Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Itabajara da Silva Vaz Junior | -- | 2074 | 2023-09-07 13:13:58 | | | |
| 2 | Lindsay Dong | Meta information modification | 2074 | 2023-09-11 02:57:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rodríguez-Durán, A.; Ullah, S.; Parizi, L.F.; Ali, A.; Da Silva Vaz Junior, I. Rabbits as Animal Models for Anti-Tick Vaccine Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/48923 (accessed on 07 February 2026).
Rodríguez-Durán A, Ullah S, Parizi LF, Ali A, Da Silva Vaz Junior I. Rabbits as Animal Models for Anti-Tick Vaccine Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/48923. Accessed February 07, 2026.
Rodríguez-Durán, Arlex, Shafi Ullah, Luís Fernando Parizi, Abid Ali, Itabajara Da Silva Vaz Junior. "Rabbits as Animal Models for Anti-Tick Vaccine Development" Encyclopedia, https://encyclopedia.pub/entry/48923 (accessed February 07, 2026).
Rodríguez-Durán, A., Ullah, S., Parizi, L.F., Ali, A., & Da Silva Vaz Junior, I. (2023, September 07). Rabbits as Animal Models for Anti-Tick Vaccine Development. In Encyclopedia. https://encyclopedia.pub/entry/48923
Rodríguez-Durán, Arlex, et al. "Rabbits as Animal Models for Anti-Tick Vaccine Development." Encyclopedia. Web. 07 September, 2023.
Copy Citation
Studies evaluating candidate tick-derived proteins as anti-tick vaccines in natural hosts have been limited due to high costs. To overcome this problem, animal models are used in immunization tests. The most commonly used rabbit breeds were New Zealand (73.8%), Japanese white (19%), Californians (4.8%) and Flemish lop-eared (2.4%) rabbits. Anti-tick vaccines efficacy resulted in up to 99.9%. Haemaphysalis longicornis (17.9%) and Ornithodoros moubata (12.8%) were the most common tick models in vaccination trials. Experiments with rabbits have revealed that some proteins (CoAQP, OeAQP, OeAQP1, Bm86, GST-Hl, 64TRP, serpins and voraxin) can induce immune responses against various tick species.
antigen
humoral and adaptive response
immunization
rabbit
tick
1. Introduction
Ticks are obligate blood-sucking ectoparasites that parasitize a large number of terrestrial and semi-terrestrial vertebrates, including humans [1][2][3]. Although they have been considered cosmopolitan parasites, most tick species are restricted to specific habitats, especially in tropical and subtropical regions [4][5]. Ticks transmit a wide variety of pathogens, being the second most important vectors of pathogens affecting humans, and the main vector in domestic and wild animals [6][7].
Traditional methods to control these arthropods are mainly based on the use of synthetic acaricides [8][9][10]. However, the application of these products has disadvantages, including the selection of resistant tick populations, environmental contamination, and residues in products of animal origin such as milk and meat [11].
These issues raise the need to develop alternative control methods, including the selection of parasite-resistant breeds [12][13]; biological control using entomopathogenic fungi (Metarhizium spp., Beauveria spp.) [14][15]; entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) [16][17]; regulator ants (Solenopsis germinata, S. saevissima, Camponotus rengira, and Ectatomma quadridens) [18][19]; pesticides [20][21]; and immunological control through the application of anti-tick vaccines [22][23][24].
The evaluation of tick vaccines in natural hosts has limitations, mainly due to the high costs of maintaining and using farm or wild animals in experiments. For this reason, animal models such as hamsters, guinea pigs, and rabbits are commonly used [25][26][27]. These animals have been used as models for basic and applied research, not only to test immune responses generated by anti-tick vaccines, but also to study resistance to chemical acaricides and tick-borne pathogen infection under laboratory conditions [28][29][30][31].
The use of hamsters, guinea pigs, and rabbits in tick vaccination experiments generally has low maintenance costs, minimal space requirements, short reproductive cycles and larger numbers of pups produced per year compared to some natural hosts [32][33][34]. However, there are distinct benefits and disadvantages to each of these models. For instance, the use of hamsters is limited by low blood volume, compared to the use of guinea pigs and rabbits [35][36]. On the other hand, guinea pigs have thick skin, which makes blood collection relatively difficult, sometimes even requiring anesthetic techniques to collect small volumes, in contrast to rabbits, which do not require anesthetic techniques for blood collection [37].
Another limitation in experimental animal models is the number of ticks that can be used when performing the infestation. Studies in rabbits have reported that these animals can support a higher burden of adult ticks [23][38] compared to mice, hamsters or guinea pigs [39][40]. Interestingly, the rabbit model was the first animal model used in several immunological studies and was crucial, for example, in the development of Louis Pasteur’s rabies vaccine in 1881 [41]. In 1976, the World Health Organization (WHO) [42] highlighted rabbits as among the most important laboratory animals for the study of different diseases [42][43][44][45]. The most common breeds of laboratory rabbits are derived from the European rabbit (Oryctolagus cuniculus) [46].
Laboratory rabbits have proven to be the most suitable and accessible hosts for all life-stages of various tick species during infestation and vaccination experiments [32][47]. This is because it has several advantages over the use of laboratory mice and rats, such as: (i) a longer life span than mice and rats [48]; (ii) a larger body size (up to four times larger than rats); (iii) higher blood volume, cell and tissue samples [49]; (iv) the production of copious antiserum [42][50]; and (v) easy maintenance and breeding [50].
