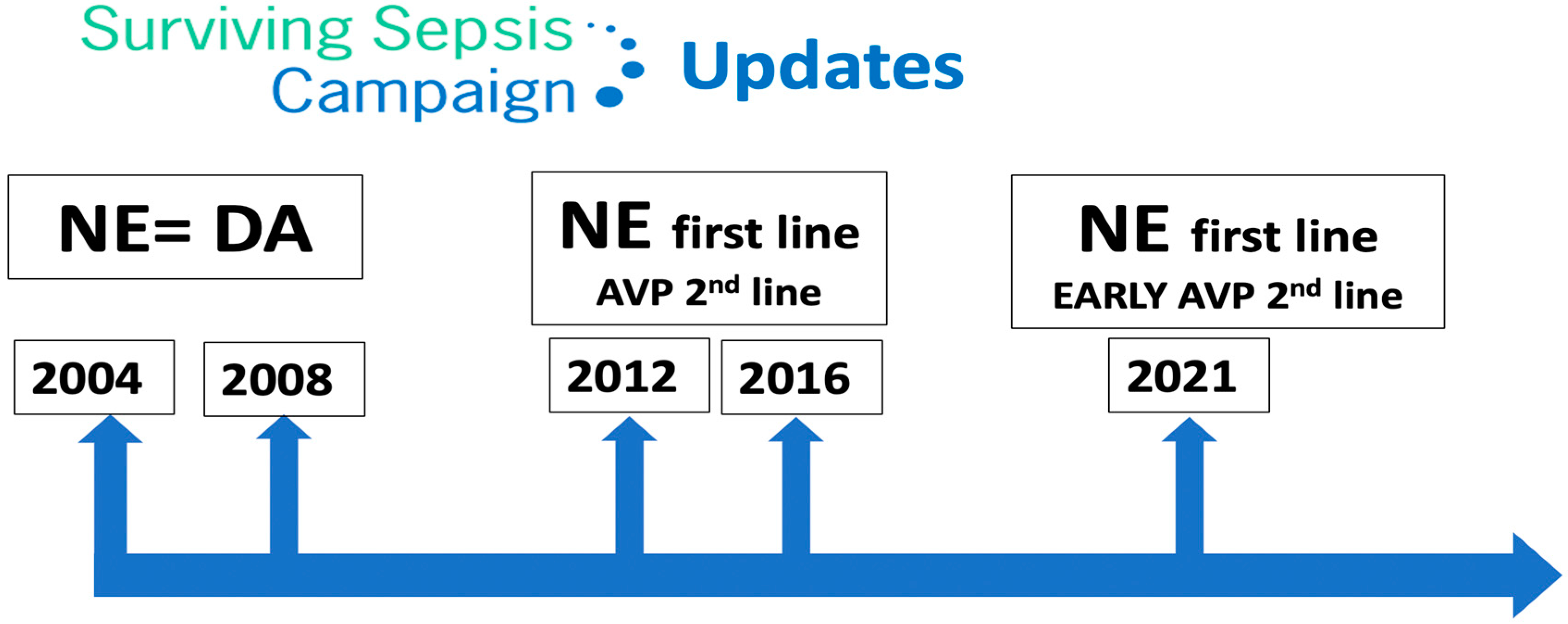 Figure 2.Surviving Sepsis Campaign updates. NE: norepinephrine; DA: dopamine; AVP: arginine vasopressin.Table 2.Pivotal trials of vasopressors in septic shock.
