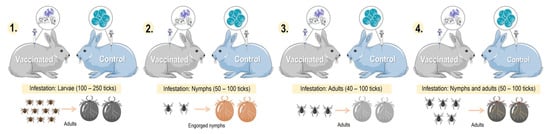
Studies evaluating candidate tick-derived proteins as anti-tick vaccines in natural hosts have been limited due to high costs. To overcome this problem, animal models are used in immunization tests. The most commonly used rabbit breeds were New Zealand (73.8%), Japanese white (19%), Californians (4.8%) and Flemish lop-eared (2.4%) rabbits. Anti-tick vaccines efficacy resulted in up to 99.9%. Haemaphysalis longicornis (17.9%) and Ornithodoros moubata (12.8%) were the most common tick models in vaccination trials. Experiments with rabbits have revealed that some proteins (CoAQP, OeAQP, OeAQP1, Bm86, GST-Hl, 64TRP, serpins and voraxin) can induce immune responses against various tick species.
- antigen
- humoral and adaptive response
- immunization
- rabbit
- tick
1. Introduction
2. Vaccination in Rabbits
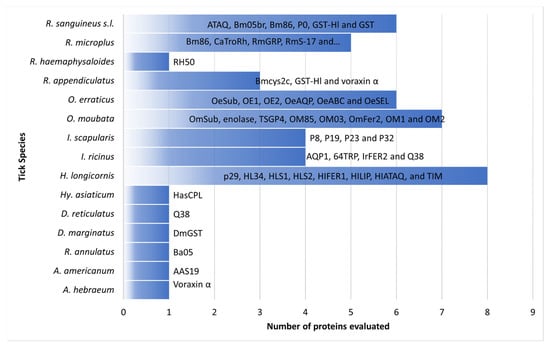
2.1. Haemaphysalis spp.
2.2. Ornithodoros spp.
2.3. Rhipicephalus spp.
2.4. Ixodes spp.
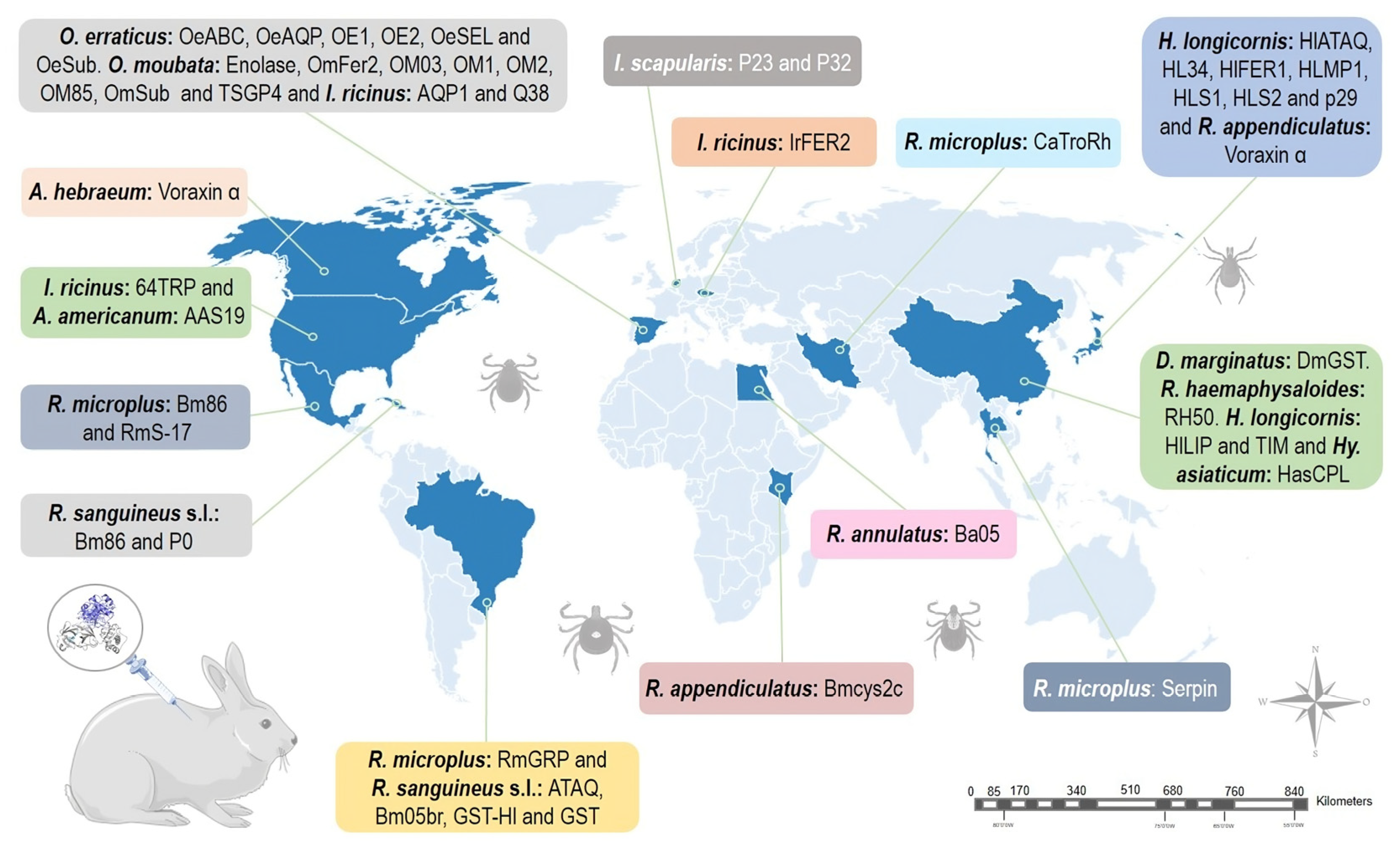
2.5. Dermacentor spp.
3. Summary
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12091117
References
- Brisola, C. Mites (ticks and others). In Medical and Veterinary Entomology; Publishing Athens: Athens, Greece, 2011; pp. 263–315.
- Alcantara, E.; Ferreira da Silva, C.; Ávila, R.; Pacheco, R.; Muñoz, L.; Honorio, D. Ticks (Acari: Argasidae and Ixodidae) infesting amphibians and reptiles in northeastern Brazil. Syst. Appl. Acarol. 2018, 23, 1497.
