2. Pancreatic Cancer and Pharmacological Approaches
PaC is an underhanded pathology with a poor prognosis. The paucity of specific symptoms hinders the diagnosis, which is often made very late, when surgical resection is no longer possible. Depending on the extent of the disease, most clinicians use a four-tiered staging system: resectable, borderline resectable, locally advanced, and metastatic disease [
5]. For each stage of PaC, specific pharmacological treatments are actionable. Gemcitabine in mono- or combo therapy with nab-paclitaxel, or nab-paclitaxel alone or with cisplatin and capecitabine, or even the multidrug FOLFIRINOX are prevalently used [
6]. Besides these cytotoxic treatments, other drugs are usable to treat PaC, such as Trametinib, Palbociclib, Trastuzumab, Bevacizumab, Vismodegib, and PEGPH20 [
7,
8]. Some of these are studied in clinical trials and are already approved by the Food and Drug Administration (FDA). Unfortunately, their use is limited to some PaC subtypes or patients [
7] that must have molecular and genetic testing to check if their mutations are specifically targeted by these therapies [
7,
8].
Both resectable and borderline resectable PaCs are treated with a postoperative systemic therapy setting, and different trials demonstrated that 6 months of multidrug FOLFIRINOX treatment is the most efficient therapy, increasing the median overall survival (MOS) from 35 to 54.4 months and the disease-free survival (DFS) from 12.8 to 21.6 months [
6]. Anyway, gemcitabine, alone or combined with capecitabine, remains the treatment of choice for patients that do not tolerate multidrug FOLFIRINOX [
6].
Locally advanced cancer differs from the first two subtypes for an extensive vascular involvement that precludes surgical resection. Depending on the performance status of the patient and their tolerance to the treatment, this kind of PaC is commonly treated with gemcitabine, with gemcitabine plus nab-paclitaxel, or with FOLFIRINOX [
9,
10,
11]. The support of systemic chemotherapy (gemcitabine in the absence or presence of erlotinib) with radiotherapy does not absolutely prolong the patient’s OS [
12].
Unfortunately, metastatic disease is diagnosed in at least 50% of PaC patients. At that stage, chemotherapy remains the only chance and it is used palliatively for cancer-related symptoms and to prolong the life expectancy. Even in this PaC clinical subtype, FOLFIRINOX is the most efficient first-line therapy, because it improves the OS from 6.8 to 11.1 months as compared with gemcitabine [
13]. Nonetheless, even in this case, gemcitabine remains the elite treatment for debilitated patients. A first-line three-phase study, comparing gemcitabine versus gemcitabine plus-paclitaxel, demonstrated that patients treated with the combined therapy showed an OS of 8.7 months compared with the 6.7 months OS of patients treated with the monotherapy [
14]. The therapy with Olaparib, a PARP inhibitor, is successfully used in BRCA1- and BRCA2-positive metastatic PaC [
15]. This represents the first biomarker-based therapy in PaC. In metastatic PaC, gemcitabine is also used as second-line therapy in patients who had progressed on first-line FOLFIRINOX, while a combination of fluorouracil plus leucovorin with nano-liposomal irinotecan is used in patients who had progressed on first-line gemcitabine-based therapy [
6,
16].
Despite the significant number of therapeutical drugs used in PaC therapy, this cancer often develops resistance. The mechanisms leading to chemoresistance are various, ranging from genetic factors to the tumor microenvironment influence, without neglecting the most recent discoveries about the ability of exosomes in inducing drug resistance. This aspect will be extensively discussed in the subsequent sections of this review.
3. Exosomes Composition
Exosomes, a particular and specific group of extracellular vesicles (EVs), are released by different cell types, and much of the evidence indicates that cancer cells produce a higher number of exosomes as compared with normal cells [
17,
18,
19]. These vesicles substantially differ in the formation process from the ectosomes, a second broad group of EVs [
17]. While ectosomes are generated through the budding of the plasma membrane, exosomes are of endosomal origin and are initially produced as multivesicular bodies (MVBs), whose cargo is influenced by their interactions with intracellular organelles and vesicles [
17,
18,
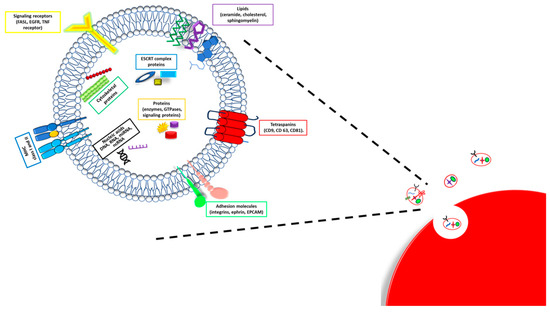
19]. Even if collected in one unique group, the exosome size and composition substantially vary. They show a diameter ranging from 30 to 150 nm and are made up of a lipidic bilayer-enveloping cytosol without organelles but enriched with protein and nucleic acids (
Figure 1) [
18].