Figure 2.Surviving Sepsis Campaign updates. NE: norepinephrine; DA: dopamine; AVP: arginine vasopressin.Table 2.Pivotal trials of vasopressors in septic shock.TRIAL
Intervention
Control
Intervention 28 Day Mortality
Control 28 Day Mortality
Absolute Difference (95%CI)
p-Value
Increased adenosine levels
-
Ischemia-modified albumin
VASST [32]
Norepinephrine
Vasopressin
35.4%
39.3%
3.9 (−2.9–10.7)
0.26
VANISH [33]
Norepinephrine
Vasopressin
30.9%
27.5%
3.4 (−5.4–12.3)
SOAP II [34]
Norepinephrine
Dopamine
48.5%
52.5%
1.17 (0.97–1.42)
0.10
ATHOS-3 [35]
Angiotensin II
Placebo
46%
54%
Hazard ratio 0.78 (0.57–1.07)
0.12
CAT [36]
Epinephrine
Norepinephrine
23%
27%
Hazard ratio 0.87 (0.48–1.58)
0.65
CATS [37]
Epinephrine
Norepinephrine + dobutamine
40%
34%
Relative risk 0.86 (0.65–1.14)
0.31
5. AVP in Vasodilatory Shock in Heart Surgery
Vasodilatory shock in heart surgery is a well-recognized syndrome occurring in 9–44% of all patients undergoing cardiopulmonary bypass (CPB) procedures, with an associated mortality rate of up to 25% [106][38]. It is also known as vasoplegic syndrome, and like the early stages of septic shock, it is characterized by low mean arterial pressure (<60–65 mmHg), markedly low systemic vascular resistance, a normal or elevated cardiac index and a poor or insufficient response to fluid or catecholamine administration. Among other risk factors (Table 43), vasoplegic syndrome occurs more frequently in populations with congenital heart disease undergoing heart surgery and in patients with heart failure requiring the implantation of a ventricular assist device or heart transplant [107,108][39][40].Table 43.Risk factors associated with vasoplegia in heart surgery.Predominant and independent risk factors
OHT: orthotopic heart transplant; LVAD: left ventricular assist device; CHD: congenital heart disease; VAD: ventricular assist device; ACEIs: angiotensin-converting enzyme inhibitors; CPB: cardiopulmonary bypass; AVP: arginine vasopressin [102][41].
6. AVP in Cardiac Arrest
Historically, both epinephrine and AVP have been used in the context of circulatory arrest. However, the present evidence shows no benefit in using AVP in this setting, and the current international resuscitation guidelines do not recommend AVP as vasopressor therapy [124][42].A systematic review of vasopressors in adult cardiac arrest was conducted by the International Liaison Committee on Resuscitation Advanced Life Support Task Force and published in 2019. Three RCTs comparing AVP versus epinephrine during out-of-hospital cardiac arrest (OHCA) were included. The results showed no significant differences between the groups in terms of return to spontaneous circulation (ROSC), survival to hospital admission, survival to hospital discharge or survival to hospital discharge with favorable neurological outcome. Subgroup analysis based on initial rhythm also did not reveal statistically significant differences. Additionally, this review included three RCTs comparing the combined use of epinephrine and AVP to epinephrine alone during OHCA, and no significant differences were found in terms of ROSC, survival to hospital admission or survival to discharge [125][43].At present, epinephrine is the only recommended vasopressor during cardiac arrest, with a strong grade of recommendation both in situations of shockable rhythm after an unsuccessful first shock and in situations of non-shockable rhythms. There are recommendations against the administration of AVP instead of or in addition to epinephrine during cardiac arrest [124][42].7. Authorized Indications and Dosages in Europe and the United States of America (USA)
The European Medicines Agency (EMA) authorized AVP for the management of catecholamine refractory hypotension following septic shock in patients older than 18 years. AVP should be administered through continuous intravenous infusion of 0.01 IU per minute using a motor pump. Dependent on the clinical response, the dose may be increased every 15–20 min up to a maximum of 0.03 IU/min.On the other hand, the USA authorized AVP for vasodilatory shock in adults (sepsis and post-cardiotomy); for septic shock, it recommends starting with a dose of 0.01 IU/min (maximum 0.07 IU/min), and for post-cardiotomy shock, starting with a dose of 0.03 units/minute (maximum 0.1 IU/min).8. Adverse Effects of AVP
Due to its potent vasoconstrictor effect, there have been concerns about the potential impact of AVP on splanchnic circulation, with a fear of splanchnic ischemia and liver dysfunction [13][12]. Experimental studies have refuted these concerns, demonstrating that with adequate fluid resuscitation, mesenteric blood flow and ileal microcirculation remain preserved [126][44]. Furthermore, clinical trials have not reported any adverse effects upon splanchnic circulation with the use of AVP [126][44].The rate of serious adverse effects was comparable between the AVP and NE groups in the VASST study [81][45]. However, there were more cardiac arrests in patients in the NE group and higher occurrence of digital ischemia and hyponatremia with the AVP group. Other side effects of both AVP and NE include reduced cardiac output, skin necrosis and intestinal ischemia.AVP induces vasoconstriction in cutaneous blood vessels, and this effect is dose-dependent. A retrospective study found that nearly one-third of patients exposed to AP experienced ischemic skin lesions [127][46]. Risk factors associated with the development of ischemic cutaneous lesions included being overweight, receiving a high dose of NE, receiving platelets and fresh frozen plasma transfusions, having a history of peripheral arterial occlusive disease and the occurrence of septic shock. After conducting a multivariate analysis, only the latter two factors remained associated with the occurrence of cutaneous complications.