- Santos, M.; Bahiense, T.; Silva, A.; Onofrio, V.; Barral, T.; Souza, B.; Lira-da-Silva, R.; Biondi, I.; Meyer, R.; Portela, R. Ticks and associated pathogens from rescued wild animals in rainforest fragments of northeastern Brazil. Front. Vet. Sci. 2020, 7, 177.
- Cortés-Vecino, J. Changes in the distribution and abundance of ticks and their relationship with global warming. J. Vet. Med. Zoot. 2010, 57, 65–75.
- Guglielmone, A.; Nava, S.; Robbins, R. Geographic distribution of the hard ticks (Acari: Ixodida: Ixodidae) of the world by countries and territories. Zootaxa 2023, 5251, 1–274.
- Jongejan, F.; Uilenberg, G. The global importance of tick. Parasitology 2004, 129, 3–14.
- De la Fuente, J.; Estrada-Pena, A.; Venzal, J.; Kocan, K.; Sonenshine, D. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2007, 13, 6938–6946.
- Abbas, R.; Zaman, M.; Colwell, D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20.
- Food and Agriculture Organization of the United Nations. Expert Consultation on the Sustainable Management of Parasites in Livestock Challenged by the Global Emergence of Resistance-Part 1: Current Status and Management of Acaricide Resistance in Livestock Ticks; FAO Animal Production and Health Report No. 17; FAO: Rome, Italy, 2022; pp. 9–10.
