
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rahul Prasad Singh | -- | 4964 | 2023-10-19 09:48:39 | | | |
| 2 | Lindsay Dong | -71 word(s) | 4893 | 2023-10-23 02:30:56 | | |
Video Upload Options
The world is currently facing global energy crises and escalating environmental pollution, which are caused by the extensive exploitation of conventional energy sources. The limited availability of conventional energy sources has opened the door to the search for alternative energy sources. In this regard, microalgae have emerged as a promising substitute for conventional energy sources due to their high photosynthetic rate, high carbohydrate and lipid content, efficient CO2 fixation capacity, and ability to thrive in adverse environments. The research and development of microalgal-based biofuel as a clean and sustainable alternative energy source has been ongoing for many years, but it has not yet been widely adopted commercially. However, it is currently gaining greater attention due to the integrated biorefinery concept. In conclusion, algae-based biofuels offer a viable alternative to traditional fuels for meeting the growing demand for energy.
1. Introduction
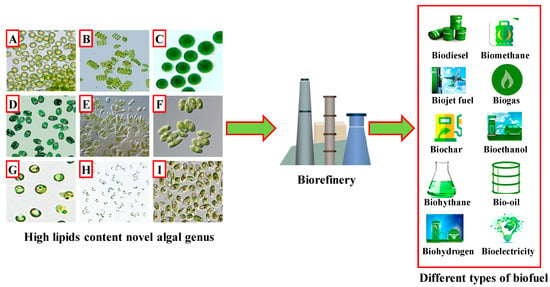
2. Microalgal Species and Biorefinery for Sustainable Biofuel Feedstock
3. Microalgae Cultivation Systems
| Cultivation Mode | Carbon Source | Energy Supply | Light Availability | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Autotrophic | Inorganic carbon | Light | Obligatory | Low cost, low energy consumption, high pigments production. | Low growth rate and biomass, specific photobioreactor required. |
| Heterotrophic | Organic carbon | Organic | No requirement | High biomass productivity and lipid accumulation due to high growth rate, process of scaling up is simplified, organic substrates can be used to alter biomass composition. | Higher cost, easy to be contaminated by other microorganisms, only a few microalgal species that can grow in a heterotrophic environment, inability to synthesize metabolites triggered by light. |
| Mixotrophic | Inorganic and organic carbon |
Light and organic carbon |
No obligatory | Increased growth rate, biomass, density and lipid accumulation, extended phase of exponential growth, stopping the photoinhibition effect and reducing biomass loss due to respiration during the dark hours, switch between photoautotroph and heterotroph regimens at any time. | High cost, contamination problems, limited microalgae species will grow. |
| Algal turf scrubber | Organic and inorganic carbon |
Light and organic carbon |
Obligatory | Improved nutrient status, pollutant removal, high biomass productivity rate, easy harvesting and low maintaince, decreased the overall production cost | Requirement of sufficient space and infrastructure |
4. Microalgae Harvesting Techniques
| Technique with Image | Principle | Advantages | Disadvantages |
|---|---|---|---|
Flocculation |
Aggregation of cells is achieved by enlarging their size through the addition of a flocculant, which can be in the form of chemicals (such as ferric chloride, ferric sulfate, and ammonium sulfate) or microbes (bacteria). | Fast and easy technique, used for large scale, less cell damage, applied to a wide variety of species, less energy requirements. | Chemicals may be expensive, high pH required, separating the coagulant from harvested biomass is difficult, limited culture medium recycling, increased microbial contamination. |
