
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eduardo Costa | -- | 2936 | 2023-09-21 17:18:02 | | | |
| 2 | Peter Tang | Meta information modification | 2936 | 2023-09-22 03:18:47 | | |
Video Upload Options
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that is the result of various environmental, bacterial and genetic stimuli, which culminate in the disruption of the skin’s barrier function. Characterized by highly pruritic skin lesions, xerosis and an array of comorbidities among which skin infections are the most common, this condition results in both a significant loss of quality of life and in the need for life-long treatments (e.g., corticosteroids, monoclonal antibodies and regular antibiotic intake), all of which may have harmful secondary effects. This, in conjunction with AD’s rising prevalence, made the development of alternative treatment strategies the focus of both the scientific community and the pharmaceutical industry. Given their potential to both manage the skin microbiome, fight infections and even modulate the local immune response, the use of antimicrobial peptides (AMPs) from more diverse origins has become one of the most promising alternative solutions for AD management, with some being already used with some success towards this end.
1. Introduction
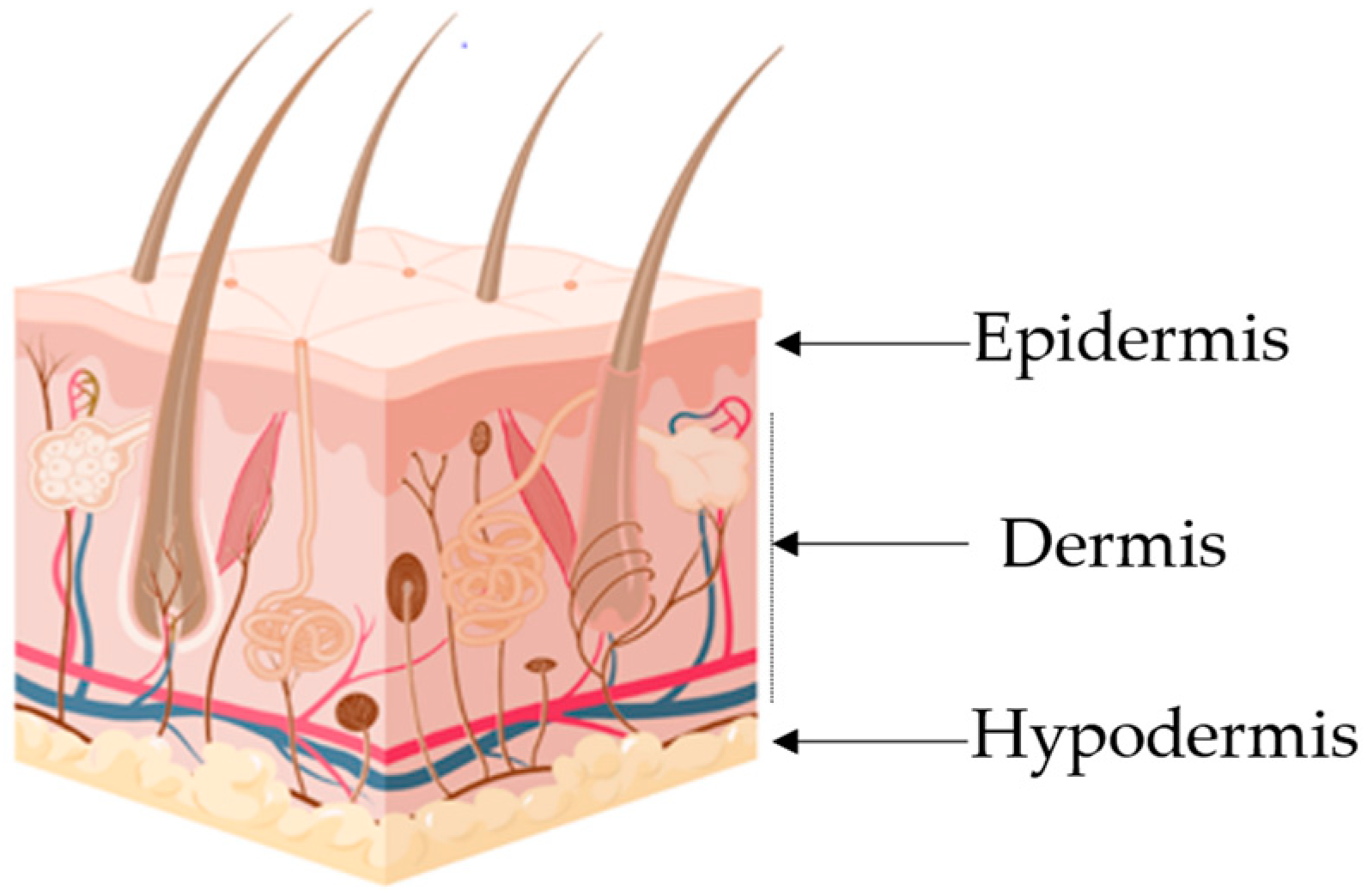
|
Layer |
Major cellular Constituents |
Major Functions |
References |
|---|---|---|---|
|
Hypodermis |
Adipocytes, fibroblasts, endothelial and muscle cells |
Insulation, mechanical integrity, support, conductance of vascular and neural signals |
|
|
Dermis |
Endothelial cells, fibroblasts, Langerhans and muscle cells |
Mechanical integrity, support, thermal barrier, energy storage, protection from physical injury |
|
|
Epidermis |
Keratinocytes, melanocytes, Langerhans and Markel cells |
Outermost barrier, immune function, protection from oxidative and mechanical stress |
2. Atopic Dermatitis
3. Antimicrobial Peptides
4. AMPs in Clinical Trials
|
AMP Name |
Clinical Trial ID |
Phase |
Target |
Reference |
|---|---|---|---|---|
|
AP-214 |
NCT00903604 |
II a |
Post-surgical organ failure |
[60] |
