Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wu, Y.; Yang, H.; Gao, H.; Huang, X.; Geng, L.; Zhang, R. Classification of Various Ligands. Encyclopedia. Available online: https://encyclopedia.pub/entry/49564 (accessed on 07 February 2026).
Wu Y, Yang H, Gao H, Huang X, Geng L, Zhang R. Classification of Various Ligands. Encyclopedia. Available at: https://encyclopedia.pub/entry/49564. Accessed February 07, 2026.
Wu, Yufeng, Hui Yang, Haojie Gao, Xiaoyi Huang, Liyuan Geng, Rui Zhang. "Classification of Various Ligands" Encyclopedia, https://encyclopedia.pub/entry/49564 (accessed February 07, 2026).
Wu, Y., Yang, H., Gao, H., Huang, X., Geng, L., & Zhang, R. (2023, September 24). Classification of Various Ligands. In Encyclopedia. https://encyclopedia.pub/entry/49564
Wu, Yufeng, et al. "Classification of Various Ligands." Encyclopedia. Web. 24 September, 2023.
Copy Citation
Enantioselective gold catalysis has flourished due to its distinct performance. In particular, a series of valuable chiral ligands have been identified, including aryl phosphine ligands bearing the proximal chiral sulfinamide motif, phosphoramidite and phosphonite ligands, phosphine ligands comprising the ferrocene scaffold, bifunctional phosphine ligands, biphosphine ligands, chiral counterion-based ligands derived from chiral phosphoric acids and chiral carbene ligands for gold(I) catalysis, along with unique ligand frameworks for cyclometalated gold(III) catalysis.
chiral ligand
asymmetric
gold catalysis
π-acidic activation
1. Introduction
Catalysis is an advanced technology in both the chemical industry and academic research [1][2][3]. Transition-metal catalyzed organic transformations have attracted a great deal of attention for the efficient crack and formations of chemical bonds [4][5][6][7]; therefore, they have been widely applied in the research and development of drugs, pesticides, fine chemicals and other functional materials [8][9][10]. Compared to other transition metals, the d10 electron configuration of gold(I) results in superior π affinity for multiple carbon–carbon bonds (Figure 1a) [11][12][13][14][15]. In addition, the gold center adopted by linear coordination places the ancillary ligand and substrate in opposite positions (Figure 1b). Thus, the conventional chelating mode of the ligands used in catalytic reactions involving other transition metals could not be employed for the design of ligand-modified gold catalysts. Furthermore, the gold(I)-catalyzed carbophilic addition of nucleophiles to carbon–carbon triple bonds proceeds through outer-sphere activation [16][17][18][19][20][21][22][23][24]. These factors make asymmetric modulation in gold(I) catalysis challenging. On the other hand, several gold(III) catalysts have also been reported to be active for such transformations because of their inherent square-planar geometry that promotes the formation of chiral centers.
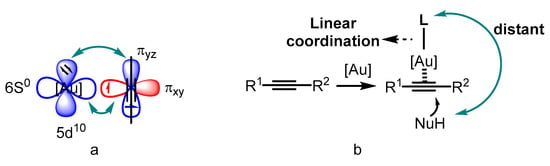
Figure 1. The superior π affinity mode (a) and the linear coordination format (b).
The practical atom economy and step economy of enantioselective gold catalysis are attributed to its unique catalytic mode for constructing sets of complex molecules and efficient tandem processes, even when dealing with remote substrates and chiral ligand fragments [25][26][27][28][29][30]. Enantioselective gold catalysis has flourished due to its distinct performance. In particular, a series of valuable chiral ligands have been identified, including aryl phosphine ligands bearing the proximal chiral sulfinamide motif, phosphoramidite and phosphonite ligands, phosphine ligands comprising the ferrocene scaffold, bifunctional phosphine ligands, biphosphine ligands, chiral counterion-based ligands derived from chiral phosphoric acids and chiral carbene ligands for gold(I) catalysis, along with unique ligand frameworks for cyclometalated gold(III) catalysis. Notably, these types of chiral ligands and structural modifications are of crucial significance.
2. Classification of Various Ligands
2.1. Phosphoramidite and Phosphonite Ligands
In the gold(I)-catalyzed [4 + 3] or [4 + 2] cyclization, electron-withdrawing phosphite ligands are believed to fit the excellent activation of the corresponding gold(I) catalysts [31][32]. To construct better chiral modulation for the challenging asymmetric gold(I) catalysis, phosphoramidite ligands have also been incorporated into the catalytic systems owing to their similar electronic properties with phosphites and π–π interactions with substrates resulting from the attenuated flexibility around the gold center as well as the closer chiral information to the new carbon stereocenters. For the enantioselective intramolecular cycloaddition of allene-tethered 1,3-dienes 1, the bicyclic products 2 and 3 can be furnished smoothly, and phosphoramidite ligand L1-ligated gold(I) catalyst displayed superior chemoselectivity (16:1.2) and enantioselectivity (91% enantiomeric excess (ee)) over the chiral bisphophine (S)-(−)-2,2′-bis(diphenylphosphino)-1,1′-binaphthalene (S-BINAP)-coordinated gold(I) analogue (Scheme 1) [33]. The proposed mechanism begins with the activation of an allene through the coordination of a gold complex. This activation leads to a concerted [4 + 3] cycloaddition, resulting in the formation of gold carbene intermediate III. The selectivity between [1][2]-R migration and ring contraction was demonstrated with density functional theory (DFT) calculations. The calculation results indicate that the ring contraction pathway possesses a lower energy barrier than that of the [1,2]-R migration pathway.
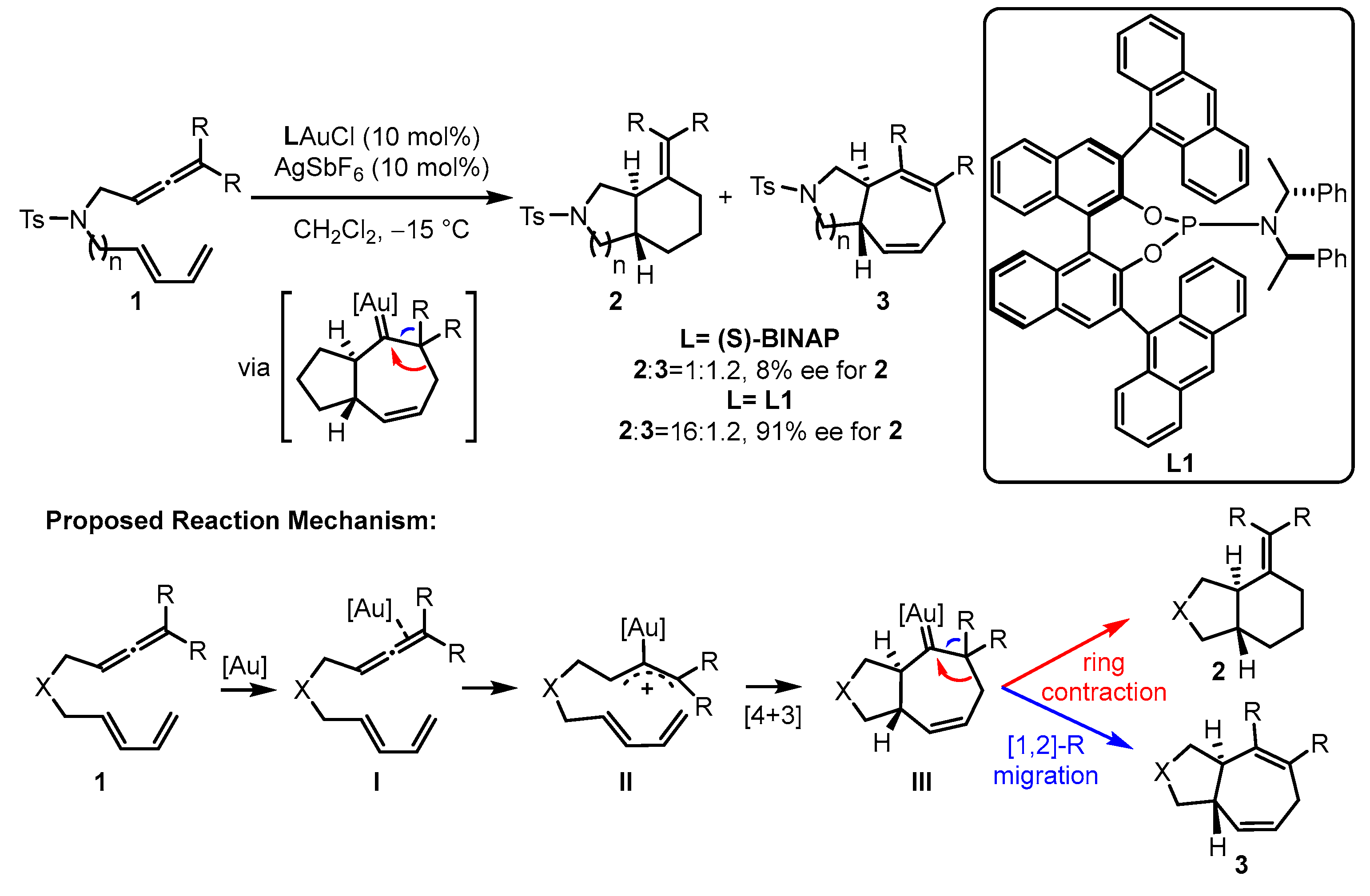
Scheme 1. The chiral phosphoramidite-tethered gold(I)-catalyzed asymmetric cycloaddition.
