Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nahum Mendez-Sanchez | -- | 1711 | 2023-09-25 20:42:42 | | | |
| 2 | Jessie Wu | + 10 word(s) | 1721 | 2023-09-26 04:07:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mendez-Sanchez, N.; Coronel-Castillo, C.E.; Cordova-Gallardo, J.; Qi, X. Reevaluating Therapeutic Use of Antibiotics in Liver Cirrhosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49609 (accessed on 07 February 2026).
Mendez-Sanchez N, Coronel-Castillo CE, Cordova-Gallardo J, Qi X. Reevaluating Therapeutic Use of Antibiotics in Liver Cirrhosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49609. Accessed February 07, 2026.
Mendez-Sanchez, Nahum, Carlos Esteban Coronel-Castillo, Jacqueline Cordova-Gallardo, Xingshun Qi. "Reevaluating Therapeutic Use of Antibiotics in Liver Cirrhosis" Encyclopedia, https://encyclopedia.pub/entry/49609 (accessed February 07, 2026).
Mendez-Sanchez, N., Coronel-Castillo, C.E., Cordova-Gallardo, J., & Qi, X. (2023, September 25). Reevaluating Therapeutic Use of Antibiotics in Liver Cirrhosis. In Encyclopedia. https://encyclopedia.pub/entry/49609
Mendez-Sanchez, Nahum, et al. "Reevaluating Therapeutic Use of Antibiotics in Liver Cirrhosis." Encyclopedia. Web. 25 September, 2023.
Copy Citation
Impairments in liver function lead to different complications. As chronic liver disease progresses (CLD), hypoalbuminemia and alterations in bile acid compositions lead to changes in gut microbiota and, therefore, in the host–microbiome interaction, leading to a proinflammatory state. Alterations in gut microbiota composition and permeability, known as gut dysbiosis, have important implications in CLD; alterations in the gut–liver axis are a consequence of liver disease, but also a cause of CLD. Furthermore, gut dysbiosis plays an important role in the progression of liver cirrhosis and decompensation, particularly with complications such as hepatic encephalopathy and spontaneous bacterial peritonitis.
dysbiosis
gut microbiota
liver cirrhosis
antibiotics
bile acids
1. Antibiotic Effects on Portal Hypertension
The portal vein serves as a major conduit for nutrients, toxins, and microbial products from the gut to the liver. Disruption of the gut–liver axis can lead to dysbiosis, which has been implicated in the pathogenesis of chronic liver disease (CLD). Studies have shown that alterations in gut microbiota composition and function contribute to liver inflammation, fibrosis, and portal hypertension. The dysbiosis-induced increased intestinal permeability to gut microbial metabolites, such as lipopolysaccharide (LPS), secondary bile acids (BAs), and trimethylamine N-oxide (TMAO), has been shown to influence hepatic vascular tone and contribute to portal hypertension [1]. Moreover, evidence suggests that when those metabolites escape to the systemic circulation, they may induce systemic hypertension [2][3].
Recent research on factors influencing gut microbiome (GM) with regard to portal hypertension has opened new avenues for therapeutic interventions. Modulating the gut microbiota through strategies such as probiotics, prebiotics, antibiotics, and fecal microbiota transplantation might represent promising therapies to improve liver-related complications and reduce portal hypertension. Additionally, targeting gut microbial metabolites and their receptors may offer novel therapeutic options for the management of portal hypertension [1][4]. In fact, bacterial-derived products may increase hyperdynamic circulation and intrahepatic vascular resistance, promoting a further increase in portal pressure and the risk of bleeding [5][6][7].
Regarding infections, when compared with controls, patients with liver cirrhosis and increased populations of Bacteroides, Escherichia, Shigella, and Prevotella have severe portal hypertension and high levels of IL-8 in their hepatic veins [8]. Furthermore, it seems that patients with variceal bleeding have a higher rate of bacterial infections, and the administration of intravenous antibiotics, such as norfloxacin or ampicillin/sulbactam, may improve complications [6].