|
C16G2 |
NCT03004365 |
II c |
Streptococcus mutans |
[61] |
|
CZEN-002 |
NCT03145220 |
II a |
Antifungal |
[62] |
|
Daptomycin |
NCT01922011; NCT00093067; NCT01104662; NCT02972983 |
III/IV c |
Skin infection/bacteremia |
[63] |
|
Delmitide (RDP58) |
ISRCTN84220089 |
II c |
Inflammatory bowel disease |
[64] |
|
DPK-060 |
NCT01447017; NCT01522391 |
II c |
Acute external otitis, topical treatment of microbial infections |
[65] |
|
EA-230 |
NCT03145220 |
II d |
Sepsis/renal failure |
[62] |
|
Friulimicin |
NCT00492271 |
I a |
MRSA/pneumonia |
[66] |
|
Ghrelin |
NCT00763477 |
II c |
Chronic respiratory infection |
|
|
Gramicidin |
NCT00534391 |
III d |
Infected wounds and ulcers |
[69] |
|
GSK1322322 |
NCT01209078 |
II c |
Bacterial skin infection |
[70] |
|
hLF1-11 |
NCT00430469 |
I/II a |
Bacterial/fungal infections |
|
|
Iseganan (IB-367) |
NCT00118781; NCT00022373 |
III a |
Pneumonia/oral mucositis |
[73] |
|
LFF571 |
NCT01232595 |
II c |
C. difficile |
[74] |
|
LL-37 |
EUCTR2012-002100-41 |
II a |
Leg ulcers |
[75] |
|
LTX-109 |
NCT01803035; NCT01158235 |
I/II c |
MRSA/impetigo, antiviral |
[76] |
|
Mel4 |
ACTRN1261500072556 |
II/III c |
Contact lenses antimicrobial |
[77] |
|
Melittin |
NCT02364349, NCT01526031 |
I/II c |
Inflammation |
[78] |
|
Murepavadin |
EUCTR2017-003933-27-EE |
II b |
P. aeruginosa, K. pneumoniae |
[79] |
|
Nal-P-113 |
ChiCTR-OIC-16010250 |
III c |
Periodontal disease |
[80] |
|
Neuprex® |
NCT00462904 |
III a |
Pediatric meningococcemia |
[57] |
|
Nisin |
NCT02928042; NCT02467972 |
n.a. c |
Gram-positive bacteria |
[81] |
|
Novexatin (NP213) |
NCT02933879 |
II a |
Fungal nail infection |
[82] |
|
NVB-302 |
ISRCTN40071144 |
I a |
C. difficile |
[57] |
|
Omiganan |
NCT00231153; NCT02456480 |
II/III c |
Antisepsis/catheter, Atopic dermatitis |
[83] |
|
OP-145 |
ISRCTN84220089 |
I/II c |
Chronic middle ear infection |
[84] |
|
PAC113 |
NCT00659971 |
II c |
Oral candidiasis |
|
|
P60.4Ac |
ISRCTN12149720 |
II c |
Chronic ear infections |
[87] |
|
Pexiganan (MSI-78) |
NCT00563394; NCT00563433; NCT01590758; NCT01594762 |
III a |
Diabetic foot ulcers |
[88] |
|
PMX-30063 |
NCT01211470; NCT02052388 |