Fürstner et al. implemented enantioselective cyclopropanation of styrene 4 catalyzed by a gold(I) catalyst bearing a bulky steric chiral phosphoramidite ligand associated with the 1-(2-hydroxynaphthalen-1-yl)naphthalen-2-ol (BINOL) core [34]. Nevertheless, the performance of structural modifications on this type of ligand is difficult. Accordingly, the authors further developed chiral gold catalysts with phosphoramidites featuring the α,α′-(dimethyl-1,3-dioxolane-4,5-diyl)bis(diphenylmethanol) (TADDOL) backbone (Scheme 2). With a certain adjustment of aryl substitution in the TADDOL part and amine component, L3 was found to be an ideal companion for the gold catalyst in asymmetric [2 + 2] cyclization with a 91% yield and 99% ee value, while inferior results were obtained with the employment of L2 [35]. The crystal structure of L2AuCl suggests that the two phenyl groups from the TADDOL part along with one phenyl ring from the amine part can form a cone-shaped binding pocket surrounding the gold atom. This pocket effectively restricts the flexibility of the gold center and conveys the chirality information to the final product. Another study illustrated that L4 was highly effective to realize the gold-catalyzed enantioselective cycloisomerization of 1,6-enyne 6, leading to the formation of the bicyclic product 7 in 89% yield and 95% ee (Scheme 3) [36]. Despite being an ancillary point to the gold catalysts, this ligand type displays remarkable chiral transformation for π-acidic activation.
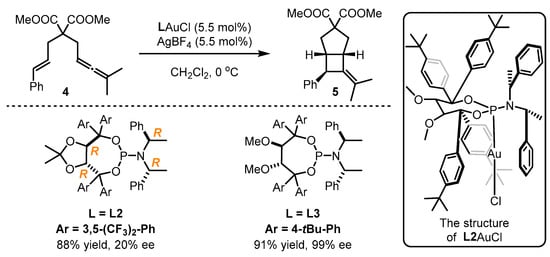
Scheme 2. The application of TADDOL-containing ligands L2 and L3 in asymmetric gold catalysis.
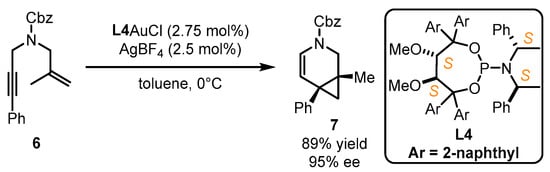
Scheme 3. The application of TADDOL-containing ligand L4 in asymmetric gold catalysis.
In pursuit of greater success, the Alcarazo group adopted an elaborated preparation of chiral cationic ancillary phosphonites and developed asymmetric cyclization of diyne 8 (Scheme 4) [37]. Their design utilized the TADDOL backbone to provide an appropriate chiral pocket for this catalytic process, while the cationic imidazolium unit introduced a positive charge to enhance the catalytic activity of the corresponding gold(I) template, leading to the facile synthesis of chiral helicene 10 with excellent regioselectivity and enantioselectivity when the L6-coordinated gold(I) catalyst was employed. However, L5 was unsatisfactory in this regard. The single-crystal structure of L6AuCl clearly reveals that the gold atom can immerse into a chiral pocket resulting from two CF3-phenyl groups and one mesityl group. Compared with L5AuCl, the formation of a seven-membered ring without the constrains of annulation was observed for L6AuCl, which leads to a closer contact between the gold atom and the three vicinal aryl substituents.
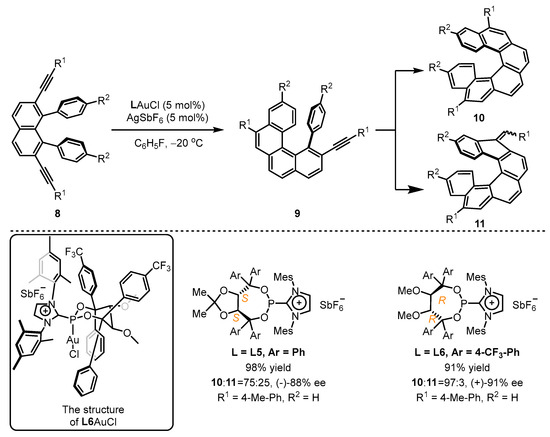
Scheme 4. Chiral cationic ancillary phosphonites applied in gold(I) catalysis.
Given the success of TADDOL-type phosphonites in gold(I)-catalyzed enantioselective hydroarylation, the Alcarazo group attempted to construct one helical and two axial stereogenic elements in one molecule using similar ligands (Scheme 5) [38]. They anchored multi-substituted diyne 12 for the attempt of gold(I) catalysts comprising chiral cationic ancillary phosphonites. It was found that L7 led to the simultaneous isolation of 13a and 13b with a remarkable 91% ee value for 13a. However, L8 was not up to the task, resulting in an unsatisfactory 8% ee value for 13a despite exhibiting excellent catalytic activity with a total yield of 90% for 13a and 13b.
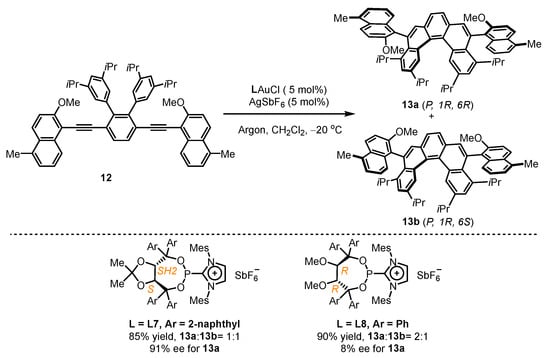
Scheme 5. Chiral cationic ancillary phosphonites applied in gold(I) catalysis.
2.2. Aryl Phosphine Ligand with a Proximal Chiral Sulfinamide
Apart from the phosphoramidite and phosphonite ligands mentioned above, aryl phosphine ligands bearing one chiral sulfinamide motif have also been developed. As reported by Zhang and coworkers, the in situ formed monogold cationic catalyst generated from digold chloride [(AuCl)2] always showcased a better catalytic activation ability than the pre-prepared monogold cationic catalyst [39][40]. Zhang et al. provided an elaborate design of the MingPhos series (including L9 and L10), where the chiral sulfinamide moiety containing adjustable steric groups was located on the ortho-position of the aryl phosphines (Scheme 6) [41]. The L9/L10-tethered gold(I) complexes performed good activity for the intermolecular asymmetric cycloaddition of 2-(1-alkynyl)-2-alken-1-one 14 and nitrone 15 with the L10-based gold complex exhibiting higher performance than the L9-containing analogue in terms of enantioselectivity. Moreover, a proposed mechanism was presented.
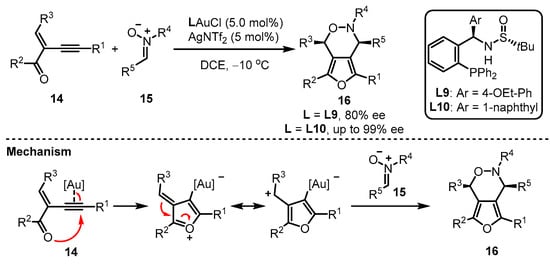
Scheme 6. Asymmetric synthesis of chiral α-Allyl-α,β-butenolides.
PC-Phos ligands, featuring a bigger chiral cave and bigger retortion of the sulfinamide part to the proximal phosphine part, were later exploited by Zhang et al. (Scheme 7) [42]. As expected, the catalytic system accommodated protected N-allenamide 18 smoothly with a 99% yield and 96% ee. The ligand L11 and a suitable protecting group were crucial for the control of high regioselectivity and enantioselectivity in this catalytic process. The authors proposed an asymmetric induction model based on the structure of L11AuCl and product 18. According to the model, the indole part only attacks the gold-activated allene bond at the Si face. Otherwise, if the attack occurs from the other side, the nucleophilic attack will be blocked by the steric obstacle from the OMe group in phosphine. Additionally, the chirality transfer can be facilitated by the hydrogen bonding interaction between the sulfonyl group and the N–H bond from the amino group, as suggested by the model of the substrates and the chiral PC-Phos ligands.
