
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pia Giovannelli | -- | 2723 | 2023-10-19 11:49:08 | | | |
| 2 | Peter Tang | Meta information modification | 2723 | 2023-10-20 04:35:56 | | |
Video Upload Options
Pancreatic cancer (PaC) is one of the most lethal tumors worldwide, difficult to diagnose, and with inadequate therapeutical chances. The most used therapy is gemcitabine, alone or in combination with nanoparticle albumin-bound paclitaxel (nab-paclitaxel), and the multidrug FOLFIRINOX. Unfortunately, PaC develops resistance early, thus reducing the already poor life expectancy of patients. The mechanisms responsible for drug resistance are not fully elucidated, and exosomes seem to be actively involved in this phenomenon, thanks to their ability to transfer molecules regulating this process from drug-resistant to drug-sensitive PaC cells. These extracellular vesicles are released by both normal and cancer cells and seem to be essential mediators of intercellular communications, especially in cancer, where they are secreted at very high numbers.
1. Introduction
2. Pancreatic Cancer and Pharmacological Approaches
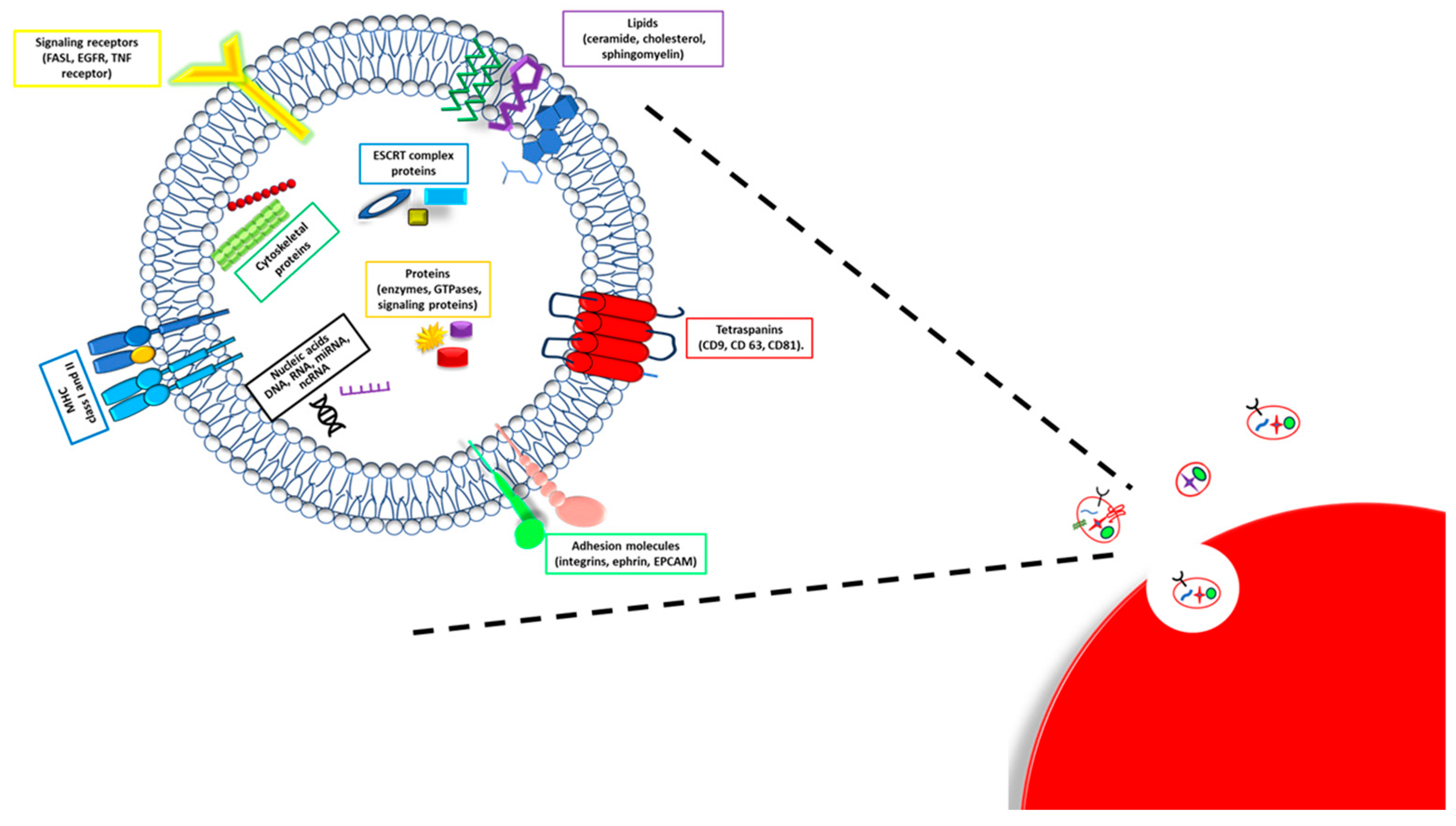
3. Exosomes Composition
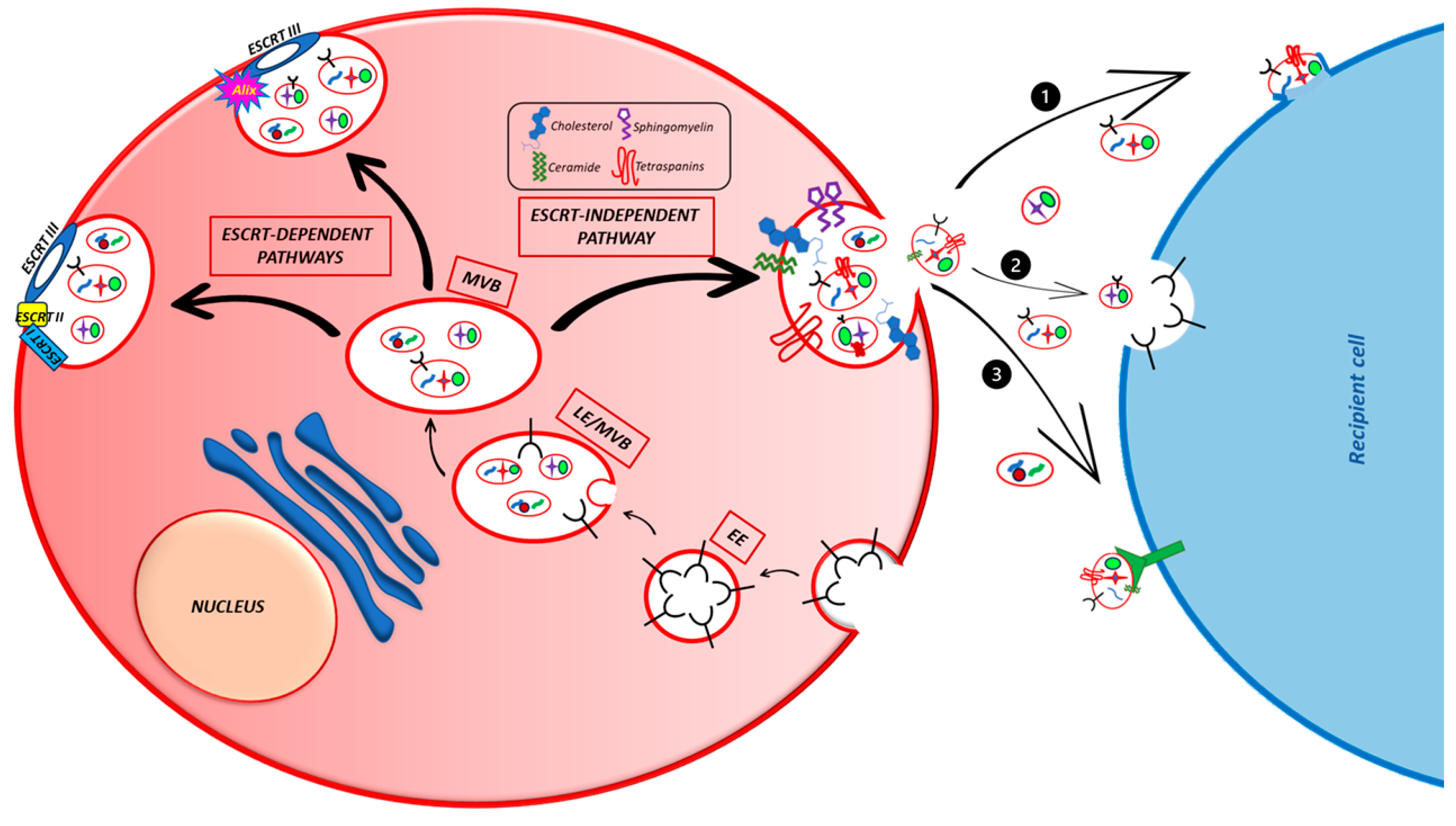
4. Exosomes Biogenesis and Secretion
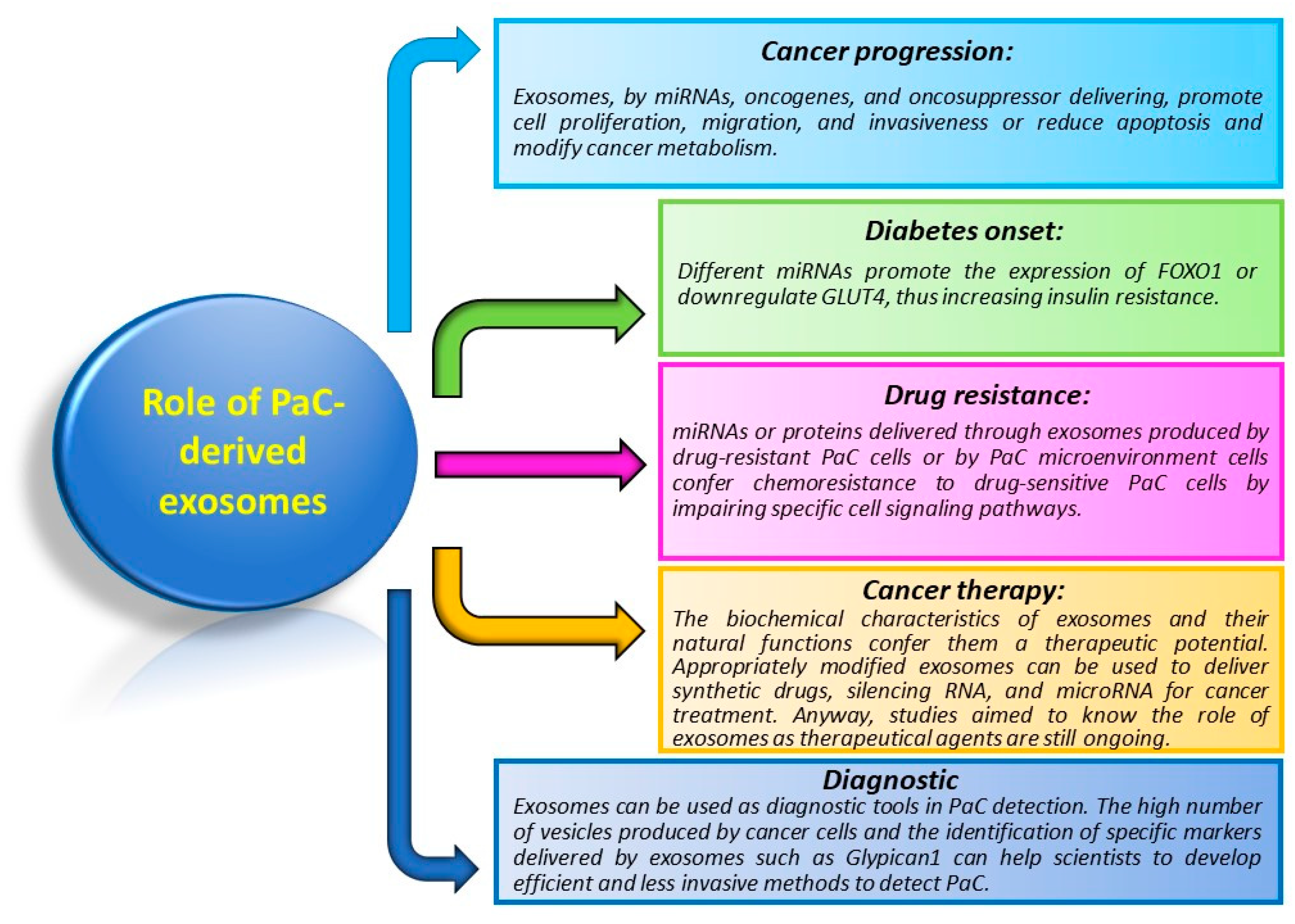
5. Role of Exosomes in PaC
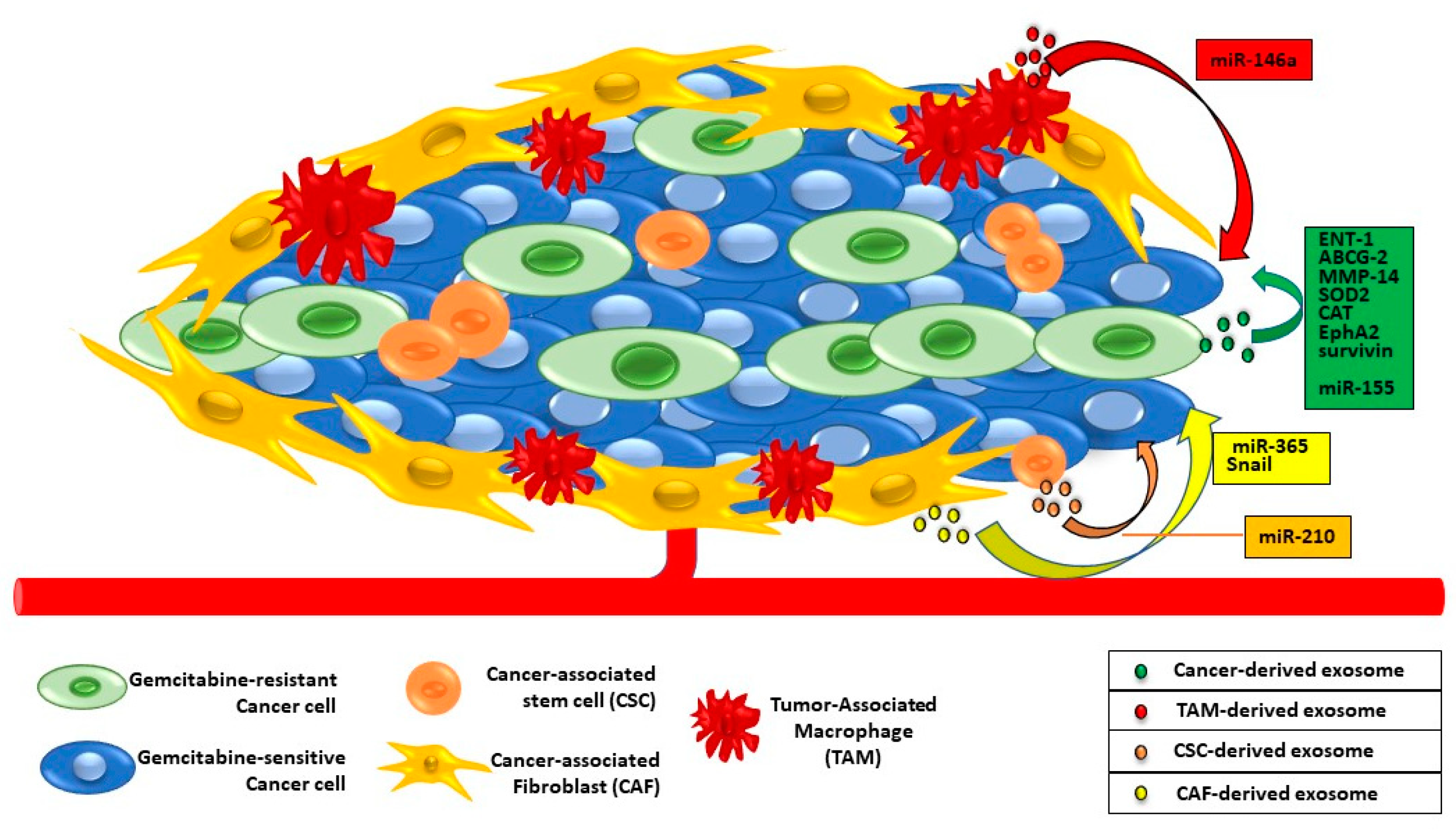
6. Exosomes and PaC Drug Resistance
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27.
- Yu, S.; Zhang, C.; Xie, K.-P. Therapeutic Resistance of Pancreatic Cancer: Roadmap to Its Reversal. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188461.
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 Identifies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature 2015, 523, 177–182.
- Jiang, Z.; Wang, H.; Mou, Y.; Li, L.; Jin, W. Functions and Clinical Applications of Exosomes in Pancreatic Cancer. Mol. Biol. Rep. 2022, 49, 11037–11048.