A recent study published by Mendoza et al [9]. showed that the use of rifaximin or norfloxacin did not cause a significant reduction in hepatic venous pressure gradient (HPVG) in patients with cirrhosis, but the use of antibiotics for longer periods in association with non-selective beta blockers (NSBB) did decrease HPVG significantly [9]. The use of rifaximin has been shown to reduce portal hypertension when associated with NSBB, compared to the use of propranolol alone [10] (Figure 1). However, norfloxacin did not perform better than the placebo in reducing HVPG [11]. Moreover, the use of probiotic VSL#3 has been shown to improve the effect of propranolol in reducing HPVG [12].
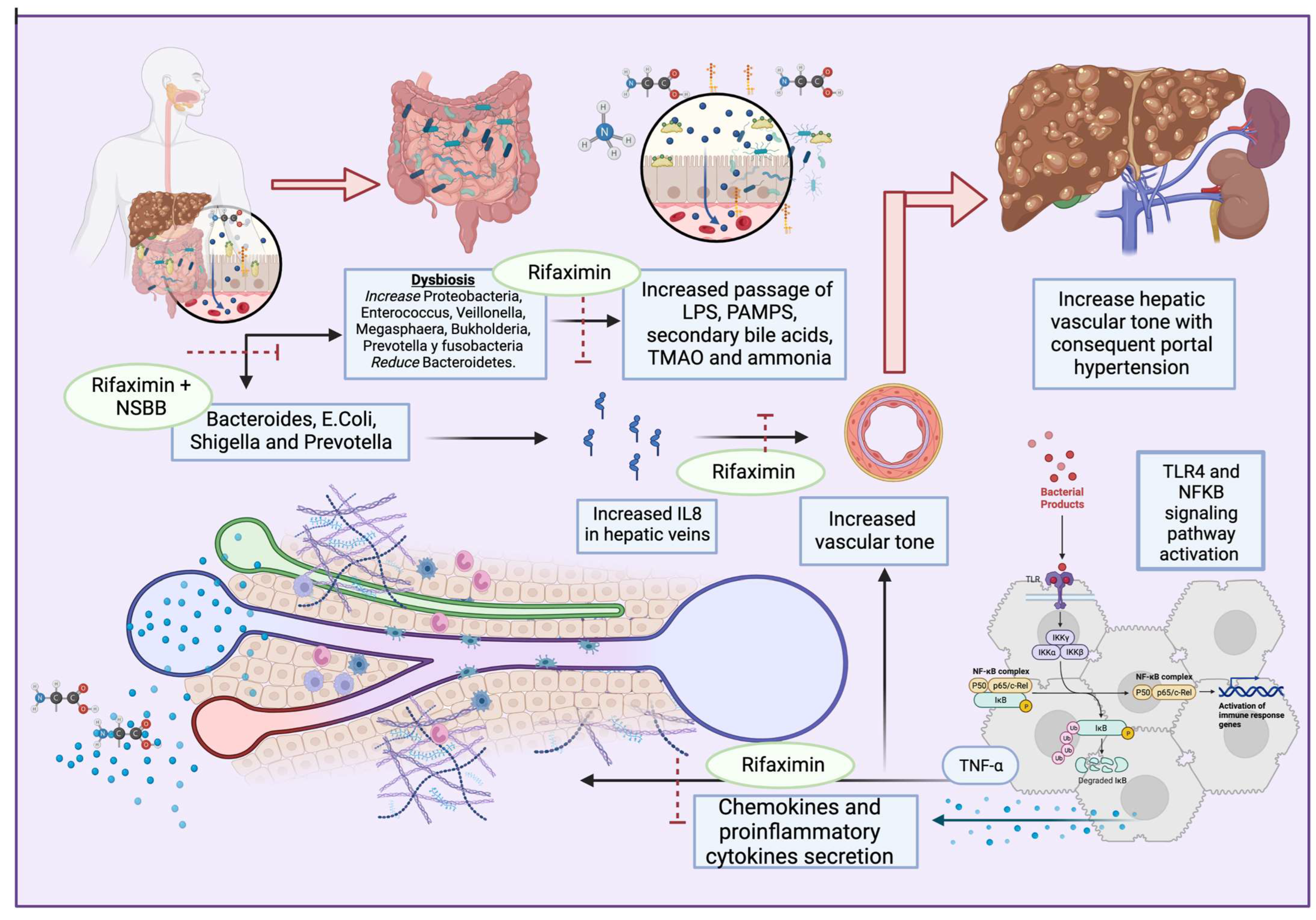
Figure 1. Dysbiosis enhances the secretion of PAMPs, secondary bile acids, TMAO, and ammonia, and activates TLR4 and NFkB pathways. This results in proinflammatory cytokine and chemokyne secretion, with increases in TNFα and IL8 that lead to portal hypertension. Antibiotics such as rifaximin seem to exert beneficial effects on multiple dysbiosis-reducing targets, IL8-producing bacteria, and the passage of bacterial products, with consequent proinflammatory pathway activation.