Filtration |
Large cells (size > 70 µm) can be filtered under pressure or suction whereas smaller cells (size < 30 µm require ultrafilters to be harvested. | High recovery efficiency, cost effective, no chemical required, low energy consumption, low shear stress. | Slow hence requires pressure or vacuum, not effective for small algae, membrane fouling/clogging and replacement increases operational and maintenance costs. |
Flotation |
Trapping algal cells by bubbling air. | Well-suited for large-scale applications, economically efficient with minimal space demands, short operation time. | Depends on bubble distribution into the suspension, needs surfactants. |
Centrifugation |
Sedimentation based on the velocity, cell size and density. | Fast and effective technique, high recovery efficiency (>90), applicable to all microalgae. | Expensive technique with high energy requirement, high operation and maintenance costs, risk of cell destruction. |
Precipitation |
Certain algae undergo self-precipitation, they settle at the bottom when circulation is halted. | No energy or chemicals are needed, it occurs naturally. | Species-specific, time periods vary depending on the species, not every species is self-precipitated. |
5. Approaches to Stimulate Lipid Production through Abiotic Stresses
5.1. Effect of Carbon Dioxide
5.2. Effect of Nitrogen Starvation
5.3. Effect of Light Intensity and Wavelength
5.4. Effect of Temperature
5.5. Effect of Salinity
5.6. Effect of pH
5.7. Effect of Metal
5.8. Effect of Sulfur Starvation
5.9. Effect of Phytohormones
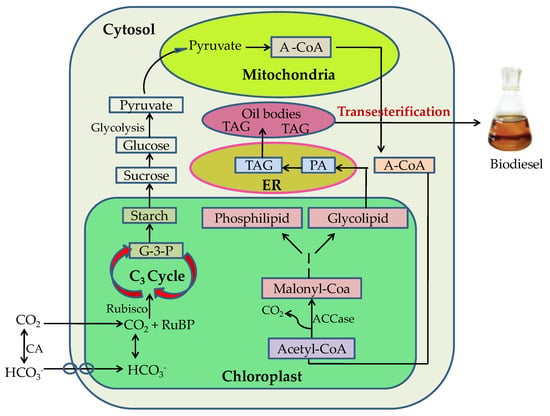
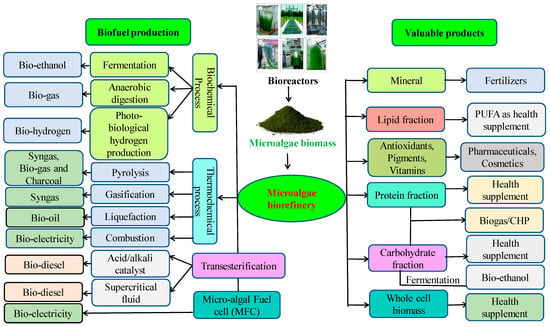
6. Microalgal Biorefinery for Biofuels Production
References
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Cooke, P.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel 2012, 97, 822–831.
- Ahamed, T.S.; Brindhadevi, K.; Krishnan, R.; Phuong, T.N.; Alharbi, S.A.; Chinnathambi, A.; Mathimani, T. In vivo detection of triacylglycerols through Nile red staining and quantification of fatty acids in hyper lipid producer Nannochloropsis sp. cultured under adequate nitrogen and deficient nitrogen condition. Fuel 2022, 322, 124179.
- Jeyakumar, N.; Hoang, A.T.; Nižetić, S.; Balasubramanian, D.; Kamaraj, S.; Pandian, P.L.; Sirohi, R.; Nguyen, P.Q.P.; Nguyen, X.P. Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel 2022, 329, 125362.
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Gurav, R.; Bhatia, R.K.; Govarthanan, M.; Tyagi, V.K.; Kumar, V.; Pugazendhi, A.; Banu, J.R.; et al. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Bioresour. Technol. 2022, 358, 127437.
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Al-Arjani, A.B.F.; Abd_Allah, E.F.; Rodríguez Dorantes, A.; Gupta, R.K. Physiological and biochemical responses of bicarbonate supplementation on biomass and lipid content of green algae Scenedesmus sp. BHU1 isolated from wastewater for renewable biofuel feedstock. Front. Microbiol. 2022, 13, 839800.
- Tiwari, O.N.; Bhunia, B.; Bandyopadhyay, T.K.; Oinam, G. Strategies for improved induction of lipid in Leptolyngbya sp. BTA 287 for biodiesel production. Fuel 2019, 256, 115896.