- Soloff, E.V.; Zaheer, A.; Meier, J.; Zins, M.; Tamm, E.P. Staging of Pancreatic Cancer: Resectable, Borderline Resectable, and Unresectable Disease. Abdom. Radiol. 2018, 43, 301–313.
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic Cancer. Lancet 2020, 395, 2008–2020.
- Shaya, J.; Kato, S.; Adashek, J.J.; Patel, H.; Fanta, P.T.; Botta, G.P.; Sicklick, J.K.; Kurzrock, R. Personalized Matched Targeted Therapy in Advanced Pancreatic Cancer: A Pilot Cohort Analysis. Npj Genom. Med. 2023, 8, 1.
- Leroux, C.; Konstantinidou, G. Targeted Therapies for Pancreatic Cancer: Overview of Current Treatments and New Opportunities for Personalized Oncology. Cancers 2021, 13, 799.
- West, H.J.; Jin, J.O. Performance Status in Patients With Cancer. JAMA Oncol. 2015, 1, 998.
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for Locally Advanced Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. Lancet Oncol. 2016, 17, 801–810.
- Hammel, P.; Lacy, J.; Portales, F.; Sobrero, A.F.; Pazo Cid, R.A.; Manzano Mozo, J.L.; Terrebonne, E.; Dowden, S.D.; Shiansong Li, J.; Ong, T.J.; et al. Phase II LAPACT Trial of Nab-Paclitaxel (Nab-P) plus Gemcitabine (G) for Patients with Locally Advanced Pancreatic Cancer (LAPC). J. Clin. Oncol. 2018, 36, 204.
- Hammel, P.; Huguet, F.; van Laethem, J.-L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844.
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825.
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703.
- The ASCO Post Staff. FDA Approves Olaparib for gBRCAm Metastatic Pancreatic Adenocarcinoma; The ASCO Post Staff: Huntington, NY, USA, 2019.
- Yu, H.-Y.; Lee, C.-Y.; Lin, L.-G.; Chao, Y.; Li, C.-P. Nanoliposomal Irinotecan with 5-Fluorouracil and Folinic Acid in Metastatic Pancreatic Cancer after Previous Gemcitabine-Based Therapy: A Real-World Experience. J. Chin. Med. Assoc. 2021; publish ahead of print.
- Rädler, J.; Gupta, D.; Zickler, A.; Andaloussi, S.E. Exploiting the Biogenesis of Extracellular Vesicles for Bioengineering and Therapeutic Cargo Loading. Mol. Ther. 2023, 31, 1231–1250.
- Kalluri, R.; LeBleu, V.S. The Biology Function and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977.
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.m.; Molaei, F.; Alahari, S.K. Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol. Cancer 2019, 18, 75.
- Robinson, S.M.; Fan, L.; White, S.A.; Charnley, R.M.; Mann, J. The Role of Exosomes in the Pathogenesis of Pancreatic Ductal Adenocarcinoma. Int. J. Biochem. Cell Biol. 2016, 75, 131–139.
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337.
- Henderson, M.C.; Azorsa, D.O. The Genomic and Proteomic Content of Cancer Cell-Derived Exosomes. Front. Oncol. 2012, 2, 38.
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145.
- Hagey, D.W.; Kordes, M.; Görgens, A.; Mowoe, M.O.; Nordin, J.Z.; Moro, C.F.; Löhr-Matthias, J.; El Andaloussi, S. Extracellular Vesicles Are the Primary Source of Blood-borne Tumour-derived Mutant KRAS DNA Early in Pancreatic Cancer. J. Extracell. Vesicles 2021, 10, e12142.
- Sexton, R.E.; Mpilla, G.; Kim, S.; Philip, P.A.; Azmi, A.S. Ras and Exosome Signaling. Semin. Cancer Biol. 2019, 54, 131–137.
- Buenafe, A.C.; Dorrell, C.; Reddy, A.P.; Klimek, J.; Marks, D.L. Proteomic Analysis Distinguishes Extracellular Vesicles Produced by Cancerous versus Healthy Pancreatic Organoids. Sci. Rep. 2022, 12, 3556.
- Bastos, N.; Ruivo, C.F.; da Silva, S.; Melo, S.A. Exosomes in Cancer: Use Them or Target Them? Semin. Cell Dev. Biol. 2018, 78, 13–21.
- Graham, T.R.; Kozlov, M.M. Interplay of Proteins and Lipids in Generating Membrane Curvature. Curr. Opin. Cell Biol. 2010, 22, 430–436.