Figure 1. Exosome composition. A schematic illustration of exosome structure and composition.
The bilayer presents a lipidic part composed of sphingomyelin, cholesterol, phosphatidylserine, ceramide, and a protein fraction. In particular, the proteins expressed on the exosome membrane are responsible for the selection of target cells and the exosome uptake [
20]. Different proteins are distributed inside and on the membrane surface of exosomes; some among them are specific types of exosomes and characteristics of the origin cells and tissues, while others are nonspecific types of exosome proteins present in all exosomes, regardless of the origin tissues. Specific types of exosome proteins are adhesion molecules (integrins, ephrin, and, mostly, the epithelial cell adhesion molecule—EPCAM), specific tetraspanins (CD9, CD63, and CD81), major histocompatibility complex (MHC) I and II class molecules and growth factors [
19], and also heat shock, cytoskeleton, apoptotic, and cell signaling proteins. Nonspecific types of exosome proteins are, instead, components of the exosome biogenesis process, such as Rab GTPases (Rab2 and Rab7), ALIX, and flotillin [
19].
Exosome content is also made up of different kinds of DNA and RNA molecules, including single- and double-stranded DNA (ssDNA and dsDNA); messenger, transfer, and ribosomal RNA (mRNA, tRNA, and rRNA); microRNA (miRNA); and non-coding RNA (ncRNA) [
19,
21]. In sum, the composition of exosomes mainly reflects the parental cells and origin tissue, except for the proteins involved in their biogenesis, which are shared by all the exosomes.
Exosomes can transport the genomic and proteomic signatures typical of the tumor cells from which they derive. These unique signatures make exosomes ideal for cancer detection but, mainly, confer to exosomes derived from cancer cells the ability to promote cancer progression and transform healthy cells [
22,
23]. Oncogenes associated with various cancers can often be found in the exosomes secreted by tumor cells [
22] as happens for KRAS
+, present at a high concentration in exosomes from patients with PaC [
24,
25,
26].
4. Exosomes Biogenesis and Secretion
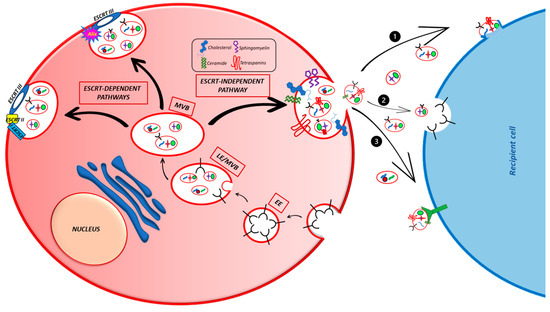
Exosomes are naturally and constitutively secreted into the extracellular space by both the healthy and sick cells of living beings, where they allow intercellular communication and take part in a wide variety of physiological or pathological processes, such as the immune response, neuronal communication, organ or cancer development, and growth. The formation process of PaC-derived exosomes is the same as the other cells and is tightly associated with the endocytic pathway (Figure 2).
Figure 2. Principal mechanisms of exosome formation in cells. The figure illustrates the ESCRT-dependent and -independent pathways involved in exosome formation and the three ways used by exosomes to enter the target cells. (1) Direct fusion with the plasma membrane; (2) endocytosis; (3) direct interaction with plasma membrane receptors.