- Ranganathan, P.; Pandey, A.K.; Sirohi, R.; Hoang, A.T.; Kim, S.H. Recent advances in computational fluid dynamics (CFD) modelling of photobioreactors: Design and applications. Bioresour. Technol. 2022, 350, 126920.
- Mehmood, M.A.; Shahid, A.; Malik, S.; Wang, N.; Javed, M.R.; Haider, M.N.; Verma, P.; Ashraf, M.U.F.; Habib, N.; Syafiuddin, A.; et al. Advances in developing metabolically engineered microbial platforms to produce fourth-generation biofuels and high-value biochemicals. Bioresour. Technol. 2021, 337, 125510.
- Anto, S.; Karpagam, R.; Renukadevi, P.; Jawaharraj, K.; Varalakshmi, P. Biomass enhancement and bioconversion of brown marine microalgal lipid using heterogeneous catalysts mediated transesterification from biowaste derived biochar and bio nanoparticle. Fuel 2019, 255, 115789.
- Japar, A.S.; Takriff, M.S.; Yasin, N.H.M. Microalgae acclimatization in industrial wastewater and its effect on growth and primary metabolite composition. Algal Res. 2021, 53, 102163.
- Bibi, F.; Ali, M.I.; Ahmad, M.; Bokhari, A.; Khoo, K.S.; Zafar, M.; Asif, S.; Mubashir, M.; Han, N.; Show, P.L. Production of lipids biosynthesis from Tetradesmus nygaardii microalgae as a feedstock for biodiesel production. Fuel 2022, 326, 124985.
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755.
- Ali, S.S.; Mastropetros, S.G.; Schagerl, M.; Sakarika, M.; Elsamahy, T.; El-Sheekh, M.; Sun, J.; Kornaros, M. Recent advances in wastewater microalgae-based biofuels production: A state-of-the-art review. Energy Rep. 2022, 8, 13253–13280.
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 2020, 274, 117841.
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599.
- Patel, P.; Patel, B.; Vekaria, E.; Shah, M. Biophysical economics and management of biodiesel, a harbinger of clean and sustainable energy. Int. J. Energy Water Resour. 2020, 4, 411–423.
- Goswami, R.K.; Mehariya, S.; Obulisamy, P.K.; Verma, P. Advanced microalgae-based renewable biohydrogen production systems: A review. Bioresour. Technol. 2021, 320, 124301.
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated approach for wastewater treatment and biofuel production in microalgae biorefineries. Energies 2021, 14, 2282.
- Salama, E.S.; Abou-Shanab, R.A.; Kim, J.R.; Lee, S.; Kim, S.H.; Oh, S.E.; Kim, H.C.; Roh, H.S.; Jeon, B.H. The effects of salinity on the growth and biochemical properties of Chlamydomonas mexicana GU732420 cultivated in municipal wastewater. Environ. Technol. Innov. 2014, 35, 1491–1498.
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179.
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110.
- Mathimani, T.; Uma, L.; Prabaharan, D. Formulation of low-cost seawater medium for high cell density and high lipid content of Chlorella vulgaris BDUG 91771 using central composite design in biodiesel perspective. J. Clean. Prod. 2018, 198, 575–586.
- Yadav, P.; Singh, R.P.; Alodaini, H.A.; Hatamleh, A.A.; Santoyo, G.; Kumar, A.; Gupta, R.K. Impact of dehydration on the physiochemical properties of Nostoc calcicola BOT1 and its untargeted metabolic profiling through UHPLC-HRMS. Front. Plant Sci. 2023, 14, 1147390.
- Cheng, D.; He, Q. Assessment of environmental stresses for enhanced microalgal biofuel production–an overview. Front. Energy Res. 2014, 2, 26.
- Miranda, A.M.; Hernandez-Tenorio, F.; Ocampo, D.; Vargas, G.J.; Sáez, A.A. Trends on CO2 capture with microalgae: A bibliometric analysis. Molecules 2022, 27, 4669.