II c |
Acute bacterial skin infection |
[89] |
|
Polymyxin B |
NCT00490477; NCT00534391 |
III d |
Gram-negative bacteria |
[90] |
|
Polymyxin E (Colistin) |
NCT01292031; NCT02573064 |
III c |
A. baumannii/pneumonia |
[91] |
|
PXL01 |
NCT01022242 |
II/III c |
Postsurgical adhesions |
|
|
SGX942(Dusquetide) |
NCT03237325 |
III c |
Oral mucositis |
|
|
Surotomycin (CB-315) |
NCT01597505 |
III a |
C. difficile |
[96] |
|
XF-73(Exeporfinium chloride) |
NCT03915470 |
II c |
Staphylococcal infection |
[97] |
References
- Moniz, T.; Costa Lima, S.A.; Reis, S. Human skin models: From healthy to disease-mimetic systems; characteristics and applications. Br. J. Pharmacol. 2020, 177, 4314–4329.
- Lima, S.C.; Reis, S. Nanoparticles in Life Sciences and Biomedicine; CRC Press: Boca Raton, FL, USA, 2018.
- Chinnappan, M.; Harris-Tryon, T.A. Novel mechanisms of microbial crosstalk with skin innate immunity. Exp. Dermatol. 2021, 30, 1484–1495.
- Uchida, Y.; Park, K.; Kabashima, K. Immunology of the Skin: Basic and Clinical Sciences in Skin Immune Responses; Springer: Berlin/Heidelberg, Germany, 2016.
- Lynch, B.; Pageon, H.; Le Blay, H.; Brizion, S.; Bastien, P.; Bornschlögl, T.; Domanov, Y. A mechanistic view on the aging human skin through ex vivo layer-by-layer analysis of mechanics and microstructure of facial and mammary dermis. Sci. Rep. 2022, 12, 849.
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.W.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743.
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16.
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293.
- Kowalska-Olędzka, E.; Czarnecka, M.; Baran, A. Epidemiology of atopic dermatitis in Europe. J. Drug Assess. 2019, 8, 126–128.
- Mallol, J.; Crane, J.; von Mutius, E.; Odhiambo, J.; Keil, U.; Stewart, A. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: A global synthesis. Allergol. Immunopathol. 2013, 41, 73–85.
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130.
- Joshi, A.A.; Vocanson, M.; Nicolas, J.-F.; Wolf, P.; Patra, V. Microbial derived antimicrobial peptides as potential therapeutics in atopic dermatitis. Front. Immunol. 2023, 14, 1125635.