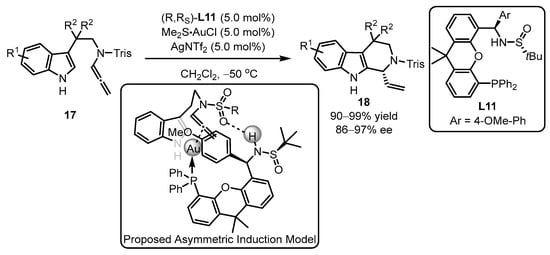
Scheme 7. PC-Phos ligands ancillary to gold complex for the asymmetric arylation.
Recently, MingPhos ligands were introduced into gold(I)-catalyzed intermolecular asymmetric [3 + 2] cycloaddition of N-allenamides 19 and nitrone 20 by Zhang et al. (Scheme 8) [43]. To note, the opposite enantiomers of oxazolidin-2-one 21 or 22 were achieved via the usage of (R,Rs)-L12 or the corresponding (S,Rs)-L12. The interactions between the sulfinamide N−H group and the pentafluorophenyl substituent are responsible for the effective allocation of chirality transfer.
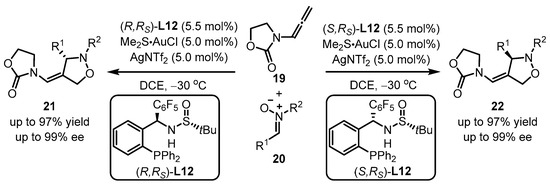
Scheme 8. MingPhos ligands ancillary to gold complexes for asymmetric arylation.
2.3. Phosphine Ligands Comprising the Ferrocene Scaffold
Phosphine ligands bearing the ferrocene backbone are a typical class of chiral ligands used for enantioselective gold(I) catalysis due to their easy accessibility and possibility to modulate chiral formation. Hayashi and colleagues reported the successful cooperation between chiral bisphosphines with the ferrocene backbone decorated with an amino group and a gold complex, in situ generating an chiral gold species for the asymmetric aldol reaction of aldehyde 23 and isocyanoacetate 24 (Scheme 9) [44]. The diastereoselectivity and enantioselectivity depend on the selection of a bulky aldehyde and a suitable ligand. Furthermore, the L13-tethered gold template provided divergent chiral oxazoline derivative 25, while the similar ligand (L14) did not efficiently catalyze the process.
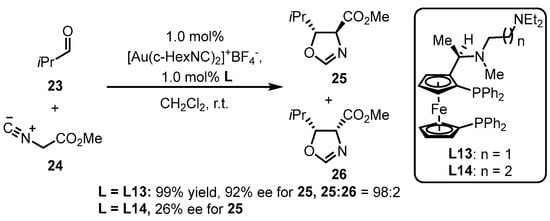
Scheme 9. Phosphine ligands possessing the ferrocene backbone for asymmetric gold catalysis.
Owing to the outer-sphere catalytic mode between a gold(I)-activated carbon–carbon triple bond and a nucleophile, the intermolecular asymmetric [2 + 2] cycloaddition of terminal alkynes 27 with alkenes 28 is challenging to conduct in gold(I) catalysis. High-throughput methods, including the screening of 90 chiral ligands, were employed to accelerate the formation of chiral cyclobutene via gold(I) catalysis (Scheme 10) [45]. Non-C2 symmetric bisphosphine ligand L15 with the ferrocene skeleton was found to be an excellent promoter for the corresponding gold(I) complex, providing catalytic reactivity under the accurate ratio of the gold(I) complex to NaBArF4 in a 1:1 ratio.
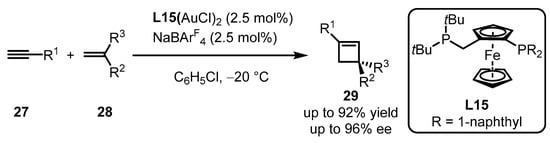
Scheme 10. Ferrocenyl bisphosphine-ligated gold catalyzed asymmetric [2 + 2] cycloaddition.
In light of the chiral JohnPhos-type scaffold, the Echavarren group synthesized a set of planar chiral monodentate 1,3-disubstituted ferrocenyl phosphines for the assembly of a sterogenic gold(I) complex (Scheme 11) [46]. It was suggested that the ancillary bulky adamantyl in ferrocene was required for the excellent performance of L16 in the desired [4 + 2] cyclization [47]. From the DFT calculations, the T-shaped π–π interaction between 3,5-(CF3)2-aryl from the phosphine ligand and an aryl group from the substrate as well as the Si face interaction of an alkenyl group with an alkynyl group meet the lowest energy for the chirality transfer.
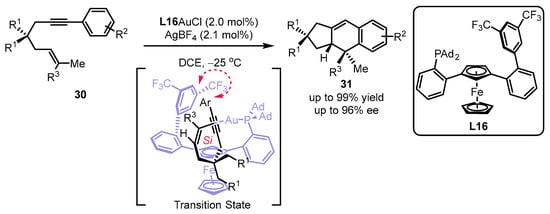
Scheme 11. Design of planar chiral phosphines for the asymmetric [4 + 2] cyclization.
2.4. Bifunctional Phosphine Ligand
Benefitting from enol chemistry that the α-C−H bond of a carbonyl could be clearly attenuated by the activation from an acid (Lewis acid or protic acid) to the carbonyl group, the Zhang group developed bifunctional phosphine ligands for gold(I)-catalyzed asymmetric reactions [48]. In such reactions, a Lewis acid acts as a ‘pull’, and a weak base functions as a ‘push’. The weak base Et3N (pKa in DMSO, 9.0) can be employed to remove α-H of a carbonyl or an imine group (pKa in DMSO ∼16–30) [49]. Without the utilization of a strong base, the reactions could accommodate more base-sensitive substances (Scheme 12A) [50][51]. From the initial design, it is anticipated that the α-H of a C−C triple bond, i.e., a propargylic proton (estimated pKa in DMSO, >30) [52][53], could be removed by a weak base with the ‘pull’ of a gold(I) catalyst to an alkynyl group (Scheme 12B,C). As a typical example, biphenyl 2-ylphosphines with remote tertiary amino groups for gold(I) catalysis were conceived by Zhang and coworkers (Scheme 13) [50]. As expected, the ligated tertiary amino group could play the role of ‘push’ for the removal of the propargylic proton to initiate new allene reactions, conjugated alkene reactions and aldol-type reactions in alkyne chemistry. With the introduction of asymmetric elements into the ligand, chiral products could be effectively achieved. As per the principle, the authors successfully converted racemic β,γ-butenolides 32 into chiral α,β-butenolides 33 promoted by chiral bifunctional phosphine ligand (S-L17)-ligated gold(I) catalysts (Scheme 13) [54]. In contrast to the JohnPhos-tethered gold(I) catalyst exhibiting 6% product yield and no ee value, the (S)-L17-tethered gold(I) catalyst showed excellent asymmetric catalysis for the γ-protonation process (99% yield and 99% ee).
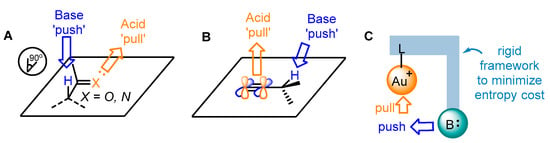
Scheme 12. The idea of enol chemistry (A) and the inspiration for the propargyl chemistry (B) and designed approach for bifunctional phosphine ligands in asymmetric gold catalysis (C).
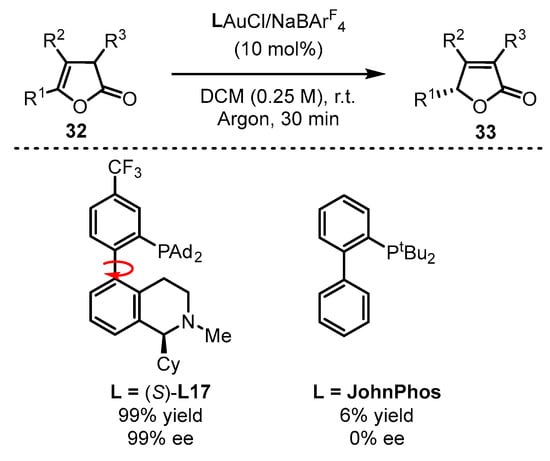
Scheme 13. Enantioselective isomerization of β,γ-butenolides to chiral α,β-butenolides.
The bifunctional phosphine ligand (S)-L17 features a fluxional biphenyl axis and contains a remote tertiary amino group possessing a vicinal chiral group to remove the α-H of an alkynyl group or a carbonyl group (Scheme 14). Though the biphenyl motif is fluxional, the chiral group can shield the amino group from one conformer to conduct the deprotonation step, which displays the chiral modulation for the process. Moreover, the cooperative ‘pull’ and ‘push’ of the bifunctional phosphine ligand-ligated gold catalysts were rationalized. Upon gold activation to the carbonyl group, the α-H is anticipated to be more acidic, allowing it to be removed by a weak base such as a tertiary amino group. Consequently, the metal enolate generated from soft enolization and the chiral ammonium cation constitute a chiral ion pair. Eventually, the chiral protonation process becomes possible, catering to the principle of soft deprotonation and chiral protonation for the asymmetric isomerization into α,β-butenolides 33 with high enantioselectivity from β,γ-butenolides 32.