2. Prophylactic Antibiotic Use for Cirrhosis
Current guidelines recommend antibiotic prophylaxis in specific situations. For patients with a history of SBP, long-term prophylaxis with oral norfloxacin or trimethoprim–sulfamethoxazole is recommended to prevent recurrence. Additionally, short-term prophylaxis with intravenous antibiotics is advised for cirrhotic patients with gastrointestinal bleeding, as it reduces the risk of infections and improves survival rates [13][14]. Regarding the latter, consensus guidelines recommend the prophylactic use of oral or intravenous antibiotics in this population. Furthermore, quinolones and beta-lactams, either alone or in combination, were effective in reducing rebleeding rates and hospital stay length in cirrhosis patients with gastrointestinal bleeding, according to a metanalysis. On the other hand, MDRO bacterial infections have reduced the efficacy of commonly used antibiotics, necessitating combined antibiotic therapy. Combination therapy with quinolones and beta-lactams has been associated with reduced mortality, rebleeding, and hospitalization lengths [14].
Patients with liver cirrhosis experience about 36% spontaneous infections, such as with SBP [15]. When SBP is suspected, empiric antibiotics are used, with third-generation cephalosporins used commonly, except in the context of MDRO risk factors, where the first option is piperacillin/tazobactam. In the case of prophylaxis, norfloxacin and ciprofloxacin are the first options for both primary and secondary prevention, followed by trimethoprim–sulfamethoxazole [16][17][18][19]. The empirical antibiotics discussed above seem to exert similar effects against SBP, but response-guided therapy, by performing a second paracentesis at 48 h to assess antibiotic response, should be considered [17]. The use of prophylactic norfloxacin might increase the risk of MDR bacterial infections, and practitioners should be aware of this after the first month of liver transplantation [20]. Hence, MDR bacterial infection remains controversial, so norfloxacin prophylaxis should be indicated in carefully selected patients [21].
Another novel strategy is selective digestive decontamination (SDD), which consists of the combination of topical nonabsorbable antibiotics or antifungal agents applied to the upper gastrointestinal tract with a short course of intravenous antibiotics. Its use began in patients with neutropenia, and it is a topic of interest in critically ill patients despite controversial evidence [22][23]. In cirrhosis, SDD was used to treat both gastrointestinal bleeding and SBP, at first with oral nonabsorbable antibiotics such as polymyxin, neomycin, gentamycin and colistin, and then with trimethoprim–sulfamethoxazole and fluroquinolones. Still, the disrupting effects of antibiotics in GM may be linked to the asymptomatic colonization of the gut by MDROs. This colonization not only represents a potential source of infection for the affected patient, but also contributes to the transmission of MDRO infections within healthcare settings. Consequently, until comprehensive studies have been conducted across multiple centers, investigating the impact of SDD on rates of multidrug resistance at both the individual and population levels, the use of SDD should be restricted to cirrhosis patients who face the highest risk of developing an infection [24]. To address this issue, the use of rifaximin is proposed; this non-absorbable antibiotic possesses distinctive effects on the gut microbiota [19]. However, the results of a recent study found that, overall, systemic antibiotic prophylaxis is more effective than rifaximin in SBP prevention and should be the standard of care for patients with advanced cirrhosis and a high risk of SBP [25].