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of Exosome Production and Cargo Sorting. Int. J. Biol. Sci. 2021, 17, 163–177.
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 1126, 5553–5565.
- Guan, L.; Wu, B.; Li, T.; Beer, L.A.; Sharma, G.; Li, M.; Lee, C.N.; Liu, S.; Yang, C.; Huang, L.; et al. HRS Phosphorylation Drives Immunosuppressive Exosome Secretion and Restricts CD8+ T-Cell Infiltration into Tumors. Nat. Commun. 2022, 13, 4078.
- Ferreira, J.V.; da Rosa Soares, A.; Ramalho, J.; Máximo Carvalho, C.; Cardoso, M.H.; Pintado, P.; Carvalho, A.S.; Beck, H.C.; Matthiesen, R.; Zuzarte, M.; et al. LAMP2A Regulates the Loading of Proteins into Exosomes. Sci. Adv. 2022, 8, eabm1140.
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 Controls the Sorting of miRNAs into Exosomes through Binding to Specific Motifs. Nat. Commun. 2013, 4, 2980.
- Cha, D.J.; Franklin, J.L.; Dou, Y.; Liu, Q.; Higginbotham, J.N.; Demory Beckler, M.; Weaver, A.M.; Vickers, K.; Prasad, N.; Levy, S.; et al. KRAS-Dependent Sorting of miRNA to Exosomes. eLife 2015, 4, e07197.
- McKenzie, A.J.; Hoshino, D.; Hong, N.H.; Cha, D.J.; Franklin, J.L.; Coffey, R.J.; Patton, J.G.; Weaver, A.M. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016, 15, 978–987.
- De Lellis, L.; Florio, R.; Di Bella, M.C.; Brocco, D.; Guidotti, F.; Tinari, N.; Grassadonia, A.; Lattanzio, R.; Cama, A.; Veschi, S. Exosomes as Pleiotropic Players in Pancreatic Cancer. Biomedicines 2021, 9, 275.
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA Packaging into Extracellular Vesicles: An Orchestra of RNA-binding Proteins? J. Extracell. Vesicles 2020, 10, e12043.
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 Marks and Controls an ESCRT-Independent Exosome Pathway. Cell Res. 2021, 31, 157–177.
- Feng, D.; Zhao, W.-L.; Ye, Y.-Y.; Bai, X.-C.; Liu, R.-Q.; Chang, L.-F.; Zhou, Q.; Sui, S.-F. Cellular Internalization of Exosomes Occurs Through Phagocytosis. Traffic 2010, 11, 675–687.
- Tian, T.; Zhu, Y.-L.; Zhou, Y.-Y.; Liang, G.-F.; Wang, Y.-Y.; Hu, F.-H.; Xiao, Z.-D. Exosome Uptake through Clathrin-Mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery. J. Biol. Chem. 2014, 289, 22258–22267.
- Adamska, A.; Elaskalani, O.; Emmanouilidi, A.; Kim, M.; Abdol Razak, N.B.; Metharom, P.; Falasca, M. Molecular and Cellular Mechanisms of Chemoresistance in Pancreatic Cancer. Adv. Biol. Regul. 2018, 68, 77–87.
- Tsukasa, K.; Ding, Q.; Yoshimitsu, M.; Miyazaki, Y.; Matsubara, S.; Takao, S. Slug Contributes to Gemcitabine Resistance through Epithelial-Mesenchymal Transition in CD133+ Pancreatic Cancer Cells. Hum. Cell 2015, 28, 167–174.
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes Derived from Cancer Stem Cells of Gemcitabine-Resistant Pancreatic Cancer Cells Enhance Drug Resistance by Delivering miR-210. Cell. Oncol. 2020, 43, 123–136.
- Fan, J.; Wei, Q.; Koay, E.J.; Liu, Y.; Ning, B.; Bernard, P.W.; Zhang, N.; Han, H.; Katz, M.H.; Zhao, Z.; et al. Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer. Theranostics 2018, 8, 5986–5994.
- Patel, G.K.; Khan, M.A.; Bhardwaj, A.; Srivastava, S.K.; Zubair, H.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes Confer Chemoresistance to Pancreatic Cancer Cells by Promoting ROS Detoxification and miR-155-Mediated Suppression of Key Gemcitabine-Metabolising Enzyme, DCK. Br. J. Cancer 2017, 116, 609–619.