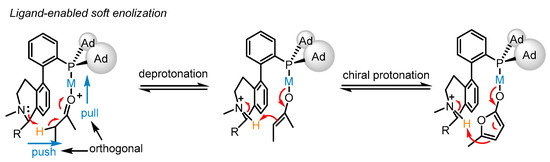
Scheme 14. Enantioselective isomerization of β,γ-butenolides to chiral α,β-butenolides.
The Zhang group achieved the implementation of a reaction from propargylic sulfonamides into chiral polysubstituted pyrroles via the cooperative catalysis of gold(I) and chiral bifunctional phosphine ligand (S)-L17 (Scheme 15) [55]. The innate diastereoselectivity of this reaction and the ‘matched’ geometry of the (S)-L17-associated gold(I) catalyst with substrates contributed to an excellent diastereo ratio (d.r.) with two chiral centers (2-methyl-5-cyclohexyl pyrrole derivative(36a–36c). The key procedure is the formation of allenes generated from the asymmetric isomerization of sulfonamides. Notably, the asymmetric reaction could tolerate high reaction temperature despite the routine pattern of low temperature to achieve higher enantioselectivity. However, the desired product was obtained in poor yield and low enantioselectivity when (R)-L17 was exposed to the catalytic system.
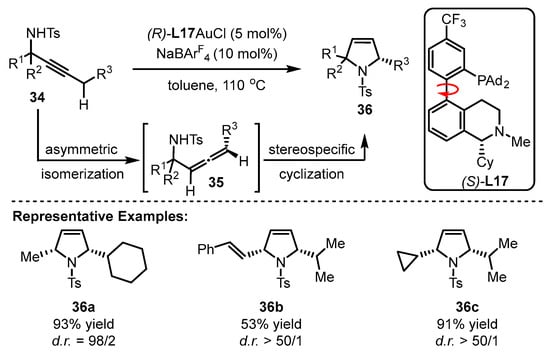
Scheme 15. Enantioselective isomerization of β,γ-butenolides to chiral α,β-butenolides.
Additionally, the Zhang group reported that the asymmetric version of ynolates 37 to chiral α-Allyl-α,β-butenolides 38 was achieved with the aid of a (S)-L17-ligated gold catalyst (Scheme 16) [56]. As described, in this tandem reaction, the ‘push’ and ‘pull’ processes were involved several times for the generation of an allene intermediate, a furan intermediate and the isomerization product. According to their reports, no ee value was detected for the desired product if a racemic L18 ligand was introduced instead of (S)-L17, despite the excellent yield obtained [57].
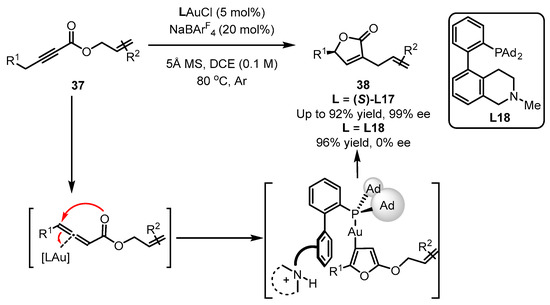
Scheme 16. Asymmetric synthesis of chiral α-Allyl-α,β-butenolides.
Later, the same group reported gold(I)-catalyzed enantioselective dearomatization of phenol 39 to drug-valuable chiral spirocyclic enone products 40, employing the strategy of gold(I)-chiral ligand cooperation (Scheme 17) [58]. The distal phosphonate presented in the binaphthyl framework L19 displayed better catalytic performance compared to the JohnPhos ligand. It is believed that the H-bond interaction of the phosphate with the hydroxyl group in phenol is the crucial step that dictates asymmetric selectivity and accelerates the dearomatization/cyclization process.
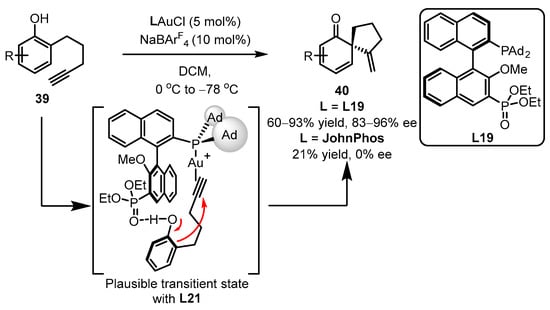
Scheme 17. Asymmetric synthesis of chiral α-Allyl-α,β-butenolides.
2.5. Biphosphine Ligands
Apart from the above monophosphine ligands, biphosphine ligands have also been developed. Thus, this section intends to present the development of these biphosphine ligands and their applications in gold-catalyzed asymmetric reactions. Although the linear coordination of a ligand with the gold(I) complex generates a single coordination site from the metal center, the asymmetric bisphosphine ligands have been successfully applied to multiple carbon–carbon bond activation reactions. The arguable Au–Au interaction is believed to play a crucial role in modulating chiral transfer due to its subtle tortuosity for the linear coordination way.
The Echavarren group first discovered the potential of a bisphosphine ligand (L20) in the gold(I)-catalyzed enantioselective tandem cycloisomerization and hydromethoxylation process (Scheme 18) [59]. Remarkably, the addition of a catalytic amount of a silver salt suggested that the monocationic Au species promoted the transformation. The reaction occurred as the electron-rich alkenyl part acted as a nucleophile to attack the chiral gold-activated alkynyl group. After the gold complex feedbacked one pair of electrons to the double bond, the alkenyl group combined with an unstable cation for the formation of a gold carbene species. Following the ring-opening and protodeauration process, the chiral alkoxylation product could be given.
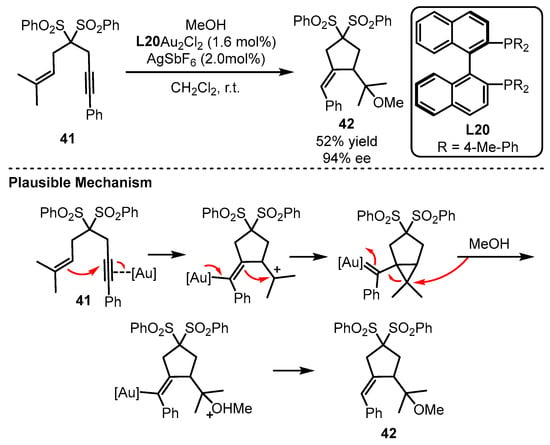
Scheme 18. Enantioselective bisphosphine-tethered gold(I) catalysis.
In the same year, the Toste group documented cyclopropantion reactions of alkyne 43 and aryl alkenes 44 using (R)-DTBM-SEGPHOS (L21) as the optimal bisphosphine ligand, affording the desired products 45 with high diasteroselectivity (>20:1) and enantioselectivity (76–94% ee) [60]. In this catalytic process, reactive gold carbene species were involved after a Rautenstrach rearrangement process. The proposed mechanism suggests that substituents from alkenes will intrinsically interact with the ligated gold complex (IIb), which disfavors the formation of trans-substituted cyclopropanes. Thus, the cis-selectivity was easily offered in most cases. However, the sterically less hindered BINAP ligand could not efficiently induce the transfer of chirality (Scheme 19), as observed from the ligand screening data.
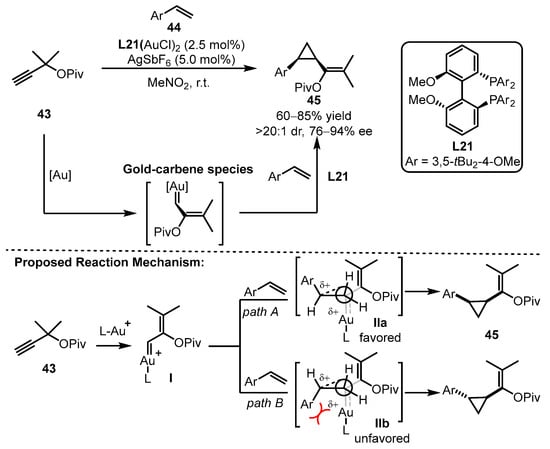
Scheme 19. Asymmetric cyclopropanation of alkynes and aryl alkenes.
Additionally, bisphosphine-containing gold complexes were also applicable to asymmetric alkoxylation of allenes, as illustrated by Widenhoefer and coworkers (Scheme 20) [61]. Specifically, bisphosphine ligand L22 was smoothly accommodated with allene 46, providing (R)-4,4-diphenyl-2-vinyltetrahydrofuran 47 with a moderate yield and high enantioselectivity (67% yield, 93% ee).
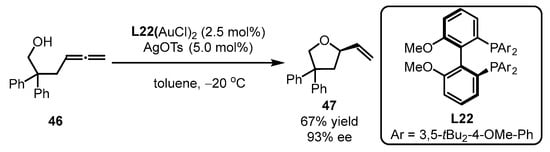
Scheme 20. Gold(I)-catalyzed asymmetric alkoxylation of allenes.