Finally, rifaximin, in combination with lactulose or L-ornithine L-aspartate, is employed for the purpose of preventing the recurrence of HE [26][27]. According to research findings, it seems that rifaximin enhances the population of beneficial intestinal bacteria, such as Bifidobacterium, Atopobium, and Faecalibacterium prausnitzii. Meanwhile, it does not significantly alter the overall composition of the gut microbiota, including the lactobacilli. Additionally, rifaximin contributes to the restoration of the intestinal barrier, potentially mitigating bacterial translocation and systemic endotoxemia in individuals with cirrhosis. This effect may be attributed to the inhibition of NF-kB activation via the pregnane X receptor (PXR) and a reduction in interleukins and TNFα expression [20][28][29].
3. Multidrug-Resistant Bacterial Infections in Patients with Cirrhosis and the Role of Gut Microbiota
Bacterial infections represent one of the leading causes of hospitalization, morbidity, and mortality in cirrhotic patients. The most frequent infections are urinary infections, pneumonia, and spontaneous bacterial peritonitis, with an increasing incidence of MDROs [30].
Owing to the increasing use of broad antibiotics in cirrhotic patients, multidrug-resistant bacterial infections have been rising; in particular, patients who received prophylactic norfloxacin for SBP experience higher risks of MDRO infection [31]. Hence, this assertion remains controversial; in a study performed by Marciano et al., they found that norfloxacin exerts a beneficial effect on SBP prophylaxis, with no increased incidence of MDRO infections [21]. To address the uncertainty as to whether antibiotic prophylaxis is beneficial or not, more clinical trials should be performed to test long-term antibiotics [32]. Furthermore, in a multicenter study in Europe, it was found that about 30% of positive cultures from infections in patients with liver cirrhosis were caused by MDROs. The most frequently isolated MDROs in this series were extended-spectrum beta-lactamase-producing Enterobacteriaceae. In that same study, in a second series of patients it was revealed that the prevalence of MDROs was 23% (392 infections out of 2587 patients), and among culture-positive infections, it was 38%. A slight increase in the rate of carbapenem-resistant Enterobacteriaceae was observed in this series [33]. In general, a global prevalence of 34% MDR bacterial infection is estimated in liver cirrhosis [34]. Antibiotic resistance is associated with poor prognosis and the failure of antibiotic strategies, particularly those based on third-generation cephalosporins or quinolones [35]. Furthermore, the main risk factors for MDRO infections in patients with cirrhosis are long-term norfloxacin prophylaxis, recent infection by multi-resistant bacteria, and the recent use of β-lactams [36].
It is important to consider the spectrum of infectious pathogens from Gram-negative bacteria in community-acquired infections compared with Gram-positive bacteria in hospital-acquired infections [37].
Antibiotics may also predispose individuals to other infections, such as invasive fungal infections. Fungal infections are much less frequent; they are usually nosocomial and associated with extremely high short-term mortality. In patients with cirrhosis, invasive fungal infections occur in approximately 3–7% of culture-positive infected individuals, and they are more commonly observed as secondary or nosocomial infections during the course of acute-on-chronic liver failure (ACLF). Among them, invasive candidiasis, or candidemia, is the most frequent, accounting for 70–90% of cases, followed by invasive aspergillosis.
Invasive fungal infections in patients with decompensated cirrhosis are generally associated with an extremely poor prognosis. Candidemia and other invasive candidiasis infections are accompanied by 28-day mortality rates ranging from 45% to 60%. ACLF complicated by IA has an even worse prognosis, with only rare cases of survival despite receiving appropriate antifungal treatment [38].
Using a targeted metagenomics approach, Delavy et al. [39] observed a high degree of interindividual diversity in healthy gut microbiota. They found that the prevalence of C. albicans was much higher than previously reported, with all subjects except one carrying C. albicans, albeit at varying levels. The administration of third-generation cephalosporins significantly altered the composition of the microbiota, and the fungal load was increased both in the short and the long term. The variations in C. albicans levels in response to third-generation cephalosporin treatment could be partially explained by changes in the levels of endogenous fecal β-lactamase activity. Subjects with higher β-lactamase activity showed lower C. albicans levels [39]. This suggests that the use of a particular antibiotic treatment may change the specific types of microorganisms, either fungal or bacterial, in the GM [40].