Moreover, it is evident that rabbit-based experiments are more cost-effective when comparing trials conducted using large animals such as bovines. Various factors contribute to the overall costs, including animal prices, the extended maintenance period, a higher demand for feed, as well as the size and complexity of the animal facilities. Bovines require a greater amount of physical space and specialized infrastructure, along with large feed quantities. As a result, more demanding waste management systems are necessary for bovine experiments.
2. Vaccination in Rabbits
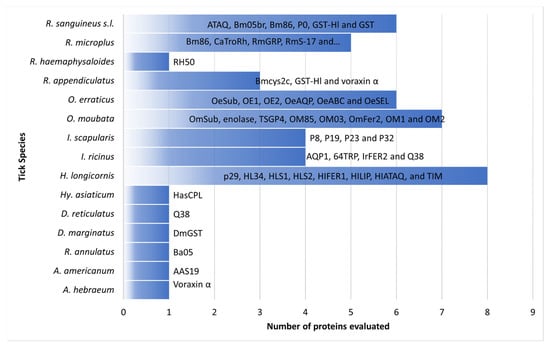
Rabbits are currently used as a model organism in anti-tick vaccines assays against ticks of the genera Amblyomma, Dermacentor, Hyalomma, Haemaphysalis, Ixodes, Ornithodoros, and Rhipicephalus (Figure 1) [23][26][51][52][53][54].

Figure 1. Tick-derived proteins evaluated in tick vaccination trials using rabbits as an animal model.
2.1. Haemaphysalis spp.
The tick Haemaphysalis longicornis tick is native to east Asia, with sparse distribution in Australia, New Zealand, and the U.S. [55][56]. It has a three-host life cycle, infesting cattle, and wild animals such as ungulates, lagomorphs, carnivores, and birds [57][58]. Immunological studies have shown different immunogenic proteins with the potential to develop a vaccine against H. longicornis from China and Japan. Japanese white rabbit and New Zealand breeds were mostly used in the infestation experiments.
2.2. Ornithodoros spp.
Ornithodoros erraticus and Ornithodoros moubata are nidicolous and endophilic argasid ticks that are widely distributed in different regions [59][60][61][62], and can intermittently feed on various vertebrates such as birds and canines [63][64]. Eight tick-derived proteins were evaluated for the development of vaccines against O. erraticus and O. moubata using rabbits as an animal model. Oleaga et al. tested the O. moubata ferritin 2 orthologues in New Zealand white rabbits, obtaining 71% efficacy for OmFer2, which corresponded to a decreased egg-hatching rate and in the subsequent number of emerging O. moubata larvae [65]. On the other hand, Pérez-Sánchez’s research group tested the immune response against aquaporin, showing moderate vaccine efficacy against O. erraticus [26].
2.3. Rhipicephalus spp.
Rhipicephalus appendiculatus, Rhipicephalus microplus, and Rhipicephalus sanguineus s.l. are medically important ixodid ticks of the genus Rhipicephalus [66]. Rhipicephalus appendiculatus is distributed in central, eastern, and southeastern Africa [67][68]. Rhipicephalus microplus and R. sanguineus s.l. are cosmopolitan ticks, distributed in the tropical and subtropical regions of the globe [6][69]. They present monoxene (R. microplus) and hetorexone (R. sanguineus s.l. and R. appendiculatus) lifecycles, preferring domestic hosts such as bovines, canines, and some wild animals, respectively. They feed on humans as incidental hosts [69][70].
The voraxin α homologue of the R. appendiculatus tick was used to immunize Japanese white rabbits, which resulted in a reduction in the weight of ticks, followed by a 50% reduction in egg mass [71]. On the other hand, a different study determined the vaccinal efficacy of rGST in New Zealand white rabbits, showing that rGST caused a reduction in the number of female R. sanguineus s.l. infestations [72].