- Kulthanan, K.; Samutrapong, P.; Jiamton, S.; Tuchinda, P. Adult-onset atopic dermatitis: A cross-sectional study of natural history and clinical manifestation. Asian Pac. J. Allergy Immunol. 2007, 25, 207.
- Silverberg, J.I. Atopic dermatitis in adults. Med. Clin. 2020, 104, 157–176.
- Silverberg, J.I. Comorbidities and the impact of atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 123, 144–151.
- Nguyen, H.L.T.; Peng, G.; Trujillo-Paez, J.V.; Yue, H.; Ikutama, R.; Takahashi, M.; Umehara, Y.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The Antimicrobial Peptide AMP-IBP5 Suppresses Dermatitis-like Lesions in a Mouse Model of Atopic Dermatitis through the Low-Density Lipoprotein Receptor-Related Protein-1 Receptor. Int. J. Mol. Sci. 2023, 24, 5200.
- Morelli, P.; Gaspari, M.; Gabriele, C.; Dastoli, S.; Bennardo, L.; Pavel, A.B.; Patruno, C.; Del Duca, E.; Nisticò, S.P. Proteomic analysis from skin swabs reveals a new set of proteins identifying skin impairment in atopic dermatitis. Exp. Dermatol. 2021, 30, 811–819.
- Thyssen, J.P.; Skov, L.; Hamann, C.R.; Gislason, G.H.; Egeberg, A. Assessment of major comorbidities in adults with atopic dermatitis using the Charlson comorbidity index. J. Am. Acad. Dermatol. 2017, 76, 1088–1092.e1081.
- Andersen, Y.M.; Egeberg, A.; Skov, L.; Thyssen, J.P. Comorbidities of atopic dermatitis: Beyond rhinitis and asthma. Curr. Dermatol. Rep. 2017, 6, 35–41.
- Silverberg, J.I.; Gelfand, J.; Margolis, D.; Boguniewicz, M.; Fonacier, L.S.; Grayson, M.H.; Ong, P.Y.; Fuxench, Z.C.; Simpson, E.L. Measurement properties of Hospital Anxiety and Depression Scale used in atopic dermatitis in adults. J. Allergy Clin. Immunol. 2019, 143, AB130.
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40.
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R. Nano-scale architecture of blood-brain barrier tight-junctions. Elife 2021, 10, e63253.
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int. J. Mol. Sci. 2021, 22, 2506.
- Akiyama, T.; Niyonsaba, F.; Kiatsurayanon, C.; Ushio, H.; Fujimura, T.; Ueno, T.; Okumura, K.; Ogawa, H.; Ikeda, S. The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J. Innate Immun. 2014, 6, 739–753.
- Katsarou, S.; Makris, M.; Vakirlis, E.; Gregoriou, S. The Role of Tight Junctions in Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2023, 12, 1538.
- Yuki, T.; Komiya, A.; Kusaka, A.; Kuze, T.; Sugiyama, Y.; Inoue, S. Impaired tight junctions obstruct stratum corneum formation by altering polar lipid and profilaggrin processing. J. Dermatol. Sci. 2013, 69, 148–158.
- Yokouchi, M.; Kubo, A.; Kawasaki, H.; Yoshida, K.; Ishii, K.; Furuse, M.; Amagai, M. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J. Dermatol. Sci. 2015, 77, 28–36.
- Seguchi, T.; Chang-Yi, C.; Kusuda, S.; Takahashi, M.; Aisu, K.; Tezuka, T. Decreased expression of filaggrin in atopic skin. Arch. Dermatol. Res. 1996, 288, 442–446.
- Jensen, J.-M.; Fölster-Holst, R.; Baranowsky, A.; Schunck, M.; Winoto-Morbach, S.; Neumann, C.; Schütze, S.; Proksch, E. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J. Investig. Dermatol. 2004, 122, 1423–1431.
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337.
- Chen, H.; Common, J.; Haines, R.; Balakrishnan, A.; Brown, S.; Goh, C.; Cordell, H.; Sandilands, A.; Campbell, L.; Kroboth, K. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br. J. Dermatol. 2011, 165, 106–114.