Shin and coworkers applied a chiral bisphosphine ligand (L23) derived from the SegPhos backbone to a gold-catalyzed enantioselective intermolecular [4 + 2] cycloaddition of propiolate 48 with substituted alkene 49 (Scheme 21) [62]. α, β-Unsaturated δ-lactone 50 was detected as the main product along with several diene side-products generated from metathesis and conjugate addition. Furthermore, the use of 1,1,2,2-Cl4-ethane as a solvent and the addition of sodium dodecyl sulfate (SDS) as a surfactant were significant for enhancing chemoselectivity and mediating chiral transfer.
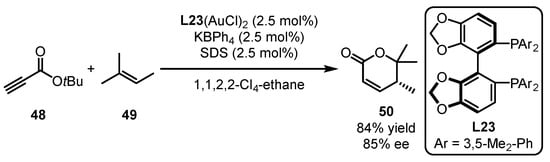
Scheme 21. Dimeric gold catalyst-promoted enantioselective [4 + 2] cycloaddition.
2.6. Chiral Counterions Derived from Chiral Phosphoric Acids
The phosphine-containing organic ligands are summarized from Scetion 2.1. to Section 2.5.. In this section, a unique type of phosphine ligand, referred to as chiral phosphoric acid-derived chiral counterion ligands, is discussed. The development of asymmetric gold catalysis via chiral ion pairs between cationic gold catalysts and chiral counterions is a successful strategy to confer to the enantioselectivity of gold-catalyzed reactions [63]. The Toste group explored the counterion strategy in gold catalysis for the intramolecular asymmetric hydroalkoxylation of allenes [64]. To achieve high enantioselectivity in the reactions, the “matched” effect of a phosphine-associated gold catalyst with a chiral silver salt should be considered (Scheme 22). Specifically, the cooperation of bis(chlorogold) bis(diphenylphosphino)methane [dppm(AuCl)2] with Ag-L24 prompted the asymmetric cyclization of allenol 51 to tetrahydrofuran 52 with excellent yield and enantioselectivity. On the contrary, inferior results were provided when Ph3PAuCl was utilized instead of dppm(AuCl)2.
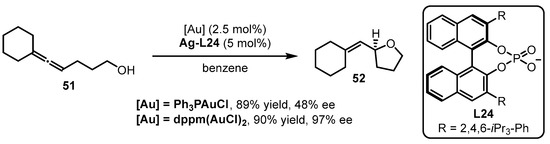
Scheme 22. Chiral counterion-induced asymmetric cyclization of allenol 51.
On the other hand, the chiral counterion induction approach could be extended to enantioselective cyclization of alkynyl hydroxylamine 53 (Scheme 23) [65]. The ‘matched’ system of dppm(AuCl)2 with Ag-L25 could induce efficient chirality mediation for the formation of vinyl isoxazolidine 54. As an alternative, if Ag-L25 was displaced with Ag-L26, the enantioselectivity of the reaction decreased dramatically.
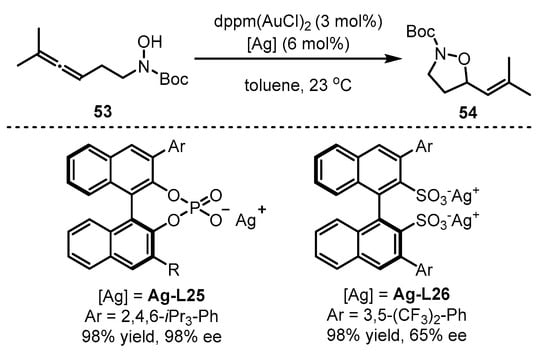
Scheme 23. Gold(I)-catalyzed asymmetric formation of vinyl isoxazolidines.
2.7. Chiral Carbene Ligand
Apart from the above phosphine-containing organic ligands, chiral carbene ligands have also been developed for asymmetric gold catalysis. As the distinct performance of gold(I)-carbene complexes in terms of chemoselectivity and regioselectivity in carbophilic activation, chiral carbene ligand-involving gold(I) catalysts were also elaborately designed to conduct a set of asymmetric transformations [66]. Considering the repertoire of an electron-rich group in offering the stability of an allene intermediate, Toste and coworkers conceived that, compared to phosphite-tethered gold complexes, the more electron-donating carbene-coordinated gold(I) catalysts could facilitate 6-endo-trig cyclization of propargyl ester 55 in a more efficient manner (Scheme 24) [67]. Undoubtedly, incorporation of different substituents into the ligand skeleton is crucial for the effective enantioselectivity control. As observed, ligand L28 bearing a 4-CF3 group was identified as an excellent auxiliary for achieving 85% yield and 91% ee, while the unsubstituted ligand (L27) triggered an inferior result.
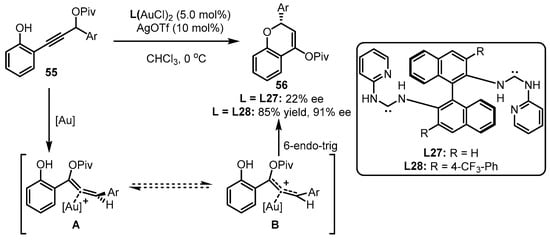
Scheme 24. Asymmetric [3 + 3] reaction catalyzed by (acyclic diaminocarbene)-gold(I) complexes.
Subsequently, Slaughter and coworkers developed impressive monodentate acyclic diaminocarbene ligands (L29, L30) for the enantioselective gold(I) catalysis based on the comprehension of the chiral (carbene)gold template (Scheme 25) [68]. The designed monodentate gold(I) complexes were introduced into tandem cyclization and nucleophilic addition of ortho-alkynylbenzaldehyde 57. Although L29 mismatched the catalytic system with a detrimental result, the modified ligand (L30) was found to be an excellent promoter for the formation of sterogenic 1H-isochromene 58 (70% yield, 99% ee). It appeared that the secondary interactions generated from the electrostatic attraction between the decorated amino group and suitable substrate promoted the efficient construction of the chiral motif. The crystal structure of L30AuCl reveals that the rotation of the gold complex with the substrates is restricted due to the interaction between the gold atom and the aryl ring (3,5-(CF3)2-Ph in binaphthyl skeleton) as well as the π–π stacking between a phenyl (in the amino part) and a naphthyl unit. This restriction facilitates chirality control during the cyclization process.
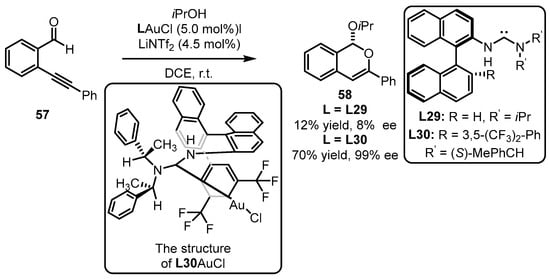
Scheme 25. Tandem cyclization reaction catalyzed by (acyclic diaminocarbene)gold(I) complexes.
Acyclic diaminocarbene gold(I) complexes were further applied to the asymmetric hydroazidation and hydroamination of allenes 59 by the Toste group (Scheme 26) [69]. The introduction of carbene ligand L31 resulted in excellent yield and ee values, whereas the other explored ligands failed to dictate the chirality formation. It is noteworthy that, with L31 as a ligand, opposite conformers can be obtained only by using different nucleophiles (TMSN3 for the formation of 60 and H2NBoc for the generation of 61).
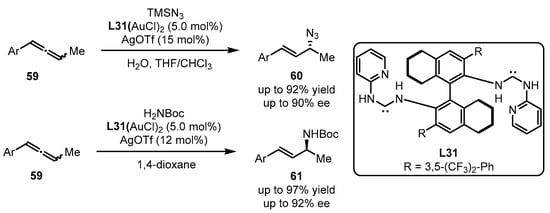
Scheme 26. Chiral carbene-tethered gold catalyzed nucleophilic addition of allenes.
The Toste group subsequently challenged enantio-induction of tandem [3,3]-sigmatropic rearrangement and [2 + 2]-cyclization of propiolate 62 containing an indolyl group with the aid of computation (Scheme 27) [70]. The bulky L32-tethered gold(I) complex was identified as the optimized catalyst for chirality transformation through the construction of three chiral carbon centers. The computational studies suggested that the intramolecular H-bond formation from vicinal amino NH and O facilitated the mediation of chirality achievement.
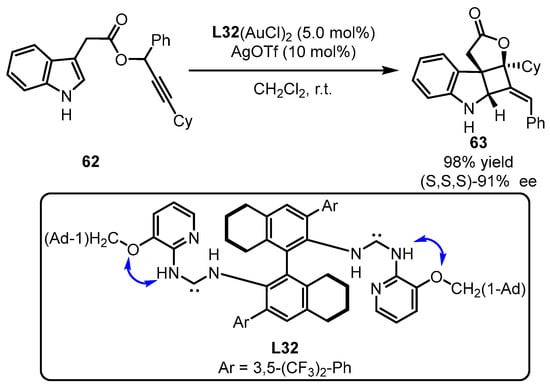
Scheme 27. (Acyclic diaminocarbene)gold(I) complexes for challenging chirality formation.