2.4. Ixodes spp.
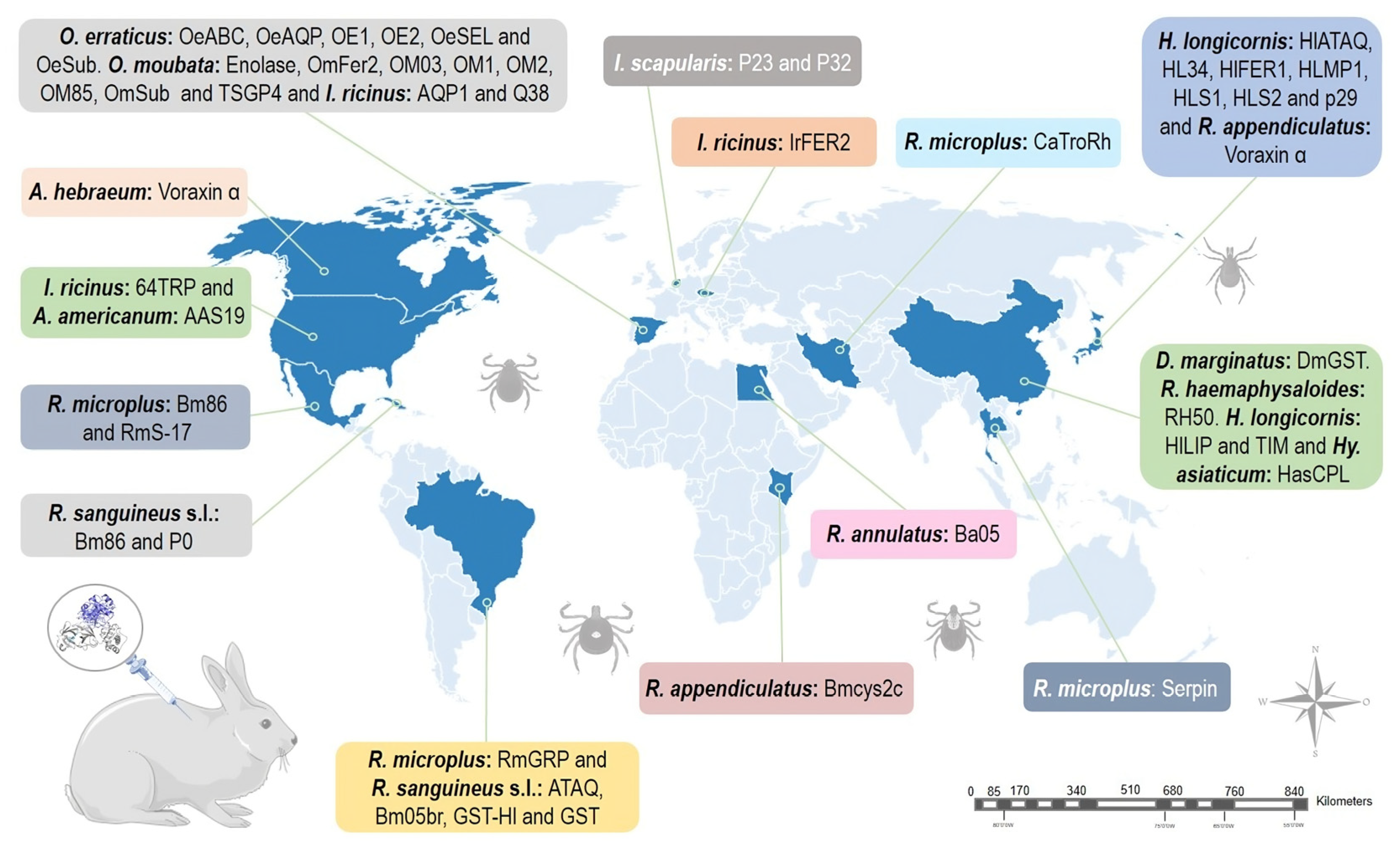
Ixodes ricinus and Ixodes scapularis are ixodid ticks that are characterized by a heteroxenous life cycle, and infest cattle, deer, dogs, and a wide variety of vertebrates, including humans [73][74][75]. The nymphal stage is most frequently responsible for transmitting pathogens to humans [76][77]. Of the 265 species of Ixodes, 55 are distributed in the neotropical regions of the planet [5]; however, I. ricinus and I. scapularis can be found only in the northern hemisphere [73]. Vaccination studies against I. ricinus and I. scapularis using the New Zealand rabbit breed were reported in the U.S., Spain, and the Netherlands (Figure 2) [39][78][79].

Figure 2. Geographical distribution of studies using rabbits as animal models to test anti-tick vaccines. Parts of the figures were drawn by using pictures from Servier Medical Art: http://smart.servier.com/ (accessed on 18 May 2023).
2.5. Dermacentor spp.
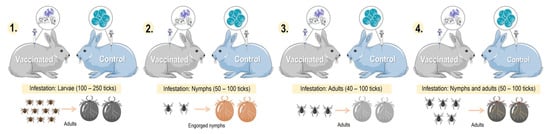
Dermacentor marginatus is an ixodid tick that has a heteroxenous life cycle and a variety of hosts including canines, horses, and humans [80][81]. It is a tick with a cosmopolitan distribution, present mainly in the Nearctic, Palearctic, and Neotropic ecozones of the planet [82][83][84]. In the search for proteins for the development of a vaccine against D. marginatus, the New Zealand white rabbit was used as an animal model in infestations and vaccination experiments. A study infested New Zealand breed rabbits with D. marginatus after administering the last dose of the immunogen of GST, recording moderate vaccine efficacy against D. marginatus (Figure 3) [85].

Figure 3. Comparison of different models of tick infestation in rabbits: 1. larval-stage tick infestation; 2. nymphal-stage tick infestation; 3. adult-stage tick infestation; and 4. nymphal- and adult-stage tick infestation. Parts of the figures were drawn by using pictures from Servier Medical Art: http://smart.servier.com/ (accessed on 21 February 2023).
3. Summary
To date, 57 tick-derived proteins have been evaluated as potential anti-tick vaccines by studying the immunogenic responses generated using rabbits as an experimental model. Rabbit models for anti-tick vaccination trials have allowed for a better understanding of the physiological mechanisms of ticks infesting mammal hosts. For example, a study of the serpins HLS1, rHLS2, rSerpin, and RmS-17 in rabbits stimulated an immune response that affected the prolonged duration of feeding, increased mortality, and reduced oviposition in ticks like H. longicornis and R. microplus [38][52][86][87].