- Elias, P.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Allergy Asthma Rep. 2009, 9, 265–272.
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446.
- Jurakic Toncic, R.; Kezic, S.; Jakasa, I.; Ljubojevic Hadzavdic, S.; Balic, A.; Petkovic, M.; Pavicic, B.; Zuzul, K.; Marinovic, B. Filaggrin loss-of-function mutations and levels of filaggrin degradation products in adult patients with atopic dermatitis in Croatia. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1789–1794.
- Clausen, M.L.; Edslev, S.; Andersen, P.; Clemmensen, K.; Krogfelt, K.; Agner, T. Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br. J. Dermatol. 2017, 177, 1394–1400.
- Thyssen, J.; Vestergaard, C.; Deleuran, M.; de Bruin-Weller, M.; Bieber, T.; Taieb, A.; Seneschal, J.; Cork, M.; Paul, C.; Flohr, C. European Task Force on Atopic Dermatitis (ETFAD): Treatment targets and treatable traits in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e839–e842.
- Briscoe, C.C.; Reich, P.; Fritz, S.; Coughlin, C.C. Staphylococcus aureus antibiotic susceptibility patterns in pediatric atopic dermatitis. Pediatr. Dermatol. 2019, 36, 482–485.
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862.
- Otero, M.E.; van den Reek, J.M.; Seyger, M.M.; van de Kerkhof, P.C.; Kievit, W.; de Jong, E.M. Determinants for drug survival of methotrexate in patients with psoriasis, split according to different reasons for discontinuation: Results of the prospective MTX-CAPTURE. Br. J. Dermatol. 2017, 177, 497–504.
- Chen, Y.; Yan, Y.; Liu, H.; Qiu, F.; Liang, C.L.; Zhang, Q.; Huang, R.Y.; Han, L.; Lu, C.; Dai, Z. Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8(+) T-cell memory in wild-type and humanized mice. Theranostics 2020, 10, 10466–10482.
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018, 48, 19–33.
- Schlessinger, J.; Shepard, J.S.; Gower, R.; Su, J.C.; Lynde, C.; Cha, A.; Ports, W.C.; Purohit, V.; Takiya, L.; Werth, J.L. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to< 24 months with mild-to-moderate atopic dermatitis: A phase IV open-label study (CrisADe CARE 1). Am. J. Clin. Dermatol. 2020, 21, 275–284.
- Lin, C.M.; Cooles, F.A.; Isaacs, J.D. Basic mechanisms of JAK inhibition. Mediterr. J. Rheumatol. 2020, 31, 100.
- Blair, H.A. Tralokinumab in Atopic Dermatitis: A Profile of Its Use. Clin. Drug Investig. 2022, 42, 365–374.
- Agboola, F.; Atlas, S.J.; Brouwer, E.; Carlson, J.J.; Hansen, R.N.; Herron-Smith, S.; Nhan, E.; Rind, D.M.; Pearson, S.D. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: Effectiveness and value. J. Manag. Care Spec. Pharm. 2022, 28, 108–114.
- Gisondi, P.; Maurelli, M.; Costanzo, A.; Esposito, M.; Girolomoni, G. The Combination of Dupilumab with Other Monoclonal Antibodies. Dermatol. Ther. 2023, 13, 7–12.
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675.
- Thakur, A.; Sharma, A.; Alajangi, H.K.; Jaiswal, P.K.; Lim, Y.-B.; Singh, G.; Barnwal, R.P. In pursuit of next-generation therapeutics: Antimicrobial peptides against superbugs, their sources, mechanism of action, nanotechnology-based delivery, and clinical applications. Int. J. Biol. Macromol. 2022, 218, 135–156.