References
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut-liver axis, cirrhosis and portal hypertension: The chicken and the egg. Hepatol. Int. 2018, 12 (Suppl. 1), 24–33.
- Di Tommaso, N.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. The Gut-Vascular Barrier as a New Protagonist in Intestinal and Extraintestinal Diseases. Int. J. Mol. Sci. 2023, 24, 1470.
- Touyz, R.M. Gut Dysbiosis–Induced Hypertension Is Ameliorated by Intermittent Fasting. Circ. Res. 2021, 128, 1255–1257.
- Li, M.; Li, K.; Tang, S.; Lv, Y.; Wang, Q.; Wang, Z.; Luo, B.; Niu, J.; Zhu, Y.; Guo, W. Restoration of the gut microbiota is associated with a decreased risk of hepatic encephalopathy after TIPS. JHEP Rep. 2022, 4, 100448.
- Lata, J.; Juránková, J.; Husová, L.; Senkyrík, M.; Díte, P.; Dastych, M.; Príbramská, V.; Kroupa, R. Variceal bleeding in portal hypertension: Bacterial infection and comparison of efficacy of intravenous and per-oral application of antibiotics–a randomized trial. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1105–1110.
- Chavez-Tapia, N.C.; Barrientos-Gutierrez, T.; Tellez-Avila, F.; Soares-Weiser, K.; Mendez-Sanchez; Gluud, C.; Uribe, M.N. Meta-analysis: Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding–An updated Cochrane review. Aliment. Pharmacol. Ther. 2011, 34, 509–518.
- Zhang, H.; Gao, J. Antibiotics and probiotics on hepatic venous pressure gradient in cirrhosis: A systematic review and a meta-analysis. PLoS ONE 2022, 17, e0273231.
- Gedgaudas, R.; Bajaj, J.S.; Skieceviciene, J.; Varkalaite, G.; Jurkeviciute, G.; Gelman, S.; Valantiene, I.; Zykus, R.; Pranculis, A.; Bang, C. Circulating microbiome in patients with portal hypertension. Gut Microbes 2022, 14, 2029674.
- Mendoza, Y.P.; Rodrigues, S.G.; Bosch, J.; Berzigotti, A. Effect of poorly absorbable antibiotics on hepatic venous pressure gradient in cirrhosis: A systematic review and meta-analysis. Dig. Liver Dis. 2020, 52, 958–965.
- Lim, Y.L.; Kim, M.Y.; Jang, Y.O.; Baik, S.K.; Kwon, S.O. Rifaximin and Propranolol Combination Therapy Is More Effective than Propranolol Monotherapy for the Reduction of Portal Pressure: An Open Randomized Controlled Pilot Study. Gut Liver. 2017, 11, 702–710.
- Kemp, W.; Colman, J.; Thompson, K.; Madan, A.; Vincent, M.; Chin-Dusting, J.; Kompa, A.; Krum, H.; Roberts, S. Norfloxacin treatment for clinically significant portal hypertension: Results of a randomised double-blind placebo-controlled crossover trial. Liver Int. 2009, 29, 427–433.
- Gupta, N.; Kumar, A.; Sharma, P.; Garg, V.; Sharma, B.C.; Sarin, S.K. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: A randomized trial. Liver Int. 2013, 33, 1148–1157.
- Ferrarese, A.; Passigato, N.; Cusumano, C.; Gemini, S.; Tonon, A.; Dajti, E.; Marasco, G.; Ravaioli, F.; Colecchia, A. Antibiotic prophylaxis in patients with cirrhosis: Current evidence for clinical practice. World J. Hepatol. 2021, 13, 840–852.
- Gao, Y.; Qian, B.; Zhang, X.; Liu, H.; Han, T. Prophylactic antibiotics on patients with cirrhosis and upper gastrointestinal bleeding: A meta-analysis. PLoS ONE 2022, 17, e0279496.
- Tay, P.W.L.; Xiao, J.; Tan, D.J.H.; Ng, C.; Lye, Y.N.; Lim, W.H.; Teo, V.X.Y.; Heng, R.R.Y.; Heng, R.R.Y.; Lum, L.H.W.; et al. An Epidemiological Meta-Analysis on the Worldwide Prevalence, Resistance, and Outcomes of Spontaneous Bacterial Peritonitis in Cirrhosis. Front. Med. 2021, 8, 693652.