2.8. Cyclometalated X-Y (X = C, O; Y = C, O) Ligand Frameworks
Enantioselective reactions with gold(I) catalysis have been extensively developed [71][72][73], as also illustrated in all the above examples. However, the linear geometry of gold(I) catalysts restricts its ability to modulate chirality formation (Scheme 28). On the other hand, the square-planar geometry of gold(III) catalysts introducing cyclometalated ligand frameworks is expected to avoid the restriction owing to the potential mediation from lithered chiral ligand proximal to the reactive center. However, the unstable risk of gold(III) complex 64 for reduction to gold(I) or unreactive state of traditional gold(III) catalyst 65 impeded its application in enantioenriched catalysis [74][75][76].
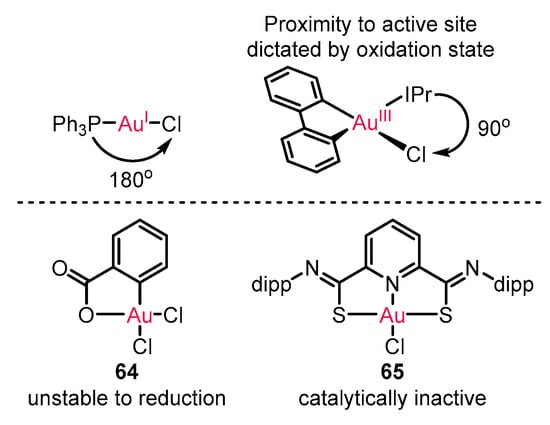
Scheme 28. The innate difference of gold(I) and gold(III) catalytic mode.
To address the above-mentioned limitations of traditional gold(III) catalysts, the Toste group developed a cyclometalated C−C ligand framework to stabilize the gold(III) cationic species [77]. This framework possesses powerful catalytic activity while maintaining a stable structure that facilitates effective reaction turnover (Scheme 29). A chiral N-heterocyclic carbene (NHC) ligand was positioned in the square vicinal to the reaction center to induce chiral formation. The resulting elaborated chiral NHC-tethered gold(III) catalysts displayed varied activity and divergent enantioselectivity in the cycloisomerization of enynes 66. Notably, (R,R)-gold(III)(biphenyl)L33Cl selectively facilitated the conversion of the (R)-enyne substrate into bicyclic adducts 67 in moderate conversion (44%) and good ee value (85%), whereas the gold(III) catalyst bearing L34 showcased a sluggish result (4% conversion, −2% ee) [78]. Meanwhile, (S)-enyne substrate 68 was provided along with the enantioconvergent cyclization via kinetic resolution. As described above, the reaction is initiated by the effective coordination of the gold complex with the triple bond of the enyne substrates, leading to the formation of complex I. Following this, a concerted cycloaddition took place, resulting in the generation of gold carbenes (II and II’). Subsequently, the bicyclic scaffold is furnished with the migration of α–H and the release of the catalyst from the system.
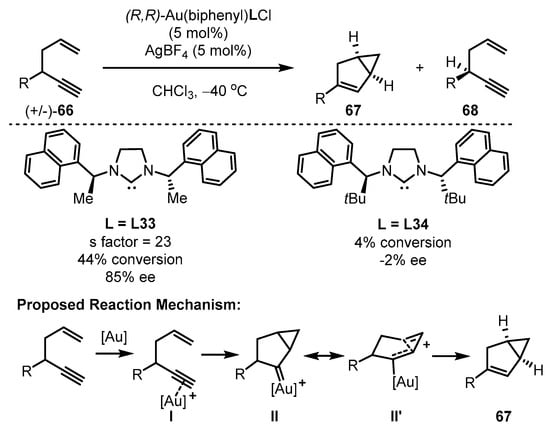
Scheme 29. The chiral gold(III)-catalyzed asymmetric cycloisomerization of enynes.
The square-planar chiral gold(III) catalysts were further applied to the asymmetric cycloaddition of 2,4-hexadienals 69 and cyclopentadiene 70 to synthesize valuable chiral bicycles 71 (Scheme 30) [79]. Different NHC ligands were prepared for the formation of chiral gold(III) catalysts. As observed, 1-naphthyl substituted NHC (L36) demonstrated superior chiral induction compared with the 2-naphthyl counterpart (L35), which suggests that a sterically bulky ligand is beneficial for this catalytic transformation.
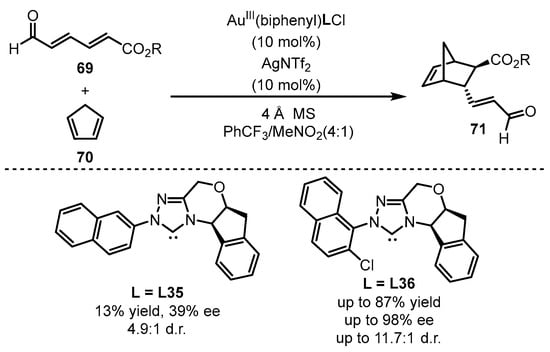
Scheme 30. The chiral gold(III)-catalyzed enantioselective Diels–Alder reaction.
Subsequently, Wong and coworkers synthesized O,O-chelated cyclometalated oxazoline gold(III) catalysts [76]. When the chiral O,O-chelated backbone was introduced into the gold(III) complexes, the combination of gold(III) catalyst (74 or 75) and L-camphorsulfonic acid (L-CSA) effectively initiated the carboalkoxylation reaction of alkyne 72 for the formation of 3-methoxyindanone 73 (Scheme 31) [80]. From the proposed transition state, the steric hindrance from the 2,4,6-trimethylphenyl group in the oxazoline part would be significant. This hindrance creates clear repulsion towards the substrate in the unfavored TSB, which contributed significantly to the improvement of enantioselectivity (75% ee for 74 vs. 90% ee for 75).
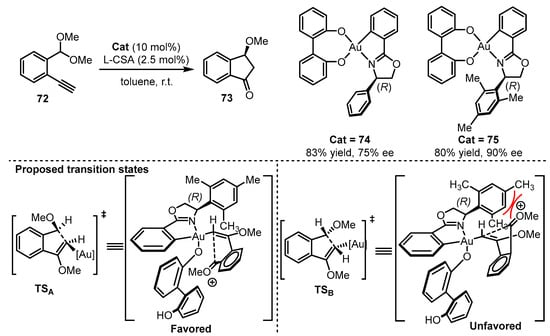
Scheme 31. Asymmetric O,O-chelated cyclometalated oxazoline gold(III) catalysis.
References
- Santi, M.; Sancineto, L.; Nascimento, V.; Braun Azeredo, J.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow Biocatalysis: A Challenging Alternative for the Synthesis of APIs and Natural Compounds. Int. J. Mol. Sci. 2021, 22, 990.
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396.
- García-Sancho, C.; Mérida-Robles, J.M.; Cecilia-Buenestado, J.A.; Moreno-Tost, R.; Maireles-Torres, P.J. The Role of Copper in the Hydrogenation of Furfural and Levulinic Acid. Int. J. Mol. Sci. 2023, 24, 2443.
- Chakrabortty, S.; Almasalma, A.A.; de Vries, J.G. Recent developments in asymmetric hydroformylation. Catal. Sci. Technol. 2021, 11, 5388–5411.
- Jentoft, F.C. Transition metal-catalyzed deoxydehydration: Missing pieces of the puzzle. Catal. Sci. Technol. 2022, 12, 6308–6358.
- Choi, J.; Fu, G.C. Transition metal-catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 2017, 356, eaaf7230.
- Kakiuchi, F.; Kochi, T. Transition-Metal-Catalyzed Carbon-Carbon Bond Formation via Carbon-Hydrogen Bond Cleavage. Synthesis 2008, 2008, 3013–3039.
- Reshi, N.U.D.; Saptal, V.B.; Beller, M.; Bera, J.K. Recent Progress in Transition-Metal-Catalyzed Asymmetric Reductive Amination. ACS Catal. 2021, 11, 13809–13837.
- Aplan, M.P.; Gomez, E.D. Recent Developments in Chain-Growth Polymerizations of Conjugated Polymers. Ind. Eng. Chem. Res. 2017, 56, 7888–7901.
- Kumar, A.; Patil, N.T. Ligand-Enabled Sustainable Gold Catalysis. ACS Sustain. Chem. Eng. 2022, 10, 6900–6918.
- Hashmi, A.S.K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211.
- Li, Z.; Brouwer, C.; He, C. Gold-Catalyzed Organic Transformations. Chem. Rev. 2008, 108, 3239–3265.