Globally, the use of rabbits has provided novel evidence on a vaccine based on salivary glycine-rich proteins in various medically important tick species. According to the findings obtained by Zhou et al., using rabbits immunized with the glycine-rich protein RH50, the protein was only expressed in the salivary glands of partially fed ticks and not in the salivary glands of unfed ticks or in the midgut, fat body, or ovary of partially fed ticks, in contrast to what was previously reported for p29 and Bm86 proteins [51][88][89].
Rabbits have been used as an immunization model to evaluate immunological responses to a given antigen (Q38, Bm86, GST, serpins and voraxin) against different tick species. For example, high vaccine efficacy against both I. ricinus and D. reticulatus was obtained with the chimeric protein Q38 containing subolesin/akirin [30].
Similarly, experiments on rabbits using voraxin α, a protein derived from the male tick and transferred to the female through copulation to stimulate female blood-feeding [90], have yielded vaccine efficiency by reducing feeding times in Amblyomma hebraeum. There is an amino acid sequence similarity between the voraxin α of A. hebraeum (85%) and that of D. variabilis (92%) and R. appendiculatus (85%) [71]. The immunization results could therefore potentially be similar, making this protein a good multispecies vaccine candidate.
The use of rabbits as animal models in the discovery of anti-tick molecules has been fundamental in enabling the testing of these molecules before inoculation of the natural hosts. It was verified that rabbits present an immune response similar to that of the natural hosts. For example, the use of the ferritin 2 protein to immunize rabbits infested with I. ricinus (IrFER2) yielded an efficiency of 98%, while the efficiency of the same protein used in bovines infested with R. microplus and R. annulatus (RmFER2) was 64% and 72%, respectively [91].
The immune responses generated by the different proteins studied in rabbits could vary depending on the challenges of ticks in immature or mature life stages. For example, the response generated by the p29 and HL34 proteins in the life stages of larvae, nymphs, and adults of H. longicornis fed on immunized rabbits suggests that these proteins may be involved in mediating key physiological functions in the tick [51][92]. Although mature and immature ticks commonly express native p29, their sensitivities to rabbit immune responses against rp29 appear to be different [51], while the native HL34 is expressed in both immature (larvae and nymphs) and adult ticks. It is thus likely that immunity against rHL34 is directed towards immature and mature ticks [92].
Additionally, studies on rabbits have allowed us to broaden our knowledge about the “exposed” and “hidden” antigens of anti-tick proteins. For example, it was reported that HLS1 acts on the expression of hidden antigens, inhibiting the secretion of rHLS1 in rabbits during feeding [52]. Also, 64TRP isoforms were characterized as “dual-acting” anti-tick proteins against R. sanguineus s.l. and I. ricinus; they target both “exposed” and “hidden” antigens, preventing attachment, and feeding by affecting the feeding site, as well as cross-reacting with ‘hidden’ midgut antigens, resulting in the death of engorged ticks [39].
Results obtained from the study of the tick saliva proteome have shown a variety of proteins that protect ticks against host immune responses and antihemostatic mechanisms [93][94][95][96][97][98]. This is because, during hematophagy, tick salivary glands undergo remarkable growth and differentiation, accompanied by a significant increase in the synthesis of different proteins [99]. Tirloni et al. identified 187 tick and 68 bovine proteins in the saliva proteome of R. microplus, demonstrating that R. microplus saliva is rich in hemolipoproteins, lipocalins, peptidase inhibitors, antimicrobial peptides, glycine, and maintenance proteins [95]. These proteins, together with pharmacological bioactive lipids, can counteract the host’s defenses and hemostatic mechanisms [93][100], while the host physiological systems can trigger changes in the feeding activity of ticks [101] by stimulating proteins to limit host defense mechanisms [102].
References
- Brisola, C. Mites (ticks and others). In Medical and Veterinary Entomology; Publishing Athens: Athens, Greece, 2011; pp. 263–315.
- Alcantara, E.; Ferreira da Silva, C.; Ávila, R.; Pacheco, R.; Muñoz, L.; Honorio, D. Ticks (Acari: Argasidae and Ixodidae) infesting amphibians and reptiles in northeastern Brazil. Syst. Appl. Acarol. 2018, 23, 1497.
- Santos, M.; Bahiense, T.; Silva, A.; Onofrio, V.; Barral, T.; Souza, B.; Lira-da-Silva, R.; Biondi, I.; Meyer, R.; Portela, R. Ticks and associated pathogens from rescued wild animals in rainforest fragments of northeastern Brazil. Front. Vet. Sci. 2020, 7, 177.
- Cortés-Vecino, J. Changes in the distribution and abundance of ticks and their relationship with global warming. J. Vet. Med. Zoot. 2010, 57, 65–75.
- Guglielmone, A.; Nava, S.; Robbins, R. Geographic distribution of the hard ticks (Acari: Ixodida: Ixodidae) of the world by countries and territories. Zootaxa 2023, 5251, 1–274.
- Jongejan, F.; Uilenberg, G. The global importance of tick. Parasitology 2004, 129, 3–14.
- De la Fuente, J.; Estrada-Pena, A.; Venzal, J.; Kocan, K.; Sonenshine, D. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2007, 13, 6938–6946.