- Park, S.; Nguyen, T.H.T.; Jin, E. Improving lipid production by strain development in microalgae: Strategies, challenges and perspectives. Bioresour. Technol. 2019, 292, 121953.
- Rastogi, R.P.; Pandey, A.; Larroche, C.; Madamwar, D. Algal Green Energy–R&D and technological perspectives for biodiesel production. Renew. Sustain. Energy Rev. 2018, 82, 2946–2969.
- Seo, J.Y.; Jeon, H.J.; Kim, J.W.; Lee, J.; Oh, Y.K.; Ahn, C.W.; Lee, J.W. Simulated-sunlight-driven cell lysis of magnetophoretically separated microalgae using ZnFe2O4 octahedrons. Ind. Eng. Chem. Res. 2018, 57, 1655–1661.
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804.
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178.
- Khandelwal, A.; Vijay, A.; Dixit, A.; Chhabra, M. Microbial fuel cell powered by lipid extracted algae: A promising system for algal lipids and power generation. Bioresour. Technol. 2018, 247, 520–527.
- Song, D.; Park, J.; Kim, K.; Lee, L.S.; Seo, J.Y.; Oh, Y.K.; Kim, Y.J.; Ryou, M.H.; Lee, Y.M.; Lee, K. Recycling oil-extracted microalgal biomass residues into nano/micro hierarchical Sn/C composite anode materials for lithium-ion batteries. Electrochim. Acta 2017, 250, 59–67.
- Beacham, T.A.; Macia, V.M.; Rooks, P.; White, D.A.; Ali, S.T. Altered lipid accumulation in Nannochloropsis salina CCAP849/3 following EMS and UV induced mutagenesis. Biotechnol. Rep. 2015, 7, 87–94.
- Deng, X.Y.; Gao, K.; Addy, M.; Li, D.; Zhang, R.C.; Lu, Q.; Ma, Y.W.; Cheng, Y.L.; Chen, P.; Liu, Y.H.; et al. Cultivation of Chlorella vulgaris on anaerobically digested swine manure with daily recycling of the post-harvest culture broth. Bioresour. Technol. 2018, 247, 716–723.
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour. Technol. 2019, 271, 368–374.
- Takeshita, T.; Ivanov, I.N.; Oshima, K.; Ishii, K.; Kawamoto, H.; Ota, S.; Yamazaki, T.; Hirata, A.; Kazama, Y.; Abe, T.; et al. Comparison of lipid productivity of Parachlorella kessleri heavy-ion beam irradiation mutant PK4 in laboratory and 150-L mass bioreactor, identification and characterization of its genetic variation. Algal Res. 2018, 35, 416–426.
- Yu, N.; Dieu, L.T.J.; Harvey, S.; Lee, D.Y. Optimization of process configuration and strain selection for microalgae-based biodiesel production. Bioresour. Technol. 2015, 193, 25–34.
- Feng, P.; Deng, Z.; Hu, Z.; Wang, Z.; Fan, L. Characterization of Chlorococcum pamirum as a potential biodiesel feedstock. Bioresour. Technol. 2014, 162, 115–122.
- Gao, Y.; Yang, M.; Wang, C. Nutrient deprivation enhances lipid content in marine microalgae. Bioresour. Technol. 2013, 147, 484–491.
- Karpagam, R.; Raj, K.J.; Ashokkumar, B.; Varalakshmi, P. Characterization and fatty acid profiling in two fresh water microalgae for biodiesel production: Lipid enhancement methods and media optimization using response surface methodology. Bioresour. Technol. 2015, 188, 177–184.
- Mao, X.; Wu, T.; Sun, D.; Zhang, Z.; Chen, F. Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour. Technol. 2018, 249, 791–798.
- Wen, X.; Geng, Y.; Li, Y. Enhanced lipid production in Chlorella pyrenoidosa by continuous culture. Bioresour. Technol. 2014, 161, 297–303.