- Obaid, M.; Islam, N.; Alouffi, A.; Zeb, A.; da Silva Vaz, I.; Tetsuya, T.; Abid, A. Acaricides resistance in ticks: Selection, diagnosis, mechanisms, and mitigation. Front. Cell. Infect. Microbiol. 2022, 12, 941831.
- Willadsen, P. Tick control: Thoughts on a research agenda. Vet. Parasitol. 2006, 138, 161–168.
- Mapholi, N.; Maiwashe, A.; Matika, O.; Riggio, V.; Banga, C.; MacNeil, M.; Dzama, K. Genetic parameters for tick counts across months for different tick species and anatomical locations in South African Nguni cattle. Trop. Anim. Health Prod. 2017, 49, 1201–1210.
- Porto-Neto, L.; Reverter, A.; Prayaga, K.; Barendse, W. The genetic architecture of climatic adaptation of tropical cattle. PLoS ONE 2014, 9, 113–118.
- Ojeda-Chi, M.; Rodriguez-Vivas, R.; Galindo-Velasco, E.; Lezama-Gutierrez, R. Laboratory and field evaluation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for the control of Rhipicephalus microplus (Acari: Ixodidae) in the Mexican tropics. Vet. Parasitol. 2010, 170, 348–354.
- Nobrega, L.; Mesquita, E.; Almeida, T.; de Oliveira, R.; Oliveira, J.; Fernandes, F.; Guedes, M.; Pinheiro, E. Encapsulation of entomopathogenic fungal conidia: Evaluation of stability and control potential of Rhipicephalus microplus. Ticks Tick Borne Dis. 2023, 14, 102184.
- Freitas-Ribeiro, G.; Furlong, J.; Vasconcelos, V.; Dolinski, C.; Ribeiro, A. Analysis of biological parameters of Boophilus microplus Canestrini, 1887 exposed to entomopathogenic nematodes Steinernema carpocapsae Santa Rosa and ALL strains (Steinernema: Rhabditidae). Braz. Arch. Biol. Technol. 2005, 48, 911–919.
- De Oliveira, C.; da Silva Matos, R.; Xavier, L.; de Souza, W.; Rita, V.; Pinheiro, E.; Dolinski, C.; de Azevedo, C. First report of pathogenicity of entomopathogenic nematodes of the genus Heterorhabditis on partially engorged females of Dermacentor nitens (Acari: Ixodidae). Biol. Control 2014, 69, 78–81.
- Zingg, S.; Dolle, P.; Voordouw, M.; Kern, M. The negative effect of wood ant presence on tick abundance. Parasit. Vectors 2018, 11, 164.
- Platts-Mills, T.; Retterer, M.; Workman, L.; Wilson, J. A consistent “shortage” of cases of the alpha-gal syndrome (AS) on the gulf coast: Possible relevance of fire ants as a predator of lone star ticks. J. Allergy Clin. Immunol. 2019, 143, AB278.
- Adenubi, O.; Ahmed, A.; Fasina, F.; McGaw, L.; Eloff, J.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crops Prod. 2018, 123, 779–806.
- Charlie-Silva, I.; Giglioti, R.; Magalhaes, P.; Sousa, I.; AnnFoglio, M.; Oliveira, M.; Chagas, A. Lack of impact of dietary inclusión of dried Artemisia annua leaves for cattle on infestation by Rhipicephalus (Boophilus) microplus tick. Ticks Tick Borne Dis. 2018, 9, 1115–1119.
- de la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376.
- Parizi, L.; Konrdörfer, C.; Alves, G.; Fagundes, B.; Kiio, I.; Amaral, M.; da Silva, R.; Camargo-Mathias, M.; Seixas, A.; Konnai, S.; et al. Rhipicephalus microplus cystatin as a potential cross-protective tick vaccine against Rhipicephalus appendiculatus. Ticks Tick Borne Dis. 2020, 11, 101378.