- Abbas, R.; Zaman, M.; Colwell, D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20.
- Food and Agriculture Organization of the United Nations. Expert Consultation on the Sustainable Management of Parasites in Livestock Challenged by the Global Emergence of Resistance-Part 1: Current Status and Management of Acaricide Resistance in Livestock Ticks; FAO Animal Production and Health Report No. 17; FAO: Rome, Italy, 2022; pp. 9–10.
- Obaid, M.; Islam, N.; Alouffi, A.; Zeb, A.; da Silva Vaz, I.; Tetsuya, T.; Abid, A. Acaricides resistance in ticks: Selection, diagnosis, mechanisms, and mitigation. Front. Cell. Infect. Microbiol. 2022, 12, 941831.
- Willadsen, P. Tick control: Thoughts on a research agenda. Vet. Parasitol. 2006, 138, 161–168.
- Mapholi, N.; Maiwashe, A.; Matika, O.; Riggio, V.; Banga, C.; MacNeil, M.; Dzama, K. Genetic parameters for tick counts across months for different tick species and anatomical locations in South African Nguni cattle. Trop. Anim. Health Prod. 2017, 49, 1201–1210.
- Porto-Neto, L.; Reverter, A.; Prayaga, K.; Barendse, W. The genetic architecture of climatic adaptation of tropical cattle. PLoS ONE 2014, 9, 113–118.
- Ojeda-Chi, M.; Rodriguez-Vivas, R.; Galindo-Velasco, E.; Lezama-Gutierrez, R. Laboratory and field evaluation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for the control of Rhipicephalus microplus (Acari: Ixodidae) in the Mexican tropics. Vet. Parasitol. 2010, 170, 348–354.
- Nobrega, L.; Mesquita, E.; Almeida, T.; de Oliveira, R.; Oliveira, J.; Fernandes, F.; Guedes, M.; Pinheiro, E. Encapsulation of entomopathogenic fungal conidia: Evaluation of stability and control potential of Rhipicephalus microplus. Ticks Tick Borne Dis. 2023, 14, 102184.
- Freitas-Ribeiro, G.; Furlong, J.; Vasconcelos, V.; Dolinski, C.; Ribeiro, A. Analysis of biological parameters of Boophilus microplus Canestrini, 1887 exposed to entomopathogenic nematodes Steinernema carpocapsae Santa Rosa and ALL strains (Steinernema: Rhabditidae). Braz. Arch. Biol. Technol. 2005, 48, 911–919.
- De Oliveira, C.; da Silva Matos, R.; Xavier, L.; de Souza, W.; Rita, V.; Pinheiro, E.; Dolinski, C.; de Azevedo, C. First report of pathogenicity of entomopathogenic nematodes of the genus Heterorhabditis on partially engorged females of Dermacentor nitens (Acari: Ixodidae). Biol. Control 2014, 69, 78–81.
- Zingg, S.; Dolle, P.; Voordouw, M.; Kern, M. The negative effect of wood ant presence on tick abundance. Parasit. Vectors 2018, 11, 164.
- Platts-Mills, T.; Retterer, M.; Workman, L.; Wilson, J. A consistent “shortage” of cases of the alpha-gal syndrome (AS) on the gulf coast: Possible relevance of fire ants as a predator of lone star ticks. J. Allergy Clin. Immunol. 2019, 143, AB278.
- Adenubi, O.; Ahmed, A.; Fasina, F.; McGaw, L.; Eloff, J.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crops Prod. 2018, 123, 779–806.
- Charlie-Silva, I.; Giglioti, R.; Magalhaes, P.; Sousa, I.; AnnFoglio, M.; Oliveira, M.; Chagas, A. Lack of impact of dietary inclusión of dried Artemisia annua leaves for cattle on infestation by Rhipicephalus (Boophilus) microplus tick. Ticks Tick Borne Dis. 2018, 9, 1115–1119.
- de la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376.
- Parizi, L.; Konrdörfer, C.; Alves, G.; Fagundes, B.; Kiio, I.; Amaral, M.; da Silva, R.; Camargo-Mathias, M.; Seixas, A.; Konnai, S.; et al. Rhipicephalus microplus cystatin as a potential cross-protective tick vaccine against Rhipicephalus appendiculatus. Ticks Tick Borne Dis. 2020, 11, 101378.
- Wang, D.; Lihong, L.; Pinxing, W.; Hongmeng, D.; Shuwen, X.; Jingze, L.; Yonghong, H. Gene cloning, analysis and effect of a new lipocalin homologue from Haemaphysalis longicornis as a protective antigen for an anti-tick vaccine. Vet. Parasitol. 2021, 290, 109–358.
- Gomes, H.; Moraes, J.; Githaka, N.; Martins, R.; Isezaki, M.; da Silva Vaz, I.; Logullo, C.; Konnai, C.; Ohashi, K. Vaccination with cyclin-dependent kinase tick antigen confers protection against Ixodes infestation. Vet. Parasitol. 2015, 211, 266–273.
- Pérez-Sánchez, R.; Manzano-Román, R.; Obolo-Mvoulouga, P.; Oleaga, A. Function-guided selection of midgut antigens from Ornithodoros erraticus ticks and an evaluation of their protective efficacy in rabbits. Vet. Parasitol. 2019, 272, 1–12.