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Gines, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, Evaluation and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome. Hepatology 2021, 74, 1014–1048.
- Yim, H.J.; Kim, T.H.; Suh, S.J.; Yim, S.Y.; Jung, Y.K.; Seo, Y.S.; Kang, S.H.; Kim, M.Y.; Baik, S.K.; Kim, H.S. Response-Guided Therapy with Cefotaxime, Ceftriaxone, or Ciprofloxacin for Spontaneous Bacterial Peritonitis: A Randomized Trial: A Validation Study of 2021 AASLD Practice Guidance for SBP. Am. J. Gastroenterol. 2023, 118, 654–663.
- Facciorusso, A.; Papagiouvanni, I.; Cela, M.; Buccino, V.R.; Sacco, R. Comparative efficacy of long-term antibiotic treatments in the primary prophylaxis of spontaneous bacterial peritonitis. Liver Int. 2019, 39, 1448–1458.
- Feuerstadt, P.; Hong, S.J.; Brandt, L.J. Chronic Rifaximin Use in Cirrhotic Patients Is Associated with Decreased Rate of C. difficile Infection. Dig. Dis. Sci. 2020, 65, 632–638.
- Pérez-Cameo, C.; Oriol, I.; Lung, M.; Lladó, L.; Dopazo, C.; Nuvials, X.; Los-Arcos, I.; Sabé, N.; Castells, L.; Len, O. Impact of Prophylactic Norfloxacin in Multidrug Resistant Bacterial Infections in the Early Liver Posttransplant Period. Exp. Clin. Transplant. 2023, 21, 236–244.
- Hurley, J.C. Selective digestive decontamination, a seemingly effective regimen with individual benefit or a flawed concept with population harm? Crit. Care 2021, 25, 323.
- Myburgh, J.; Seppelt, I.M.; Goodman, F.; Billot, L.; Correa, M.; Davis, J.S.; Gordon, A.C.; Hammond, N.E.; Iredell, J.; Li, Q. Effect of Selective Decontamination of the Digestive Tract on Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation. JAMA 2022, 328, 1911–1921.
- Garcia-Tsao, G. Prophylactic Antibiotics in Cirrhosis: Are They Promoting or Preventing Infections? Clin. Liver Dis. 2019, 14, 98–102.
- Lutz, P.; Parcina, M.; Bekeredjian-Ding, I.; Nischalke, H.D.; Nattermann, J.; Sauerbruch, T.; Hoerauf, A.; Strassburg, C.P.; Spengler, U. Impact of Rifaximin on the Frequency and Characteristics of Spontaneous Bacterial Peritonitis in Patients with Liver Cirrhosis and Ascites. PLoS ONE 2014, 9, e93909.
- Higuera-de-la-Tijera, F.; Servín-Caamaño, A.I.; Salas-Gordillo, F.; Pérez-Hernández, J.L.; Abdo-Francis, J.M.; Camacho-Aguilera, J.; Alla, S.N.; Jiménez-Ponce, F. Primary Prophylaxis to Prevent the Development of Hepatic Encephalopathy in Cirrhotic Patients with Acute Variceal Bleeding. Can. J. Gastroenterol. Hepatol. 2018, 2018, 3015891.
- Coronel-Castillo, C.E.; Contreras-Carmona, J.; Frati-Munari, A.C.; Uribe, M.; Méndez-Sánchez, N. Eficacia de la rifaximina en los diferentes escenarios clínicos de la encefalopatía hepáticaEfficacy of rifaximin in the different clinical scenarios of hepatic encephalopathy. Rev. Gastroenterol. Mex. (Engl. Ed.) 2020, 85, 56–68.
- Ponziani, F.R.; Zocco, M.A.; D’Aversa, F.; Pompili, M.; Gasbarrini, A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J. Gastroenterol. 2017, 23, 4491–4499.
- Yu, X.; Jin, Y.; Zhou, W.; Xiao, T.; Wu, Z.; Su, J.; Gao, H.; Shen, P.; Zheng, B.; Luo, Q. Rifaximin Modulates the Gut Microbiota to Prevent Hepatic Encephalopathy in Liver Cirrhosis Without Impacting the Resistome. Front. Cell. Infect. Microbiol. 2021, 11, 761192.