- Nguyen, H.L.T.; Trujillo-Paez, J.V.; Umehara, Y.; Yue, H.; Peng, G.; Kiatsurayanon, C.; Chieosilapatham, P.; Song, P.; Okumura, K.; Ogawa, H.; et al. Role of Antimicrobial Peptides in Skin Barrier Repair in Individuals with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 7607.
- Clausen, M.-L.; Slotved, H.-C.; Krogfelt, K.A.; Andersen, P.S.; Agner, T. In vivo expression of antimicrobial peptides in atopic dermatitis. Exp. Dermatol. 2016, 25, 3–9.
- Kanda, N.; Hau, C.; Tada, Y.; Sato, S.; Watanabe, S. Decreased serum LL-37 and vitamin D 3 levels in atopic dermatitis: Relationship between IL-31 and oncostatin M. Allergy 2012, 67, 804–812.
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992.
- Răileanu, M.; Borlan, R.; Campu, A.; Janosi, L.; Turcu, I.; Focsan, M.; Bacalum, M. No country for old antibiotics! Antimicrobial peptides (AMPs) as next-generation treatment for skin and soft tissue infection. Int. J. Pharm. 2023, 642, 123169.
- Do, N.; Weindl, G.; Grohmann, L.; Salwiczek, M.; Koksch, B.; Korting, H.C.; Schäfer-Korting, M. Cationic membrane-active peptides–anticancer and antifungal activity as well as penetration into human skin. Exp. Dermatol. 2014, 23, 326–331.
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281.
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349.
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122.
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12.
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134.
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022, 42, 1377–1422.
- Guo, L.; McLean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shi, W. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574.
- Van Groenendael, R.; Beunders, R.; Kox, M.; van Eijk, L.T.; Pickkers, P. The Human Chorionic Gonadotropin Derivate EA-230 Modulates the Immune Response and Exerts Renal Protective Properties: Therapeutic Potential in Humans. Semin. Nephrol. 2019, 39, 496–504.
- Huang, H.W. DAPTOMYCIN, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183395.
- Travis, S.; Yap, L.M.; Hawkey, C.; Warren, B.; Lazarov, M.; Fong, T.; Tesi, R. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm. Bowel Dis. 2005, 11, 713–719.
- Håkansson, J.; Ringstad, L.; Umerska, A.; Johansson, J.; Andersson, T.; Boge, L.; Rozenbaum, R.T.; Sharma, P.K.; Tollbäck, P.; Björn, C.; et al. Characterization of the in vitro, ex vivo, and in vivo Efficacy of the Antimicrobial Peptide DPK-060 Used for Topical Treatment. Front. Cell. Infect. Microbiol. 2019, 9, 174.
- Schneider, T.; Gries, K.; Josten, M.; Wiedemann, I.; Pelzer, S.; Labischinski, H.; Sahl, H.-G. The lipopeptide antibiotic Friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate. Antimicrob. Agents Chemother. 2009, 53, 1610–1618.
- Miki, K.; Kitada, S.; Miki, M.; Hui, S.-P.; Shrestha, R.; Yoshimura, K.; Tsujino, K.; Kagawa, H.; Oshitani, Y.; Kida, H.; et al. A phase II, open-label clinical trial on the combination therapy with medium-chain triglycerides and ghrelin in patients with chronic obstructive pulmonary disease. J. Physiol. Sci. 2019, 69, 969–979.
- Gualillo, O.; Lago, F.; Gómez-Reino, J.; Casanueva, F.F.; Dieguez, C. Ghrelin, a widespread hormone: Insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003, 552, 105–109.
- Pavithrra, G.; Rajasekaran, R. Gramicidin peptide to combat antibiotic resistance: A review. Int. J. Pept. Res. Ther. 2020, 26, 191–199.
- Corey, R.; Naderer, O.J.; O’Riordan, W.D.; Dumont, E.; Jones, L.S.; Kurtinecz, M.; Zhu, J.Z. Safety, tolerability, and efficacy of GSK1322322 in the treatment of acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 2014, 58, 6518–6527.