References
- Oliver, G.; Schäfer, E.A. On the Physiological Action of Extracts of Pituitary Body and Certain Other Glandular Organs: Preliminary Communication. J. Physiol. 1895, 18, 277–279.
- Dünser, M.W.; Lindner, K.H.; Wenzel, V. A Century of Arginine Vasopressin Research Leading to New Therapeutic Strategies. Anesthesiology 2006, 105, 444–445.
- Czakó, L.; Mezei, G.; László, F.A. Treatment of Diabetes Insipidus with 1-Deamino-8-d-Arginine Vasopressin. Acta Med. Acad. Sci. Hung. 1975, 32, 75–84.
- Kehne, J.H.; Hughes, F.A.; Gompertz, M.L. The Use of Surgical Pituitrin in the Control of Esophageal Varix Bleeding; an Experimental Study and Report of Two Cases. Surgery 1956, 39, 917–925.
- Park, K.S.; Yoo, K.Y. Role of Vasopressin in Current Anesthetic Practice. Korean J. Anesthesiol. 2017, 70, 245.
- Sharshar, T.; Blanchard, A.; Paillard, M.; Raphael, J.C.; Gajdos, P.; Annane, D. Circulating Vasopressin Levels in Septic Shock. Crit. Care Med. 2003, 31, 1752–1758.
- Treschan, T.A.; Peters, J.; Warltier, D.C. The Vasopressin System. Anesthesiology 2006, 105, 599–612.
- Sklar, A.H.; Schrier, R.W. Central Nervous System Mediators of Vasopressin Release. Physiol. Rev. 1983, 63, 1243–1280.
- Sai, Y.; Okamura, T.; Amakata, Y.; Toda, N. Comparison of Responses of Canine Pulmonary Artery and Vein to Angiotensin II, Bradykinin and Vasopressin. Eur. J. Pharmacol. 1995, 282, 235–241.
- Evora, P.R.B.; Pearson, P.J.; Rodrigues, A.J.; Viaro, F.; Schaff, H.V. Effect of Arginine Vasopressin on the Canine Epicardial Coronary Artery: Experiments on V1-Receptor-Mediated Production of Nitric Oxide. Arq. Bras. Cardiol. 2003, 80, 483–494.
- Rotondo, F.; Butz, H.; Syro, L.V.; Yousef, G.M.; Di Ieva, A.; Restrepo, L.M.; Quintanar-Stephano, A.; Berczi, I.; Kovacs, K. Arginine Vasopressin (AVP): A Review of Its Historical Perspectives, Current Research and Multifunctional Role in the Hypothalamo-Hypophysial System. Pituitary 2016, 19, 345–355.
- Demiselle, J.; Fage, N.; Radermacher, P.; Asfar, P. Vasopressin and Its Analogues in Shock States: A Review. Ann. Intensive Care 2020, 10, 9.
- Birnbaumer, M. The V2 Vasopressin Receptor Mutations and Fluid Homeostasis. Cardiovasc. Res. 2001, 51, 409–415.
- Pivonello, R.; De Bellis, A.; Faggiano, A.; Di Salle, F.; Petretta, M.; Di Somma, C.; Perrino, S.; Altucci, P.; Bizzarro, A.; Bellastella, A.; et al. Central Diabetes Insipidus and Autoimmunity: Relationship between the Occurrence of Antibodies to Arginine Vasopressin-Secreting Cells and Clinical, Immunological, and Radiological Features in a Large Cohort of Patients with Central Diabetes Insipidus of Known and Unknown Etiology. J. Clin. Endocrinol. Metab. 2003, 88, 1629–1636.