- Arcadi, A. Alternative Synthetic Methods through New Developments in Catalysis by Gold. Chem. Rev. 2008, 108, 3266–3325.
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072.
- Qian, D.; Zhang, J. Gold-catalyzed cyclopropanation reactions using a carbenoid precursor toolbox. Chem. Soc. Rev. 2015, 44, 677–698.
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936.
- Shen, H.C. Recent advances in syntheses of heterocycles and carbocycles via homogeneous gold catalysis. Part 1: Heteroatom addition and hydroarylation reactions of alkynes, allenes, and alkenes. Tetrahedron 2008, 64, 3885–3903.
- Shen, H.C. Recent advances in syntheses of carbocycles and heterocycles via homogeneous gold catalysis. Part 2: Cyclizations and cycloadditions. Tetrahedron 2008, 64, 7847–7870.
- Debrouwer, W.; Heugebaert, T.S.A.; Roman, B.I.; Stevens, C.V. Homogeneous Gold-Catalyzed Cyclization Reactions of Alkynes with N- and S-Nucleophiles. Adv. Synth. Catal. 2015, 357, 2975–3006.
- Huple, D.B.; Ghorpade, S.; Liu, R.-S. Recent Advances in Gold-Catalyzed N- and O-Functionalizations of Alkynes with Nitrones, Nitroso, Nitro and Nitroxy Species. Adv. Synth. Catal. 2016, 358, 1348–1367.
- Shahzad, S.A.; Sajid, M.A.; Khan, Z.A.; Canseco-Gonzalez, D. Gold catalysis in organic transformations: A review. Synth. Commun. 2017, 47, 735–755.
- Mertens, R.T.; Awuah, S.G. Gold Catalysis: Fundamentals and Recent Developments. In Catalysis by Metal Complexes and Nanomaterials: Fundamentals and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Volume 1317, pp. 19–55.
- Collado, A.; Nelson, D.J.; Nolan, S.P. Optimizing Catalyst and Reaction Conditions in Gold(I) Catalysis–Ligand Development. Chem. Rev. 2021, 121, 8559–8612.
- Campeau, D.; León Rayo, D.F.; Mansour, A.; Muratov, K.; Gagosz, F. Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2021, 121, 8756–8867.
- Cera, G.; Bandini, M. Enantioselective Gold(I) Catalysis with Chiral Monodentate Ligands. Isr. J. Chem. 2013, 53, 848–855.
- Wang, Y.-M.; Lackner, A.D.; Toste, F.D. Development of Catalysts and Ligands for Enantioselective Gold Catalysis. Acc. Chem. Res. 2014, 47, 889–901.
- Zi, W.; Dean Toste, F. Recent advances in enantioselective gold catalysis. Chem. Soc. Rev. 2016, 45, 4567–4589.
- Li, Y.; Li, W.; Zhang, J. Gold-Catalyzed Enantioselective Annulations. Chem.-Eur. J. 2017, 23, 467–512.
- Milcendeau, P.; Sabat, N.; Ferry, A.; Guinchard, X. Gold-catalyzed enantioselective functionalization of indoles. Org. Biomol. Chem. 2020, 18, 6006–6017.
- Wu, Y.; Hu, C.; Wang, T.; Eberle, L.; Hashmi, A.S.K. Gold-Catalyzed Reaction of Anthranils with Alkynyl Sulfones for the Regioselective Formation of 3-Hydroxyquinolines. Adv. Synth. Catal. 2022, 364, 1233–1238.
- López, S.; Herrero-Gómez, E.; Pérez-Galán, P.; Nieto-Oberhuber, C.; Echavarren, A.M. Gold(I)-Catalyzed Intermolecular Cyclopropanation of Enynes with Alkenes: Trapping of Two Different Gold Carbenes. Angew. Chem. Int. Ed. 2006, 45, 6029–6032.
- Gorin, D.J.; Watson, I.D.G.; Toste, F.D. Fluorenes and Styrenes by Au(I)-Catalyzed Annulation of Enynes and Alkynes. J. Am. Chem. Soc. 2008, 130, 3736–3737.
- Alonso, I.; Trillo, B.; López, F.; Montserrat, S.; Ujaque, G.; Castedo, L.; Lledós, A.; Mascareñas, J.L. Gold-Catalyzed Cycloadditions of Allenedienes, including an Enantioselective Version with New Phosphoramidite-Based Catalysts: Mechanistic Aspects of the Divergence between and Pathways. J. Am. Chem. Soc. 2009, 131, 13020–13030.
- Flügge, S. Total Synthesis of Amphidinolide V and Analogues-Studies on Homogeneous Gold(I) Catalysis; Technische Universität Dortmund: Dortmund, Germany, 2009.
- Teller, H.; Flügge, S.; Goddard, R.; Fürstner, A. Enantioselective Gold Catalysis: Opportunities Provided by Monodentate Phosphoramidite Ligands with an Acyclic TADDOL Backbone. Angew. Chem. Int. Ed. 2010, 49, 1949–1953.
- Teller, H.; Corbet, M.; Mantilli, L.; Gopakumar, G.; Goddard, R.; Thiel, W.; Fürstner, A. One-Point Binding Ligands for Asymmetric Gold Catalysis: Phosphoramidites with a TADDOL-Related but Acyclic Backbone. J. Am. Chem. Soc. 2012, 134, 15331–15342.
- González-Fernández, E.; Nicholls, L.D.M.; Schaaf, L.D.; Farès, C.; Lehmann, C.W.; Alcarazo, M. Enantioselective Synthesis of Carbohelicenes. J. Am. Chem. Soc. 2017, 139, 1428–1431.
- Zhang, J.; Simon, M.; Golz, C.; Alcarazo, M. Enantioselective Synthesis of Helicenes Containing Two Additional Chiral Axes. Isr. J. Chem. 2022, e202200043.
- Liu, F.; Qian, D.; Li, L.; Zhao, X.; Zhang, J. Diastereo- and Enantioselective Gold(I)-Catalyzed Intermolecular Tandem Cyclization/Cycloadditions of 2-(1-Alkynyl)-2-alken-1-ones with Nitrones. Angew. Chem. Int. Ed. 2010, 49, 6669–6672.
- Zhou, G.; Liu, F.; Zhang, J. Enantioselective Gold-Catalyzed Functionalization of Unreactive sp3 C—H Bonds through a Redox–Neutral Domino Reaction. Chem. Eur. J. 2011, 17, 3101–3104.
- Zhang, Z.-M.; Chen, P.; Li, W.; Niu, Y.; Zhao, X.-L.; Zhang, J. A New Type of Chiral Sulfinamide Monophosphine Ligands: Stereodivergent Synthesis and Application in Enantioselective Gold(I)-Catalyzed Cycloaddition Reactions. Angew. Chem. Int. Ed. 2014, 53, 4350–4354.
- Wang, Y.; Zhang, P.; Di, X.; Dai, Q.; Zhang, Z.-M.; Zhang, J. Gold-Catalyzed Asymmetric Intramolecular Cyclization of N-Allenamides for the Synthesis of Chiral Tetrahydrocarbolines. Angew. Chem. Int. Ed. 2017, 56, 15905–15909.
- Xu, B.; Zhang, Z.-M.; Han, J.; Gu, G.; Zhang, J. Enantioselectivity Tunable Gold-Catalyzed Intermolecular Cycloaddition of N-Allenamides with Nitrones. Chin. J. Chem. 2022, 40, 1407–1412.
- Ito, Y.; Sawamura, M.; Hayashi, T. Catalytic asymmetric aldol reaction: Reaction of aldehydes with isocyanoacetate catalyzed by a chiral ferrocenylphosphine-gold(I) complex. J. Am. Chem. Soc. 1986, 108, 6405–6406.
- García-Morales, C.; Ranieri, B.; Escofet, I.; López-Suarez, L.; Obradors, C.; Konovalov, A.I.; Echavarren, A.M. Enantioselective Synthesis of Cyclobutenes by Intermolecular Cycloaddition with Non-C2 Symmetric Digold Catalysts. J. Am. Chem. Soc. 2017, 139, 13628–13631.
- Caniparoli, U.; Escofet, I.; Echavarren, A.M. Planar Chiral 1,3-Disubstituted Ferrocenyl Phosphine Gold(I) Catalysts. ACS Catal. 2022, 12, 3317–3322.
- Wu, Y.; Tian, B.; Hu, C.; Sekine, K.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Chemodivergent reaction of azomethine imines and 2H-azirines for the synthesis of nitrogen-containing scaffolds. Org. Biomol. Chem. 2019, 17, 5505–5508.
- Saito, S.; Yamamoto, H. Carbonyl Recognition. In Modern Carbonyl Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 33–67.
- Ren, J.; Cramer, C.J.; Squires, R.R. Superacidity and Superelectrophilicity of BF3−Carbonyl Complexes. J. Am. Chem. Soc. 1999, 121, 2633–2634.
- Rathke, M.W.; Cowan, P.J. Procedures for the acylation of diethyl malonate and ethyl acetoacetate with acid chlorides using tertiary amine bases and magnesium chloride. J. Org. Chem. 1985, 50, 2622–2624.