- Lynn, G.; Černý, L.; Kurokawa, C.; Diktaş, H.; Matias, J.; Sajid, A.; Arora, G.; DePonte, K.; Narasimhan, S.; Fikrig, E. Immunization of guinea pigs with cement extract induces resistance against Ixodes scapularis ticks. Ticks Tick Borne Dis. 2022, 13, 10201.
- Ma, M.; Chen, Z.; Liu, A.; Ren, Q.; Liu, J.; Liu, Z.; Li, Y.; Yin, H.; Guan, G.; Luo, J. Biological parameters of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) fed on rabbits, sheep, and cattle. Korean. J. Parasitol. 2016, 54, 301–305.
- Colby, L.; Quenee, L.; Zitzow, L. Considerations for infectious disease research studies using animals. Comp. Med. 2017, 67, 222–231.
- Contreras, M.; de la Fuente, J. Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 subolesin/akirin chimera. Vaccine 2016, 34, 3010–3013.
- Stokes, J.; Walker, D.; Varela-Stokes, A. The guinea pig model for tick-borne spotted fever rickettsioses: A second look. Ticks Tick Borne Dis 2020, 11, 101538.
- Burkholder, T.; Linton, G.; Hoyt, J.; Young, R. The Rabbit as an Experimental Model. In The Laboratory Rabbit, Guinea Pig, Hamster, and other Rodents; Suckow, M., Stevens, K., Wilson, R., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 529–560.
- Esteves, P.; Abrantes, J.; Baldauf, H.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; et al. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018, 50, 1–10.
- Soares, J.; Pinheiro, A.; Esteves, P. The rabbit as an animal model to study innate immunity genes: Is it better than mice? Front. Immunol. 2022, 13, 981815.
- Valentine, H.; Daugherity, E.; Singh, B.; Maurer, K. The experimental use of Syrian hamsters. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M., Stevens, K., Wilson, R., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 875–906.
- Miedel, E.; Hankenson, F. Biology and diseases of hámsters. In Laboratory Animal Medicine, 3rd ed.; Fox, J., Anderson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 209–245.
- Shomer, N.; Holcombe, H.; Harkness, J. Biology and diseases of guinea pigs. In Laboratory Animal Medicine, 3rd ed.; Fox, J., Anderson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 247–283.
- Lagunes-Quintanilla, R.; Valdez-Espinoza, U.; Hernández-Ortiz, R.; Castro-Saines, E.; Merino, O.; Mendoza-Martínez, N. Experimental vaccination in rabbits using the peptide RmS-17 antigen reduces the performance of a Mexican Rhipicephalus microplus tick strain. Ticks Tick Borne Dis. 2022, 13, 102044.
- Trimnell, A.; Davies, G.; Lissina, O.; Hails, R.; Nuttall, P. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005, 23, 4329–4341.
- Kurokawa, C.; Narasimhan, S.; Vidyarthi, A.; Sameet, C.; Meister, L.; Diktas, H.; Strank, N.; Lynn, G.; DePonte, K.; Craft, J.; et al. Repeat tick exposure elicits distinct immune responses in guinea pigs and mice. Ticks Tick Borne Dis. 2020, 11, 101529.
- Schneider, M.; Santos-Burgoa, C. Treatment against human rabies: A bit of its history. Public Health 1994, 28, 454–463.
- Russell, R.; Schilling, P. Selected topics on laboratory medicine: The rabbit. In Series of Scientific and Technical Monographs; WHO: Rome, Italy, 1976; Volume 4, pp. 12–86.
- Kirkland, W. Ultrastructural changes in the nymphal salivary glands of the rabbit tick, Haemaphysalis leporispalustris, during feeding. J. Insect Physiol. 1971, 17, 1933–1946.
- McGowan, M.; Homer, T.; Odell, G.; McNew, R.; Barker, R. Performance of ticks fed on rabbits inoculated with extracts derived from homogenized tick Amblyomma maculatum Koch (Acari: Ixodidae). J. Parasitol. 1980, 66, 42–48.
- Walker, A.; Fletcher, J. Histological study of the attachment sites of adult Rhipicephalus appendiculatus on rabbits and cattle. Int. J. Parasitol. 1986, 16, 399–413.
- Fox, R. The biology of the laboratory rabbit. In Taxonomy and Genetics; Weisbroth, S., Kraus, A., Eds.; Academic Press: New York, NY, USA, 1974; 22p.
- Graur, D.; Duret, L.; Gouy, M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 1996, 379, 333–335.
- Bryda, E. The mighty mouse: The impact of rodents on advances in biomedical research. Mo Med. 2013, 110, 207–211.
- Mullane, K.; Williams, M. Animal models of asthma: Reprise or reboot? Biochem. Pharmacol. 2014, 87, 131–139.
- ARBA. American Rabbit Breeders Association. Available online: https://www.arba.net/breeds.htm (accessed on 30 October 2018).
- Mulenga, A.; Sugimoto, Y.; Sako, K.; Musoke, A.; Mozaria, S.; Onuma, M. Molecular characterisation of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect. Immun. 1999, 40, 1652–1658.
- Sugino, M.; Imamura, S.; Mulenga, A.; Nakajima, M.; Tsuda, A.; Ohashi, K.; Onuma, M. A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine 2003, 21, 2844–2851.