- Christ-Crain, M.; Gaisl, O. Diabetes Insipidus. Presse Medicale Paris Fr. 2021, 50, 104093.
- Schurr, J.W.; Szumita, P.M.; DeGrado, J.R. Neuroendocrine Derangements in Early Septic Shock: Pharmacotherapy for Relative Adrenal and Vasopressin Insufficiency. Shock Augusta Ga 2017, 48, 284–293.
- Russell, J.A.; Walley, K.R. Vasopressin and Its Immune Effects in Septic Shock. J. Innate Immun. 2010, 2, 446–460.
- Cattaneo, M. Review of Clinical Experience of Desmopressin in Patients with Congenital and Acquired Bleeding Disorders. Eur. J. Anaesthesiol. Suppl. 1997, 14, 10–14; discussion 14–18.
- Holmes, C.L.; Patel, B.M.; Russell, J.A.; Walley, K.R. Physiology of Vasopressin Relevant to Management of Septic Shock. Chest 2001, 120, 989–1002.
- Glavaš, M.; Gitlin-Domagalska, A.; Dębowski, D.; Ptaszyńska, N.; Łęgowska, A.; Rolka, K. Vasopressin and Its Analogues: From Natural Hormones to Multitasking Peptides. Int. J. Mol. Sci. 2022, 23, 3068.
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247.
- Mårtensson, J.; Gordon, A.C. Terlipressin or Norepinephrine, or Both in Septic Shock? Intensive Care Med. 2018, 44, 1964–1966.
- Liu, Z.-M.; Chen, J.; Kou, Q.; Lin, Q.; Huang, X.; Tang, Z.; Kang, Y.; Li, K.; Zhou, L.; Song, Q.; et al. Terlipressin versus Norepinephrine as Infusion in Patients with Septic Shock: A Multicentre, Randomised, Double-Blinded Trial. Intensive Care Med. 2018, 44, 1816–1825.
- Laterre, P.-F.; Berry, S.M.; Blemings, A.; Carlsen, J.E.; François, B.; Graves, T.; Jacobsen, K.; Lewis, R.J.; Opal, S.M.; Perner, A.; et al. Effect of Selepressin vs Placebo on Ventilator- and Vasopressor-Free Days in Patients with Septic Shock: The SEPSIS-ACT Randomized Clinical Trial. JAMA 2019, 322, 1476–1485.
- Assaf, A. Adhesions after Laparoscopic Myomectomy: Effect of the Technique Used: ADHESIONS AFTER LAPAROSCOPIC MYOMECTOMY. Gynaecol. Endosc. 1999, 8, 225–229.
- Ojeda-Yuren, A.S.; Cerda-Reyes, E.; Herrero-Maceda, M.R.; Castro-Narro, G.; Piano, S. An Integrated Review of the Hepatorenal Syndrome. Ann. Hepatol. 2021, 22, 100236.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801.
- Cohen, J.; Vincent, J.-L.; Adhikari, N.K.J.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A Roadmap for Future Research. Lancet Infect. Dis. 2015, 15, 581–614.
- Vincent, J.-L.; Marshall, J.C.; Ñamendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the Worldwide Burden of Critical Illness: The Intensive Care Over Nations (ICON) Audit. Lancet Respir. Med. 2014, 2, 380–386.
- Gamper, G.; Havel, C.; Arrich, J.; Losert, H.; Pace, N.L.; Müllner, M.; Herkner, H. Vasopressors for Hypotensive Shock. Cochrane Database Syst. Rev. 2016.
- Annane, D.; Vignon, P.; Renault, A.; Bollaert, P.-E.; Charpentier, C.; Martin, C.; Troché, G.; Ricard, J.-D.; Nitenberg, G.; Papazian, L.; et al. Norepinephrine plus Dobutamine versus Epinephrine Alone for Management of Septic Shock: A Randomised Trial. Lancet Lond. Engl. 2007, 370, 676–684.