- Nibbering, P.; Ravensbergen, E.; Welling, M.; Van Berkel, L.; Van Berkel, P.; Pauwels, E.; Nuijens, J. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476.
- Brouwer, C.; Roscini, L.; Cardinali, G.; Corte, L.; Pierantoni, D.C.; Robert, V.; Rahman, M.; Welling, M.M. Structure-activity relationship study of synthetic variants derived from the highly potent human antimicrobial peptide hLF (1-11). Cohesive J. Microbiol. Infect. Dis 2018, 1, 1–19.
- Papazian, L.; Donati, S.Y. Chapter 28—Hospital-acquired pneumonia. In Infectious Diseases, 3rd ed.; Cohen, J., Opal, S.M., Powderly, W.G., Eds.; Mosby: London, UK, 2010; pp. 294–299.
- Mullane, K.; Lee, C.; Bressler, A.; Buitrago, M.; Weiss, K.; Dabovic, K.; Praestgaard, J.; Leeds, J.A.; Blais, J.; Pertel, P. Multicenter, randomized clinical trial to compare the safety and efficacy of LFF571 and vancomycin for Clostridium difficile infections. Antimicrob. Agents Chemother. 2015, 59, 1435–1440.
- Brown, K.L.; Poon, G.F.; Birkenhead, D.; Pena, O.M.; Falsafi, R.; Dahlgren, C.; Karlsson, A.; Bylund, J.; Hancock, R.E.; Johnson, P. Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J. Immunol. 2011, 186, 5497–5505.
- AS, P.H. A Double-Blind, Placebo-Controlled, Interventional Parallel Group Study to Evaluate the Antiviral Effect of a Single Nasal Application of LTX-109 3% Gel, in Comparison to Placebo Gel, in Subjects with COVID-19 Infection; WHO: Geneva, Switzerland, 2021.
- Yasir, M.; Dutta, D.; Willcox, M.D. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703.
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol. Toxicol. 2021, 22, 42.
- Martin-Loeches, I.; Dale, G.E.; Torres, A. Murepavadin: A new antibiotic class in the pipeline. Expert Rev. Anti-Infect. Ther. 2018, 16, 259–268.
- Wang, H.; Ai, L.; Zhang, Y.; Cheng, J.; Yu, H.; Li, C.; Zhang, D.; Pan, Y.; Lin, L. The Effects of Antimicrobial Peptide Nal-P-113 on Inhibiting Periodontal Pathogens and Improving Periodontal Status. BioMed Res. Int. 2018, 2018, 1805793.
- Campion, A.; Casey, P.G.; Field, D.; Cotter, P.D.; Hill, C.; Ross, R.P. In vivo activity of Nisin A and Nisin V against Listeria monocytogenesin mice. BMC Microbiol. 2013, 13, 23.
- Mercer, D.K.; Robertson, J.C.; Miller, L.; Stewart, C.S.; O’Neil, D.A. NP213 (Novexatin®): A unique therapy candidate for onychomycosis with a differentiated safety and efficacy profile. Med. Mycol. 2020, 58, 1064–1072.
- Niemeyer-van der Kolk, T.; van der Wall, H.; Hogendoorn, G.K.; Rijneveld, R.; Luijten, S.; van Alewijk, D.C.; van den Munckhof, E.H.; de Kam, M.L.; Feiss, G.L.; Prens, E.P.; et al. Pharmacodynamic effects of topical omiganan in patients with mild to moderate atopic dermatitis in a randomized, placebo-controlled, phase II trial. Clin. Transl. Sci. 2020, 13, 994–1003.
- Malanovic, N.; Leber, R.; Schmuck, M.; Kriechbaum, M.; Cordfunke, R.A.; Drijfhout, J.W.; de Breij, A.; Nibbering, P.H.; Kolb, D.; Lohner, K. Phospholipid-driven differences determine the action of the synthetic antimicrobial peptide OP-145 on Gram-positive bacterial and mammalian membrane model systems. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2437–2447.