- Maity, J.P.; Bundschuh, J.; Chen, C.Y.; Bhattacharya, P. Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives—A mini review. Energy 2014, 78, 104–113.
- Wiencke, C.; Clayton, M.N.; Gómez, I.; Iken, K.; Lüder, U.H.; Amsler, C.D.; Karsten, U.; Hanelt, D.; Bischof, K.; Dunton, K. Life strategy, ecophysiology and ecology of seaweeds in polar waters. Rev. Environ. Sci. Biotechnol. 2007, 6, 95–126.
- Kuo, C.M.; Lin, T.H.; Yang, Y.C.; Zhang, W.X.; Lai, J.T.; Wu, H.T.; Chang, J.S.; Lin, C.S. Ability of an alkali-tolerant mutant strain of the microalga Chlorella sp. AT1 to capture carbon dioxide for increasing carbon dioxide utilization efficiency. Bioresour. Technol. 2017, 244, 243–251.
- Abid, A.; Saidane, F.; Hamdi, M. Feasibility of carbon dioxide sequestration by Spongiochloris sp. microalgae during petroleum wastewater treatment in airlift bioreactor. Bioresour. Technol. 2017, 234, 297–302.
- Choi, Y.Y.; Patel, A.K.; Hong, M.E.; Chang, W.S.; Sim, S.J. Microalgae Bioenergy with Carbon Capture and Storage (BECCS): An emerging sustainable bioprocess for reduced CO2 emission and biofuel production. Bioresour. Technol. Rep. 2019, 7, 100270.
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389.
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int. J. Hydrogen Energy 2017, 42, 8505–8517.
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701.
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500.
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920.
- Raeesossadati, M.J.; Ahmadzadeh, H.; McHenry, M.P.; Moheimani, N.R. CO2 bioremediation by microalgae in photobioreactors: Impacts of biomass and CO2 concentrations, light, and temperature. Algal Res. 2014, 6, 78–85.
- Lv, J.M.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour. Technol. 2010, 101, 6797–6804.
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995.
- Ferreira, G.F.; Pinto, L.R.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466.
- Nascimento, I.A.; Cabanelas, I.T.D.; dos Santos, J.N.; Nascimento, M.A.; Sousa, L.; Sansone, G. Biodiesel yields and fuel quality as criteria for algal-feedstock selection: Effects of CO2-supplementation and nutrient levels in cultures. Algal Res. 2015, 8, 53–60.
- Bibi, F.; Jamal, A.; Huang, Z.; Urynowicz, M.; Ali, M.I. Advancement and role of abiotic stresses in microalgae biorefinery with a focus on lipid production. Fuel 2022, 316, 123192.
- Sulochana, S.B.; Arumugam, M. Targeted metabolomic and biochemical changes during nitrogen stress mediated lipid accumulation in Scenedesmus quadricauda CASA CC202. Front. Bioeng. Biotechnol. 2020, 8, 585632.
- Chen, H.; Wang, Q. Regulatory mechanisms of lipid biosynthesis in microalgae. Biol. Rev. 2021, 96, 2373–2391.
- Shtaida, N.; Khozin, G.I.; Boussiba, S. The role of pyruvate hub enzymes in supplying carbon precursors for fatty acid synthesis in photosynthetic microalgae. Photosynth. Res. 2015, 125, 407–422.
- Nagappan, S.; Devendran, S.; Tsai, P.C.; Jayaraman, H.; Alagarsamy, V.; Pugazhendhi, A.; Ponnusamy, V.K. Metabolomics integrated with transcriptomics and proteomics: Evaluation of systems reaction to nitrogen deficiency stress in microalgae. Process Biochem. 2020, 91, 1–14.
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and morphological changes triggered by nitrogen stress in the oleaginous microalga Chlorella vulgaris. Microorganisms 2022, 10, 566.
- Napolitano, G.E. The relationship of lipids with light and chlorophyll measurements in freshwater algae and periphyton 1. J. Phycol. 1994, 30, 943–950.