- Wang, D.; Lihong, L.; Pinxing, W.; Hongmeng, D.; Shuwen, X.; Jingze, L.; Yonghong, H. Gene cloning, analysis and effect of a new lipocalin homologue from Haemaphysalis longicornis as a protective antigen for an anti-tick vaccine. Vet. Parasitol. 2021, 290, 109–358.
- Gomes, H.; Moraes, J.; Githaka, N.; Martins, R.; Isezaki, M.; da Silva Vaz, I.; Logullo, C.; Konnai, C.; Ohashi, K. Vaccination with cyclin-dependent kinase tick antigen confers protection against Ixodes infestation. Vet. Parasitol. 2015, 211, 266–273.
- Pérez-Sánchez, R.; Manzano-Román, R.; Obolo-Mvoulouga, P.; Oleaga, A. Function-guided selection of midgut antigens from Ornithodoros erraticus ticks and an evaluation of their protective efficacy in rabbits. Vet. Parasitol. 2019, 272, 1–12.
- Lynn, G.; Černý, L.; Kurokawa, C.; Diktaş, H.; Matias, J.; Sajid, A.; Arora, G.; DePonte, K.; Narasimhan, S.; Fikrig, E. Immunization of guinea pigs with cement extract induces resistance against Ixodes scapularis ticks. Ticks Tick Borne Dis. 2022, 13, 10201.
- Ma, M.; Chen, Z.; Liu, A.; Ren, Q.; Liu, J.; Liu, Z.; Li, Y.; Yin, H.; Guan, G.; Luo, J. Biological parameters of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) fed on rabbits, sheep, and cattle. Korean. J. Parasitol. 2016, 54, 301–305.
- Colby, L.; Quenee, L.; Zitzow, L. Considerations for infectious disease research studies using animals. Comp. Med. 2017, 67, 222–231.
- Contreras, M.; de la Fuente, J. Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 subolesin/akirin chimera. Vaccine 2016, 34, 3010–3013.
- Stokes, J.; Walker, D.; Varela-Stokes, A. The guinea pig model for tick-borne spotted fever rickettsioses: A second look. Ticks Tick Borne Dis 2020, 11, 101538.
- Burkholder, T.; Linton, G.; Hoyt, J.; Young, R. The Rabbit as an Experimental Model. In The Laboratory Rabbit, Guinea Pig, Hamster, and other Rodents; Suckow, M., Stevens, K., Wilson, R., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 529–560.
- Esteves, P.; Abrantes, J.; Baldauf, H.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; et al. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018, 50, 1–10.
- Soares, J.; Pinheiro, A.; Esteves, P. The rabbit as an animal model to study innate immunity genes: Is it better than mice? Front. Immunol. 2022, 13, 981815.
- Valentine, H.; Daugherity, E.; Singh, B.; Maurer, K. The experimental use of Syrian hamsters. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M., Stevens, K., Wilson, R., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 875–906.
- Miedel, E.; Hankenson, F. Biology and diseases of hámsters. In Laboratory Animal Medicine, 3rd ed.; Fox, J., Anderson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 209–245.
- Shomer, N.; Holcombe, H.; Harkness, J. Biology and diseases of guinea pigs. In Laboratory Animal Medicine, 3rd ed.; Fox, J., Anderson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 247–283.
- Lagunes-Quintanilla, R.; Valdez-Espinoza, U.; Hernández-Ortiz, R.; Castro-Saines, E.; Merino, O.; Mendoza-Martínez, N. Experimental vaccination in rabbits using the peptide RmS-17 antigen reduces the performance of a Mexican Rhipicephalus microplus tick strain. Ticks Tick Borne Dis. 2022, 13, 102044.
- Trimnell, A.; Davies, G.; Lissina, O.; Hails, R.; Nuttall, P. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005, 23, 4329–4341.