- Rathke, M.W.; Nowak, M. The Horner-Wadsworth-Emmons modification of the Wittig reaction using triethylamine and lithium or magnesium salts. J. Org. Chem. 1985, 50, 2624–2626.
- Robinson, M.S.; Polak, M.L.; Bierbaum, V.M.; DePuy, C.H.; Lineberger, W.C. Experimental Studies of Allene, Methylacetylene, and the Propargyl Radical: Bond Dissociation Energies, Gas-Phase Acidities, and Ion-Molecule Chemistry. J. Am. Chem. Soc. 1995, 117, 6766–6778.
- Matthews, W.S.; Bares, J.E.; Bartmess, J.E.; Bordwell, F.G.; Cornforth, F.J.; Drucker, G.E.; Margolin, Z.; McCallum, R.J.; McCollum, G.J.; Vanier, N.R. Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution. J. Am. Chem. Soc. 1975, 97, 7006–7014.
- Cheng, X.; Li, T.; Gutman, K.; Zhang, L. Chiral Bifunctional Phosphine Ligand-Enabled Cooperative Cu Catalysis: Formation of Chiral α,β-Butenolides via Highly Enantioselective γ-Protonation. J. Am. Chem. Soc. 2021, 143, 10876–10881.
- Cheng, X.; Zhang, L. Chiral Bifunctional Phosphine Ligand Enables Gold-Catalyzed Asymmetric Isomerization and Cyclization of Propargyl Sulfonamide into Chiral 3-Pyrroline. Org. Lett. 2021, 23, 8194–8198.
- Li, T.; Dong, S.; Tang, C.; Zhu, M.; Wang, N.; Kong, W.; Gao, W.; Zhu, J.; Zhang, L. Asymmetric Construction of α,γ-Disubstituted α,β-Butenolides Directly from Allylic Ynoates Using a Chiral Bifunctional Phosphine Ligand Enables Cooperative Au Catalysis. Org. Lett. 2022, 24, 4427–4432.
- Wang, H.; Li, T.; Zheng, Z.; Zhang, L. Efficient Synthesis of α-Allylbutenolides from Allyl Ynoates via Tandem Ligand-Enabled Au(I) Catalysis and the Claisen Rearrangement. ACS Catal. 2019, 9, 10339–10342.
- Zhao, K.; Kohnke, P.; Yang, Z.; Cheng, X.; You, S.-L.; Zhang, L. Enantioselective Dearomative Cyclization Enabled by Asymmetric Cooperative Gold Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202207518.
- Muñoz, M.P.; Adrio, J.; Carretero, J.C.; Echavarren, A.M. Ligand Effects in Gold- and Platinum-Catalyzed Cyclization of Enynes: Chiral Gold Complexes for Enantioselective Alkoxycyclization. Organometallics 2005, 24, 1293–1300.
- Johansson, M.J.; Gorin, D.J.; Staben, S.T.; Toste, F.D. Gold(I)-Catalyzed Stereoselective Olefin Cyclopropanation. J. Am. Chem. Soc. 2005, 127, 18002–18003.
- Zhang, Z.; Widenhoefer, R.A. Gold(I)-Catalyzed Intramolecular Enantioselective Hydroalkoxylation of Allenes. Angew. Chem. Int. Ed. 2007, 46, 283–285.
- Kim, H.; Choi, S.Y.; Shin, S. Asymmetric Synthesis of Dihydropyranones via Gold(I)-Catalyzed Intermolecular Annulation of Propiolates and Alkenes. Angew. Chem. Int. Ed. 2018, 57, 13130–13134.
- LaLonde, R.L.; Sherry, B.D.; Kang, E.J.; Toste, F.D. Gold(I)-Catalyzed Enantioselective Intramolecular Hydroamination of Allenes. J. Am. Chem. Soc. 2007, 129, 2452–2453.
- Hamilton, G.L.; Kang, E.J.; Mba, M.; Toste, F.D. A Powerful Chiral Counterion Strategy for Asymmetric Transition Metal Catalysis. Science 2007, 317, 496–499.
- LaLonde, R.L.; Wang, Z.J.; Mba, M.; Lackner, A.D.; Toste, F.D. Gold(I)-Catalyzed Enantioselective Synthesis of Pyrazolidines, Isoxazolidines, and Tetrahydrooxazines. Angew. Chem. Int. Ed. 2010, 49, 598–601.
- Bartolomé, C.; García-Cuadrado, D.; Ramiro, Z.; Espinet, P. Synthesis and Catalytic Activity of Gold Chiral Nitrogen Acyclic Carbenes and Gold Hydrogen Bonded Heterocyclic Carbenes in Cyclopropanation of Vinyl Arenes and in Intramolecular Hydroalkoxylation of Allenes. Inorg. Chem. 2010, 49, 9758–9764.
- Wang, Y.-M.; Kuzniewski, C.N.; Rauniyar, V.; Hoong, C.; Toste, F.D. Chiral (Acyclic Diaminocarbene)Gold(I)-Catalyzed Dynamic Kinetic Asymmetric Transformation of Propargyl Esters. J. Am. Chem. Soc. 2011, 133, 12972–12975.
- Handa, S.; Slaughter, L.M. Enantioselective Alkynylbenzaldehyde Cyclizations Catalyzed by Chiral Gold(I) Acyclic Diaminocarbene Complexes Containing Weak Au–Arene Interactions. Angew. Chem. Int. Ed. 2012, 51, 2912–2915.
- Khrakovsky, D.A.; Tao, C.; Johnson, M.W.; Thornbury, R.T.; Shevick, S.L.; Toste, F.D. Enantioselective, Stereodivergent Hydroazidation and Hydroamination of Allenes Catalyzed by Acyclic Diaminocarbene (ADC) Gold(I) Complexes. Angew. Chem. Int. Ed. 2016, 55, 6079–6083.
- Niemeyer, Z.L.; Pindi, S.; Khrakovsky, D.A.; Kuzniewski, C.N.; Hong, C.M.; Joyce, L.A.; Sigman, M.S.; Toste, F.D. Parameterization of Acyclic Diaminocarbene Ligands Applied to a Gold(I)-Catalyzed Enantioselective Tandem Rearrangement/Cyclization. J. Am. Chem. Soc. 2017, 139, 12943–12946.
- Joost, M.; Amgoune, A.; Bourissou, D. Reactivity of Gold Complexes towards Elementary Organometallic Reactions. Angew. Chem. Int. Ed. 2015, 54, 15022–15045.
- Miró, J.; del Pozo, C. Fluorine and Gold: A Fruitful Partnership. Chem. Rev. 2016, 116, 11924–11966.
- Wei, Y.; Shi, M. Divergent Synthesis of Carbo- and Heterocycles via Gold-Catalyzed Reactions. ACS Catal. 2016, 6, 2515–2524.
- Johnson, M.W.; DiPasquale, A.G.; Bergman, R.G.; Toste, F.D. Synthesis of Stable Gold(III) Pincer Complexes with Anionic Heteroatom Donors. Organometallics 2014, 33, 4169–4172.
- Hashmi, A.S.K.; Blanco, M.C.; Fischer, D.; Bats, J.W. Gold Catalysis: Evidence for the In-situ Reduction of Gold(III) During the Cyclization of Allenyl Carbinols. Eur. J. Org. Chem. 2006, 2006, 1387–1389.
- Cui, J.-F.; Ko, H.-M.; Shing, K.-P.; Deng, J.-R.; Lai, N.C.-H.; Wong, M.-K. C,O-Chelated BINOL/Gold(III) Complexes: Synthesis and Catalysis with Tunable Product Profiles. Angew. Chem. Int. Ed. 2017, 56, 3074–3079.
- Wu, C.-Y.; Horibe, T.; Jacobsen, C.B.; Toste, F.D. Stable gold(III) catalysts by oxidative addition of a carbon–carbon bond. Nature 2015, 517, 449–454.
- Bohan, P.T.; Toste, F.D. Well-Defined Chiral Gold(III) Complex Catalyzed Direct Enantioconvergent Kinetic Resolution of 1,5-Enynes. J. Am. Chem. Soc. 2017, 139, 11016–11019.
- Reid, J.P.; Hu, M.; Ito, S.; Huang, B.; Hong, C.M.; Xiang, H.; Sigman, M.S.; Toste, F.D. Strategies for remote enantiocontrol in chiral gold(iii) complexes applied to catalytic enantioselective γ,δ-Diels–Alder reactions. Chem. Sci. 2020, 11, 6450–6456.
- Jiang, J.-J.; Cui, J.-F.; Yang, B.; Ning, Y.; Lai, N.C.-H.; Wong, M.-K. Chiral Cyclometalated Oxazoline Gold(III) Complex-Catalyzed Asymmetric Carboalkoxylation of Alkynes. Org. Lett. 2019, 21, 6289–6294.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
25 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