- Galay, R.; Umemiya-Shirafuji, R.; Bacolod, E.; Maeda, H.; Kusakisako, K.; Koyama, J. Two kinds of ferritin protect ixodid ticks from iron overload and consequent oxidative stress. PLoS ONE 2014, 9, e90661.
- Liang, N.; Hong-Meng, D.; Xiang-Yuan, F.; Ya-Xue, W.; Feng, Y.; Xiao-Ya, L.; Yong-Hong, H. Characterization and evaluation of a new triosephosphate isomerase homologue from Haemaphysalis longicornis as a candidate vaccine against tick infection. Ticks Tick Borne Dis. 2022, 13, 101–968.
- Egizi, A.; Bulaga-Seraphin, L.; Alt, E.; Bajwa, W.; Bernick, J.; Bickerton, M.; Fonseca, D. First glimpse into the origin and spread of the Asian longhorned tick Haemaphysalis longicornis, in the United States. Zoonoses Public Health 2020, 67, 637–650.
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X.; et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet. Health 2020, 4, e320–e329.
- Luo, L.; Zhao, L.; Wen, H.; Zhang, Z.; Liu, J.; Fang, L.; Yu, X. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. J. Emerg. Infect. Dis. 2015, 21, 1770.
- Tufts, D.; Sameroff, S.; Tagliafierro, T.; Jain, K.; Oleynik, A.; VanAcker, M.; Tokarz, R. A metagenomic examination of the pathobiome of the invasive tick species, Haemaphysalis longicornis, collected from a New York City borough, USA. Ticks Tick Borne Dis. 2020, 11, 101516.
- Pospelova-Shtrom, M. On the system of classification of ticks of the family Argasidae CAN. Acarologia 1969, 11, 1–22.
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R. African swine fever: How can global spread be prevented? Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696.
- ECDC. European Centre for Disease Prevention and Control and European Food Safety Authority. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data (accessed on 12 June 2022).
- Baizheng, W.; Xin, L.; Jingze, L.; Rong, B. Predicting the potential habitat for Ornithodoros tick species in China. Vet. Parasitol. 2022, 311, 109793.
- De Morais, J.; Lopes, I.; Nuncio, M. Spanish-African recurrent fever in Portugal: Historical and clinical-epidemic escorco. Int. Med. 2007, 14, 170–178.
- Assous, M.; Wilamowski, A. Relapsing fever borreliosis in Eurasia-forgotten, but certainly not gone! Clin. Microbiol. Infect. 2009, 15, 407–414.
- Oleaga, A.; González-Pérez, S.; Pérez-Sánchez, R. First molecular and functional characterisation of ferritin 2 proteins from Ornithodoros argasid ticks. Vet. Parasitol. 2022, 304, 109–684.
- Guglielmone, A.; Petney, T.; Robbins, R. Ixodidae (Acari: Ixodoidea): Descriptions and redescriptions of all known species from 1758 to December 31, 2019. Zootaxa 2020, 4871, 1–322.
- Walker, J.; Keirans, J.; Horak, I. The genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World, 1st ed.; Cambridge University Press: Cambridge, UK, 2000; pp. 79–104.
- Makwarela, T.; Nyangiwe, N.; Masebe, T.; Mbizeni, S.; Nesengani, L.; Djikeng, A.; Mapholi, N. Tick diversity and distribution of hard (Ixodidae) cattle ticks in South Africa. Microbiol. Res. 2023, 14, 42–59.
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26–33.
- Brophy, M.; Riehle, M.; Mastrud, N.; Ravenscraft, A.; Adamson, J.; Walker, K. Genetic variation in Rhipicephalus sanguineus s.l. ticks across Arizona. Int. J. Environ. Res. Public Health 2022, 19, 4223.
- Yamada, S.; Konnai, S.; Imamura, S.; Ito, T.; Onuma, M.; Ohashi, K. Cloning and characterization of Rhipicephalus appendiculatus voraxinα and its effect as anti-tick vaccine. Vaccine 2009, 27, 5989–5997.
- Ndawula, C.; Alves, G.; Parizi, L.; da Silva Vaz, I. Constituting a glutathione S-transferase-cocktail vaccine against tick infestation. Vaccine 2019, 37, 1918–1927.
- Gray, J. The ecology of ticks transmitting Lyme borreliosis. Exp. Appl. Acarol. 1998, 22, 249–258.
- Gilbert, L.; Maffey, G.; Ramsay, S.; Hester, A. The effect of deer management on the abundance of Ixodes ricinus in Scotland. Ecol. Appl. 2012, 22, 658–667.
- Hofmeester, T.; Sprong, H.; Jansen, P.; Prins, H.; Van, S. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in dutch forests. Parasites Vectors 2017, 10, 433.
- Ostfeld, R.; Canham, C.; Oggenfuss, K.; Winchcombe, R.; Keesing, F. Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk. PLoS ONE 2006, 4, e145.
- Pasternak, A.; Palli, S. Mapping distributions of the Lyme disease vector, Ixodes scapularis, and spirochete, Borrelia burgdorferi, in Kentucky using passive and active surveillance. Ticks Tick Borne Dis. 2022, 13, 101885.