- Gris, B.; Morosinotto, T.; Giacometti, G.M.; Bertucco, A.; Sforza, E. Cultivation of Scenedesmus obliquus in photobioreactors: Effects of light intensities and light–dark cycles on growth, productivity, and biochemical composition. Appl. Biochem. Biotechnol. 2014, 172, 2377–2389.
- Gim, G.H.; Ryu, J.; Kim, M.J.; Kim, P.I.; Kim, S.W. Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J. Ind. Microbiol. 2016, 43, 605–616.
- Severes, A.; Hegde, S.; D’Souza, L.; Hegde, S. Use of light emitting diodes (LEDs) for enhanced lipid production in micro-algae-based biofuels. J. Photochem. Photobiol. B Biol. 2017, 170, 235–240.
- Seo, Y.H.; Lee, Y.; Jeon, D.Y.; Han, J.I. Enhancing the light utilization efficiency of microalgae using organic dyes. Bioresour. Technol. 2015, 181, 355–359.
- Seo, Y.H.; Cho, C.; Lee, J.Y.; Han, J.I. Enhancement of growth and lipid production from microalgae using fluorescent paint under the solar radiation. Bioresour. Technol. 2014, 173, 193–197.
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638.
- Al-Qasmi, M.; Raut, N.; Talebi, S.; Al-Rajhi, S.; Al-Barwani, T. A review of effect of light on microalgae growth. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012; Volume 2, pp. 1–7.
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226.
- Chokshi, K.; Pancha, I.; Trivedi, K.; George, B.; Maurya, R.; Ghosh, A.; Mishra, S. Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour. Technol. 2015, 180, 162–171.
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151.
- Wang, Y.; He, B.; Sun, Z.; Chen, Y.F. Chemically enhanced lipid production from microalgae under low sub-optimal temperature. Algal Res. 2016, 16, 20–27.
- Zhu, C.J.; Lee, Y.K.; Chao, T.M. Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J. Appl. Phycol. 1997, 9, 451–457.
- Jiang, H.; Gao, K. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (bacillariophyceae) 1. J. Phycol. 2004, 40, 651–654.
- Takagi, M.; Yoshida, T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226.
- Pancha, I.; Chokshi, K.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 193, 315–323.
- Duan, X.; Ren, G.Y.; Liu, L.L.; Zhu, W.X. Salt-induced osmotic stress for lipid overproduction in batch culture of Chlorella vulgaris. Afr. J. Biotechnol. 2012, 11, 7072–7078.
- Cao, J.; Yuan, H.; Li, B.; Yang, J. Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour. Technol. 2014, 152, 177–184.
- Sajjadi, B.; Chen, W.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232.
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722.
- Mao, X.; Lao, Y.; Sun, H.; Li, X.; Yu, J.; Chen, F. Time-resolved transcriptome analysis during transitions of sulfur nutritional status provides insight into triacylglycerol (TAG) and astaxanthin accumulation in the green alga Chromochloris zofingiensis. Biotechnol. Biofuels. 2020, 13, 128.
- Wang, Q.; Zhang, Y.; Wu, H.; Xu, N.; Li, A. Effects of sulfur limitation on nitrogen and sulfur uptake and lipid accumulation in Scenedesmus acuminatus. J. Appl. Phycol. 2021, 33, 301–311.
- Xu, L.; Cheng, X.; Wang, Q. Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with Azotobacter chroococcum. Front. Plant Sci. 2018, 9, 741.
- Dao, G.H.; Wu, G.X.; Wang, X.X.; Zhuang, L.L.; Zhang, T.Y.; Hu, H.Y. Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour. Technol. 2018, 247, 561–567.
- Singh, J.; Jain, D.; Agarwal, P.; Singh, R.P. Auxin and cytokinin synergism augmenting biomass and lipid production in microalgae Desmodesmus sp. JS07. Process Biochem. 2020, 95, 223–234.