The first step for exosome generation is the formation of an endocytic vesicle containing extracellular components and its fusion with an early endosome (EE) [
18]. The EE follows two different fates: it might become recycling exosomes, thereby fusing with the plasma membrane and returning the cargo to it, or, after different maturation processes, it might differentiate into a late endosome (LE) and, lastly, into a multivesicular body (MVB) [
19,
21]. The MVB contains a certain number of intraluminal vesicles (ILVs), formed through the invagination of the membrane of the EEs, by the cargo sequestration and its distribution into the vesicles. ILVs are the effective exosome precursors [
32] and their biogenesis is mainly regulated by different mechanisms, generally divided into Endosomal Sorting Complexes Required for Transport (ESCRT)-dependent and ESCRT-independent [
18,
19,
21]. The ESCRT-mediated ILVs biogenesis depends on a sequence of processes coordinated by the four ESCRT or other support proteins such as clathrin, epsin-15, and Alix [
19] and leads, through the membrane remodeling, to the vesicles sprouting.
In the ESCRT-independent mechanisms, different molecules (tetraspanins, membrane sphingolipids, ceramides, the ceramide transport protein (CERT), and the Gi-coupled S1P1 receptor) take part in the ILV formation at various levels. Among them, tetraspanins are generally involved in more than one step of this process, such as the membrane compartmentalization into functional domains or cargo sorting [
19,
21]. Both sphingolipids and ceramide are particularly involved in membrane deformation, while CERT helps the ceramides to translocate from the Golgi and the endoplasmic reticulum to the endosomes, allowing for the membrane curvature, a local geometrical characteristic that typifies the membrane shape [
33]. Recent studies have shown that Rab31 and S1P1 are also involved in ESCRT-independent exosome formation. Rab31 is a GTPase that, once activated, induces the membrane budding, while the Gi S1P1 receptor is involved in MVB maturation [
21].
Before the formation of the vesicle, the cell picks out the appropriate cargo through a sorting process, different for each class of molecules carried by exosomes [
17,
21,
32,
34]. Monoubiquitination is the common tag to direct protein to the exosomes, even if this is not always true. Anyway, the monoubiquitinated proteins are recognized by different exosome markers containing ubiquitination recognition motifs, the better known being the Vps/27/Hrs protein, part of the ESCRT 0 complex, and the ESCRT I or II [
35]. Before being included in exosomes, deubiquitinating enzymes remove the ubiquitin from the cargo proteins. Sometimes, ubiquitination is not sufficient for protein sorting. For instance, PD-L1 is delivered by Vps27/Hrs in new vesicles in the presence of ubiquitination but also of Vp27/Hrs phosphorylation by ERK [
36]. Ubiquitination is not the only tag to address the proteins in ILVs. GPCRs can be directly recruited by Alix, another important exosome marker. Furthermore, other proteins are selected by a mechanism independent of ubiquitination as well as ESCRT [
34,
37]. These proteins possess a KFERQ domain and require the membrane protein LAMP2A that works by a mechanism dependent on the molecular chaperone HSC70, Alix, CD63, Syntenin-1, Rab31, and ceramides [
37].
Exosomes also contain non-protein cargo, such as nucleic acids. The mechanism used to direct these molecules in exosomes is poorly understood and the scant published data show that it is highly selective, because specific proteins bind a reduced number of molecules. miRNA can be addressed to exosomes by binding specific sumoylated proteins such as heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) localized into the exosome membrane [
38]. Mutant-KRAS is another protein able to alter the miRNA content in vesicles from colorectal cancer cells [
39,
40]. Tetraspanin 8 (Tspan8) is involved in the sorting of different miRNAs [
41]. Anyway, there are no general mechanisms explaining how the RNA is loaded in vesicles, and the proteins involved in this process are collected in a generic group of the RNA-binding protein class of exosomal RNA sorting [
42]. Otherwise, the mechanisms for the DNA sorting in exosomes are completely unknown and it is necessary to study this field for a major control of cancer behavior.
When the production of the ILVs is completed, MVBs have two choices [
19,
34]. They can be degraded by lysosomes or fused with the plasma membrane to release the ILVs as exosomes. Different proteins expressed on the MVB membrane are responsible for the vesicle’s fate such as the cholesterol content, the expression of sphingosine-1 phosphate (S1P), a ceramide metabolite, or several Rab GTPases, such as Rab7, Rab31, and Rab27A and B. Conversely, Rab7 favors late endosome fusion with lysosomes, thereby reducing the exosomes secretion. Rab31 counteracts the Rab7 action and recruits the GTPase-activating protein TBC1D2B, thus increasing the exosomes release [
43]. Rab27A and B are the most known mediators of exosome release. They ensure the right tethers between the SNARE proteins localized on vesicles (v-SNARE) as well as the target membrane (T-SNARE), allowing the SNARE complex formation, the completion of the fusion process, and the exosome release [
19].