- Kurokawa, C.; Narasimhan, S.; Vidyarthi, A.; Sameet, C.; Meister, L.; Diktas, H.; Strank, N.; Lynn, G.; DePonte, K.; Craft, J.; et al. Repeat tick exposure elicits distinct immune responses in guinea pigs and mice. Ticks Tick Borne Dis. 2020, 11, 101529.
- Schneider, M.; Santos-Burgoa, C. Treatment against human rabies: A bit of its history. Public Health 1994, 28, 454–463.
- Russell, R.; Schilling, P. Selected topics on laboratory medicine: The rabbit. In Series of Scientific and Technical Monographs; WHO: Rome, Italy, 1976; Volume 4, pp. 12–86.
- Kirkland, W. Ultrastructural changes in the nymphal salivary glands of the rabbit tick, Haemaphysalis leporispalustris, during feeding. J. Insect Physiol. 1971, 17, 1933–1946.
- McGowan, M.; Homer, T.; Odell, G.; McNew, R.; Barker, R. Performance of ticks fed on rabbits inoculated with extracts derived from homogenized tick Amblyomma maculatum Koch (Acari: Ixodidae). J. Parasitol. 1980, 66, 42–48.
- Walker, A.; Fletcher, J. Histological study of the attachment sites of adult Rhipicephalus appendiculatus on rabbits and cattle. Int. J. Parasitol. 1986, 16, 399–413.
- Fox, R. The biology of the laboratory rabbit. In Taxonomy and Genetics; Weisbroth, S., Kraus, A., Eds.; Academic Press: New York, NY, USA, 1974; 22p.
- Graur, D.; Duret, L.; Gouy, M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 1996, 379, 333–335.
- Bryda, E. The mighty mouse: The impact of rodents on advances in biomedical research. Mo Med. 2013, 110, 207–211.
- Mullane, K.; Williams, M. Animal models of asthma: Reprise or reboot? Biochem. Pharmacol. 2014, 87, 131–139.
- ARBA. American Rabbit Breeders Association. Available online: https://www.arba.net/breeds.htm (accessed on 30 October 2018).
- Mulenga, A.; Sugimoto, Y.; Sako, K.; Musoke, A.; Mozaria, S.; Onuma, M. Molecular characterisation of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect. Immun. 1999, 40, 1652–1658.
- Sugino, M.; Imamura, S.; Mulenga, A.; Nakajima, M.; Tsuda, A.; Ohashi, K.; Onuma, M. A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine 2003, 21, 2844–2851.
- Galay, R.; Umemiya-Shirafuji, R.; Bacolod, E.; Maeda, H.; Kusakisako, K.; Koyama, J. Two kinds of ferritin protect ixodid ticks from iron overload and consequent oxidative stress. PLoS ONE 2014, 9, e90661.
- Liang, N.; Hong-Meng, D.; Xiang-Yuan, F.; Ya-Xue, W.; Feng, Y.; Xiao-Ya, L.; Yong-Hong, H. Characterization and evaluation of a new triosephosphate isomerase homologue from Haemaphysalis longicornis as a candidate vaccine against tick infection. Ticks Tick Borne Dis. 2022, 13, 101–968.
- Egizi, A.; Bulaga-Seraphin, L.; Alt, E.; Bajwa, W.; Bernick, J.; Bickerton, M.; Fonseca, D. First glimpse into the origin and spread of the Asian longhorned tick Haemaphysalis longicornis, in the United States. Zoonoses Public Health 2020, 67, 637–650.
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X.; et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet. Health 2020, 4, e320–e329.
- Luo, L.; Zhao, L.; Wen, H.; Zhang, Z.; Liu, J.; Fang, L.; Yu, X. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. J. Emerg. Infect. Dis. 2015, 21, 1770.