- Russell, J.A.; Walley, K.R.; Singer, J.; Gordon, A.C.; Hébert, P.C.; Cooper, D.J.; Holmes, C.L.; Mehta, S.; Granton, J.T.; Storms, M.M.; et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock. N. Engl. J. Med. 2008, 358, 877–887.
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients with Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509.
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.-L. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. N. Engl. J. Med. 2010, 362, 779–789.
- Khanna, A.; English, S.W.; Wang, X.S.; Ham, K.; Tumlin, J.; Szerlip, H.; Busse, L.W.; Altaweel, L.; Albertson, T.E.; Mackey, C.; et al. Angiotensin II for the Treatment of Vasodilatory Shock. N. Engl. J. Med. 2017, 377, 419–430.
- Myburgh, J.A.; Higgins, A.; Jovanovska, A.; Lipman, J.; Ramakrishnan, N.; Santamaria, J. CAT Study investigators A Comparison of Epinephrine and Norepinephrine in Critically Ill Patients. Intensive Care Med. 2008, 34, 2226–2234.
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and Septic Shock—Basics of Diagnosis, Pathophysiology and Clinical Decision Making. Med. Clin. N. Am. 2020, 104, 573–585.
- Levin, M.A.; Lin, H.-M.; Castillo, J.G.; Adams, D.H.; Reich, D.L.; Fischer, G.W. Early On-Cardiopulmonary Bypass Hypotension and Other Factors Associated with Vasoplegic Syndrome. Circulation 2009, 120, 1664–1671.
- Omar, S.; Zedan, A.; Nugent, K. Cardiac Vasoplegia Syndrome: Pathophysiology, Risk Factors and Treatment. Am. J. Med. Sci. 2015, 349, 80–88.
- Argenziano, M.; Chen, J.M.; Choudhri, A.F.; Cullinane, S.; Garfein, E.; Weinberg, A.D.; Smith, C.R.; Rose, E.A.; Landry, D.W.; Oz, M.C. Management of Vasodilatory Shock after Cardiac Surgery: Identification of Predisposing Factors and Use of a Novel Pressor Agent. J. Thorac. Cardiovasc. Surg. 1998, 116, 973–980.
- Nakada, T.-A.; Russell, J.A.; Wellman, H.; Boyd, J.H.; Nakada, E.; Thain, K.R.; Thair, S.A.; Hirasawa, H.; Oda, S.; Walley, K.R. Leucyl/Cystinyl Aminopeptidase Gene Variants in Septic Shock. Chest 2011, 139, 1042–1049.
- Soar, J.; Berg, K.M.; Andersen, L.W.; Böttiger, B.W.; Cacciola, S.; Callaway, C.W.; Couper, K.; Cronberg, T.; D’Arrigo, S.; Deakin, C.D.; et al. Adult Advanced Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2020, 156, A80–A119.
- Holmberg, M.J.; Issa, M.S.; Moskowitz, A.; Morley, P.; Welsford, M.; Neumar, R.W.; Paiva, E.F.; Coker, A.; Hansen, C.K.; Andersen, L.W.; et al. Vasopressors during Adult Cardiac Arrest: A Systematic Review and Meta-Analysis. Resuscitation 2019, 139, 106–121.
- Martikainen, T.J.; Tenhunen, J.J.; Uusaro, A.; Ruokonen, E. The Effects of Vasopressin on Systemic and Splanchnic Hemodynamics and Metabolism in Endotoxin Shock. Anesth. Analg. 2003, 97, 1756–1763.
- Russell, J.A. Bench-to-Bedside Review: Vasopressin in the Management of Septic Shock. Crit. Care Lond. Engl. 2011, 15, 226.
- Dünser, M.W.; Mayr, A.J.; Tür, A.; Pajk, W.; Barbara, F.; Knotzer, H.; Ulmer, H.; Hasibeder, W.R. Ischemic Skin Lesions as a Complication of Continuous Vasopressin Infusion in Catecholamine-Resistant Vasodilatory Shock: Incidence and Risk Factors. Crit. Care Med. 2003, 31, 1394–1398.