Once released, exosomes enter the target cells through different mechanisms that are still not clear. They can directly fuse with the plasma membrane of the target cells or undergo different types of endocytosis (clathrin- or caveolin- or lipid raft-mediated endocytosis, pinocytosis, or phagocytosis) or directly interact with the plasma membrane receptors such as PD-L1, TRAIL, FasL, and TNF located on the membrane of cancer cells [
44,
45]. However, the most used mechanism for exosome uptake is endocytosis. Once in the cell, the cargo can carry out its function.
5. Role of Exosomes in PaC
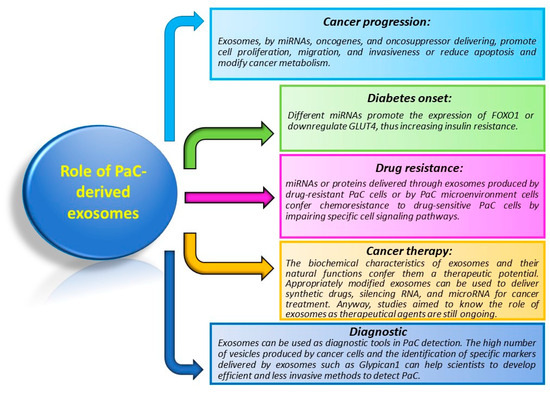
Because of their ability to carry a multitude of different molecules, exosomes are involved in distinct processes of cancer biology (Figure 3).
Figure 3. Principal functions of exosomes in PaC. The figure briefly resumes functions and mechanisms controlled by exosomes in PaC.
The role of exosomes in PaC is not quite different from exosomes acting in other cancers. As such, they alter and control cancer progression by influencing proliferation, migration, invasion, immunoregulation, and chemoresistance, both at local and systemic levels. As in other cancers, exosomes are used in the diagnosis and prognosis of PaC. Moreover, they play an exclusive role in the pathogenesis of diabetes.
6. Exosomes and PaC Drug Resistance
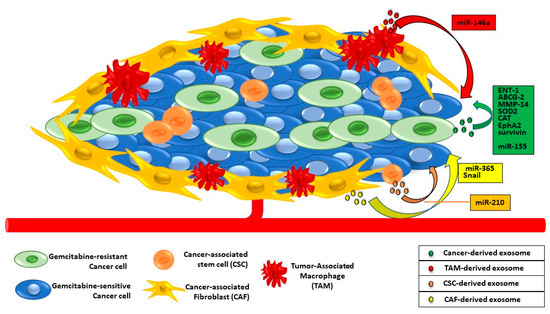
Besides the late diagnosis, one of the major plagues of PaC is the onset of chemoresistance. This attitude is due to the high heterogeneity of genetic mutations and the complex and dense stroma environment [
65]. Gemcitabine is the most used chemotherapeutic drug and, consequently, the mechanisms leading to its resistance are well studied. It might depend on genetic pressures during PaC progression as well as the stroma-derived non-coding miRNA, proteins, or miRNAs involved in the Epithelial-to-Mesenchymal transition (EMT) [
66,
67] (
Figure 4).
Figure 4. Exosomal trafficking between cancer and TME cells and gemcitabine resistance in PaC cells. Exosomes from drug-resistant PaC cells and/or from tumor-associated macrophages, fibroblasts, and stem cells contain proteins or miRNAs able to confer chemoresistance to gemcitabine-sensitive PaC cells.
The role of exosomes in cancer growth and spreading, as well as diagnostic and therapeutic factors, is now well studied and known enough, while there is less information about their involvement in drug resistance.
Exosomes might contribute in two different ways to PaC resistance. They directly work by kicking out drugs from cancer cells, or indirectly act by delivering miRNA or mutated or overexpressed proteins in recipient drug-sensitive cells [
19]. In the last case, the mechanisms of chemoresistance induced by exosomes in target cells are the same observed in drug-resistant cancer cells from which exosomes spring [
19,
65,
68,
69].
Because of the vesicular trafficking, there is an exchange of promoting chemoresistance factors between drug-resistant and drug-sensitive cells but also between cancer and microenvironment cells. One of the most basic mechanisms by which exosomes are actively involved in chemoresistance is the removal of gemcitabine from PaC cells and their microenvironment.