- Bhuyar, P.; Yusoff, M.M.; Rahim, M.H.A.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Effect of plant hormones on the production of biomass and lipid extraction for biodiesel production from microalgae Chlorella sp. J. Microbiol. Biotechnol. Food Sci. 2021, 9, 671–674.
- Che, R.; Huang, L.; Xu, J.W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Effect of fulvic acid induction on the physiology, metabolism, and lipid biosynthesis-related gene transcription of Monoraphidium sp. FXY-10. Bioresour. Technol. 2017, 227, 324–334.
- Udayan, A.; Sabapathy, H.; Arumugam, M. Stress hormones mediated lipid accumulation and modulation of specific fatty acids in Nannochloropsis oceanica CASA CC201. Bioresour. Technol. 2020, 310, 123437.
- Zabochnicka-Świątek, M.; Kamizela, T.; Kowalczyk, M.; Kalaji, H.M.; Bąba, W. Inexpensive and universal growth media for biomass production of microalgae. Glob. Nest. J. 2019, 21, 82–89.
- Ananthi, V.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. Impact of abiotic factors on biodiesel production by microalgae. Fuel 2021, 284, 118962.
- Smachetti, M.E.S.; Coronel, C.D.; Salerno, G.L.; Curatti, L. Sucrose-to-ethanol microalgae-based platform using seawater. Algal Res. 2020, 45, 101733.
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Circular bioeconomy: An introduction. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–23.
- Behera, B.; Paramasivan, B. Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour. Technol. 2022, 351, 127038.
- Choudhary, S.; Tripathi, S.; Poluri, K.M. Microalgal-based bioenergy: Strategies, prospects, and sustainability. Energy Fuel 2022, 36, 14584–14612.
- Sarangi, P.K.; Singh, A.K.; Srivastava, R.K.; Gupta, V.K. Recent Progress and Future Perspectives for Zero Agriculture Waste Technologies: Pineapple Waste as a Case Study. Sustainability 2023, 15, 3575.
- Kuppan, P.; Sudharsanam, A.; Venkateswarlu, K.; Megharaj, M. Solar technology—Closed loop synergy facilitates low-carbon circular bioeconomy in microalgal wastewater treatment. NPJ Clean Water 2023, 6, 43.
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as sustainable food and feed sources for animals and humans–biotechnological and environmental aspects. Chemosphere 2021, 271, 129800.
- Cheirsilp, B.; Maneechote, W.; Srinuanpan, S.; Angelidaki, I. Microalgae as Tools for Bio-Circular-Green Economy: Zero-waste Approaches for Sustainable Production and Biorefineries of Microalgal Biomass. Bioresour. Technol. 2023, 387, 129620.
- Idenyi, J.N.; Eya, J.C.; Nwankwegu, A.S.; Nwoba, E.G. Aquaculture sustainability through alternative dietary ingredients: Microalgal value-added products. Eng. Microbiol. 2022, 2, 100049.
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other nutrients from Haematococcus pluvialis—Multifunctional applications. Mar. Drugs 2020, 18, 459.
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84.
- Patel, A.; Krikigianni, E.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprocessing of volatile fatty acids by oleaginous freshwater microalgae and their potential for biofuel and protein production. J. Chem. Eng. 2022, 438, 135529.
- Callejón, M.J.J.; Medina, A.R.; Sánchez, M.D.M.; Moreno, P.A.G.; López, E.N.; Cerdán, L.E.; Grima, E.M. Supercritical fluid extraction and pressurized liquid extraction processes applied to eicosapentaenoic acid-rich polar lipid recovery from the microalga Nannochloropsis sp. Algal Res. 2022, 61, 102586.
- Oliver, L.; Fernández-de-Castro, L.; Dietrich, T.; Villaran, M.C.; Barrio, R.J. Production of docosahexaenoic acid and odd-chain fatty acids by microalgae Schizochytrium limacinum grown on waste-derived volatile fatty acids. Appl. Sci. 2022, 12, 3976.