- Schuijt, T.; Narasimhan, S.; Daffre, S.; de Ponte, K.; Hovius, J.; Veer, V. Identification and characterization of Ixodes scapularis antigens that elicit immunity to ticks by visualizing the yeast surface. PLoS ONE 2011, 6, e15926.
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328.
- Yunker, C.; Keirans, J.; Cliffornd, C.; Easton, E. Dermacentor ticks (Acari: Ixodoidea: Ixodidae) of the new world: A scanning electron microscope atlas. Proc. Entomol. Soc. Wash. 1986, 88, 609–627.
- Eisen, R.; Kugeler, K.; Eisen, J.; Beard, C.; Paddock, C. Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 2017, 58, 319–335.
- Dergousoff, S.; Galloway, T.; Lindsay, L.; Curry, P.; Chilton, N. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 2013, 50, 510–520.
- Zhang, G.; Zheng, D.; Tian, Y.; Li, S. A dataset of distribution and diversity of ticks in China. Sci. Data 2019, 6, 105.
- Martin, J.; Fischhoff, I.; Castellanos, A.; Han, B. Ecological predictors of zoonotic vector status among Dermacentor ticks (Acari: Ixodidae): A trait-based approach. J. Med. Entomol. 2022, 59, 2158–2166.
- Huercha, R.; Min, L.; Xinli, F.; Zhengxiang, H.; Lijiang, W.; Yongchang, L.; Wei, Z.; Yang, Z.; Yuhui, M.; Chahan, B. Characterization of glutathione S-transferase of Dermacantor marginatus and effect of the recombinant antigen as a potential anti tick vaccine. Vet. Parasitol. 2020, 279, 109043.
- Jittapalapong, S.; Kaewhom, P.; Pumhom, P.; Canales, M.; de la Fuente, J.; Stich, R. Immunization of rabbits with recombinant serine protease inhibitor reduces the performance of adult female Rhipicephalus microplus. Transbound. Emerg. Dis. 2010, 57, 103–106.
- Imamura, S.; da Silva Vaz, I.; Sugino, M.; Ohashi, K.; Onuma, M. A serine protease inhibitor (Serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 2005, 23, 1301–1311.
- Zhou, J.; Gong, H.; Zhou, Y.; Xuan, X.; Fujisaki, K. Identification of a glycine-rich protein from the tick Rhipicephalus haemaphysaloides and evaluation of its vaccine potential against tick feeding. Parasitol. Res. 2006, 100, 77–84.
- Kemp, D.; Pearson, R.; Gough, J.; Willadsen, P. Vaccination against Boophilus microplus: Localization of antigens on the tick gut cells and their interaction with the host immune system. Exp. Appl. Acarol. 1989, 7, 43–58.
- Weiss, B.; Kaufman, W. Two feeding-induced proteins from the male gonad trigger engorgement of the female tick, Amblyomma hebraeum. Proc. Natl. Acad. Sci. USA 2004, 101, 5874–5879.
- Hajdusek, O.; Almazán, C.; Loosova, G.; Villar, M.; Canales, M.; Grubhoffer, L.; Kopacek, P.; de la Fuente, J. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010, 28, 2993–2998.
- Tsuda, A.; Mulenga, A.; Sugimoto, C.; Nakajima, M.; Ohashi, K.; Onuma, M. cDNA cloning, characterization and vaccine effect analysis of Haemaphysalis longicornis tick saliva proteins. Vaccine 2001, 19, 4287–4296.
- Francischetti, I. The role of saliva in tick feeding. Front. Biosci. 2009, 14, 2051–2088.
- Mudenda, L.; Pierlé, S.; Turse, J.; Scoles, G.; Purvine, S.; Nicora, C.; Clauss, T.; Ueti, M.; Brown, W.; Brayton, K. Proteomics informed by transcriptomics identifies novel secreted proteins in Dermacentor andersoni saliva. Int. J. Parasitol. 2014, 44, 1029–1037.
- Tirloni, L.; Reck, J.; Terra, R.; Martins, J.; Mulenga, A.; Sherman, N.; Fox, J.; Yates, J.; Termignoni, C.; Pinto, A.; et al. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS ONE 2014, 9, e94831.
- Tirloni, L.; Islam, M.; Kim, T.; Diedrich, J.; Yates, J.; Pinto, A.; Mulenga, A.; You, M.; da Silva, I. Saliva from nymph and adult females of Haemaphysalis longicornis: A proteomic study. Parasit. Vectors 2015, 8, 338.
- Kim, T.; Tirloni, L.; Pinto, A.; Moresco, J.; Yates, J.; da Silva Vaz, I.; Mulenga, A. Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS Negl. Trop. Dis. 2016, 10, e0004323.
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281.
- Leboulle, G.; Rochez, C.; Louahed, J.; Ruti, B.; Brossard, M. Isolation of Ixodes ricinus salivary gland mRNA encoding factors induced during blood feeding. Am. J. Trop. Med. Hyg. 2022, 66, 225–233.
- Mans, B. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J. Innate Immun. 2011, 3, 41–51.
- Ribeiro, J.; Francischetti, I. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88.
- Wikel, S. Host immunity to ticks. Annu. Rev. Entomol. 1996, 41, 1–22.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
579
Revisions:
2 times
(View History)
Update Date:
11 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