- Patel, V.C.; Lee, S.; McPhail, M.J.W.; Da Silva, K.; Guilly, S.; Zamalloa, A.; Witherden, E.; Støy, S.; Manakkat Vijay, G.K.; Pons, N.; et al. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J. Hepatol. 2022, 76, 332–342.
- Dalbeni, A.; Mantovani, A.; Zoncapè, M.; Cattazzo, F.; Bevilacqua, M.; De Marco, L.; Paon, V.; Ieluzzi, D.; Azzini, A.M.; Carrara, E.; et al. The multi-drug resistant organisms infections decrease during the antimicrobial stewardship era in cirrhotic patients: An Italian cohort study. PLoS ONE 2023, 18, e0281813.
- Louvet, A.; Labreuche, J.; Dao, T.; Thévenot, T.; Oberti, F.; Bureau, C.; Paupard, T.; Nguyen-Khac, E.; Minello, A.; Bernard-Chabert, B.; et al. Effect of Prophylactic Antibiotics on Mortality in Severe Alcohol-Related Hepatitis: A Randomized Clinical Trial. JAMA 2023, 329, 1558–1566.
- Marciano, S.; Gutierrez-Acevedo, M.N.; Barbero, S.; Del, C.; Notar, L.; Agozino, M.; Fernandez, J.L.; Anders, M.M.; Grigera, N.; Antinucci, F.; et al. Norfloxacin prophylaxis effect on multidrug resistance in patients with cirrhosis and bacterial infections. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 481–491.
- Komolafe, O.; Roberts, D.; Freeman, S.C.; Wilson, P.; Sutton, A.J.; Cooper, N.J.; Pavlov, C.S.; Milne, E.J.; Hawkins, N.; Cowlin, M.; et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 1, CD013125.
- Piano, S.; Singh, V.; Caraceni, P.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; Mariano, M.; et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019, 156, 1368–1380.e10.
- Fernández, J.; Prado, V.; Trebicka, J.; Amoros, A.; Gustot, T.; Wiest, R.; Deulofeu, C.; Garcia, E.; Acevedo, J.; Fuhrmann, V.; et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J. Hepatol. 2019, 70, 398–411.
- Fernández, J.; Acevedo, J.; Castro, M.; Garcia, O.; de Lope, C.R.; Roca, D.; Pavesi, M.; Sola, E.; Moreira, L.; Silva, A.; et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology 2012, 55, 1551–1561.
- Kremer, W.M.; Gairing, S.J.; Kaps, L.; Ismail, E.; Kalampoka, V.; Hilscher, M.; Michel, M.; Siegel, E.; Schattenberg, J.M.; Galle, P.R.; et al. Characteristics of bacterial infections and prevalence of multidrug-resistant bacteria in hospitalized patients with liver cirrhosis in Germany. Ann. Hepatol. 2022, 27, 100719.
- Fernandez, J.; Piano, S.; Bartoletti, M.; Wey, E.Q. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J. Hepatol. 2021, 75 (Suppl. 1), S101–S117.
- Delavy, M.; Burdet, C.; Sertour, N.; Devente, S.; Docquier, J.D.; Grall, N.; Volant, S.; Ghozlane, A.; Duval, X.; Ghozlane, A.; et al. A Clinical Study Provides the First Direct Evidence That Interindividual Variations in Fecal β-Lactamase Activity Affect the Gut Mycobiota Dynamics in Response to β-Lactam Antibiotics. mBio 2022, 13, e0288022.
- Shamsaddini, A.; Gillevet, P.M.; Acharya, C.; Fagan, A.; Gavis, E.; Sikaroodi, M.; McGeorge, S.; Khoruts, A.; Albhaisi, S.; Fuchs, M.; et al. Impact of Antibiotic Resistance Genes in Gut Microbiome of Patients with Cirrhosis. Gastroenterology 2021, 161, 508–521.e7.
More
Information
Subjects:
Gastroenterology & Hepatology; Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
879
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
26 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