- Cheng, K.-T.; Wu, C.-L.; Yip, B.-S.; Chih, Y.-H.; Peng, K.-L.; Hsu, S.-Y.; Yu, H.-Y.; Cheng, J.-W. The interactions between the antimicrobial peptide P-113 and living candida albicans cells shed light on mechanisms of antifungal activity and resistance. Int. J. Mol. Sci. 2020, 21, 2654.
- Jang, W.S.; Li, X.S.; Sun, J.N.; Edgerton, M. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 2008, 52, 497–504.
- Peek, N.F.A.W.; Nell, M.J.; Brand, R.; Jansen-Werkhoven, T.; van Hoogdalem, E.J.; Verrijk, R.; Vonk, M.J.; Wafelman, A.R.; Valentijn, A.R.P.M.; Frijns, J.H.M.; et al. Ototopical drops containing a novel antibacterial synthetic peptide: Safety and efficacy in adults with chronic suppurative otitis media. PLoS ONE 2020, 15, e0231573.
- Cohen, H.; Wani, N.A.; Ben Hur, D.; Migliolo, L.; Cardoso, M.H.; Porat, Z.; Shimoni, E.; Franco, O.L.; Shai, Y. Interaction of Pexiganan (MSI-78)-Derived Analogues Reduces Inflammation and TLR4-Mediated Cytokine Secretion: A Comparative Study. ACS Omega 2023, 8, 17856–17868.
- Scorciapino, M.A.; Rinaldi, A.C. Antimicrobial peptidomimetics: Reinterpreting nature to deliver innovative therapeutics. Front. Immunol. 2012, 3, 171.
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A review of the clinical pharmacokinetics of polymyxin B. Antibiotics 2019, 8, 31.
- Al-Dulaimi, M.; Algburi, A.; Abdelhameed, A.; Mazanko, M.S.; Rudoy, D.V.; Ermakov, A.M.; Chikindas, M.L. Antimicrobial and Anti-Biofilm Activity of Polymyxin E Alone and in Combination with Probiotic Strains of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 against Clinical Isolates of Selected Acinetobacter spp.: A Preliminary Study. Pathogens 2021, 10, 1574.
- Nilsson, E.; Björn, C.; Sjöstrand, V.; Lindgren, K.; Münnich, M.; Mattsby-Baltzer, I.; Ivarsson, M.-L.; Olmarker, K.; Mahlapuu, M. A novel polypeptide derived from human lactoferrin in sodium hyaluronate prevents postsurgical adhesion formation in the rat. Ann. Surg. 2009, 250, 1021–1028.
- Wiig, M.; Dahlin, L.B.; Fridèn, J.; Hagberg, L.; Larsen, S.; Mahlapuu, M. PXL01 in Sodium Hyaluronate for Improvement of Hand Recovery After Flexor Tendon Repair Surgery: Randomized Controlled Trial: Level 1 Evidence. J. Hand Surg. 2014, 39, e45.
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Elder, J.; Sonis, S.T.; Straube, R. Dusquetide: A novel innate defense regulator demonstrating a significant and consistent reduction in the duration of oral mucositis in preclinical data and a randomized, placebo-controlled phase 2a clinical study. J. Biotechnol. 2016, 239, 115–125.
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Scott, Z.; Elder, J.; Sonis, S.T. Dusquetide: Reduction in oral mucositis associated with enduring ancillary benefits in tumor resolution and decreased mortality in head and neck cancer patients. Biotechnol. Rep. 2017, 15, 24–26.
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526.
- Zhang, C.; Li, J.; Lu, R.; Wang, S.; Fu, Z.; Yao, Z. Efficacy of a Novel Antibacterial Agent Exeporfinium Chloride,(XF-73), Against Antibiotic-Resistant Bacteria in Mouse Superficial Skin Infection Models. Infect. Drug Resist. 2023, 16, 4867–4879.