- Tufts, D.; Sameroff, S.; Tagliafierro, T.; Jain, K.; Oleynik, A.; VanAcker, M.; Tokarz, R. A metagenomic examination of the pathobiome of the invasive tick species, Haemaphysalis longicornis, collected from a New York City borough, USA. Ticks Tick Borne Dis. 2020, 11, 101516.
- Pospelova-Shtrom, M. On the system of classification of ticks of the family Argasidae CAN. Acarologia 1969, 11, 1–22.
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R. African swine fever: How can global spread be prevented? Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696.
- ECDC. European Centre for Disease Prevention and Control and European Food Safety Authority. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data (accessed on 12 June 2022).
- Baizheng, W.; Xin, L.; Jingze, L.; Rong, B. Predicting the potential habitat for Ornithodoros tick species in China. Vet. Parasitol. 2022, 311, 109793.
- De Morais, J.; Lopes, I.; Nuncio, M. Spanish-African recurrent fever in Portugal: Historical and clinical-epidemic escorco. Int. Med. 2007, 14, 170–178.
- Assous, M.; Wilamowski, A. Relapsing fever borreliosis in Eurasia-forgotten, but certainly not gone! Clin. Microbiol. Infect. 2009, 15, 407–414.
- Oleaga, A.; González-Pérez, S.; Pérez-Sánchez, R. First molecular and functional characterisation of ferritin 2 proteins from Ornithodoros argasid ticks. Vet. Parasitol. 2022, 304, 109–684.
- Guglielmone, A.; Petney, T.; Robbins, R. Ixodidae (Acari: Ixodoidea): Descriptions and redescriptions of all known species from 1758 to December 31, 2019. Zootaxa 2020, 4871, 1–322.
- Walker, J.; Keirans, J.; Horak, I. The genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World, 1st ed.; Cambridge University Press: Cambridge, UK, 2000; pp. 79–104.
- Makwarela, T.; Nyangiwe, N.; Masebe, T.; Mbizeni, S.; Nesengani, L.; Djikeng, A.; Mapholi, N. Tick diversity and distribution of hard (Ixodidae) cattle ticks in South Africa. Microbiol. Res. 2023, 14, 42–59.
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26–33.
- Brophy, M.; Riehle, M.; Mastrud, N.; Ravenscraft, A.; Adamson, J.; Walker, K. Genetic variation in Rhipicephalus sanguineus s.l. ticks across Arizona. Int. J. Environ. Res. Public Health 2022, 19, 4223.
- Yamada, S.; Konnai, S.; Imamura, S.; Ito, T.; Onuma, M.; Ohashi, K. Cloning and characterization of Rhipicephalus appendiculatus voraxinα and its effect as anti-tick vaccine. Vaccine 2009, 27, 5989–5997.
- Ndawula, C.; Alves, G.; Parizi, L.; da Silva Vaz, I. Constituting a glutathione S-transferase-cocktail vaccine against tick infestation. Vaccine 2019, 37, 1918–1927.
- Gray, J. The ecology of ticks transmitting Lyme borreliosis. Exp. Appl. Acarol. 1998, 22, 249–258.
- Gilbert, L.; Maffey, G.; Ramsay, S.; Hester, A. The effect of deer management on the abundance of Ixodes ricinus in Scotland. Ecol. Appl. 2012, 22, 658–667.
- Hofmeester, T.; Sprong, H.; Jansen, P.; Prins, H.; Van, S. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in dutch forests. Parasites Vectors 2017, 10, 433.
- Ostfeld, R.; Canham, C.; Oggenfuss, K.; Winchcombe, R.; Keesing, F. Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk. PLoS ONE 2006, 4, e145.
- Pasternak, A.; Palli, S. Mapping distributions of the Lyme disease vector, Ixodes scapularis, and spirochete, Borrelia burgdorferi, in Kentucky using passive and active surveillance. Ticks Tick Borne Dis. 2022, 13, 101885.
- Schuijt, T.; Narasimhan, S.; Daffre, S.; de Ponte, K.; Hovius, J.; Veer, V. Identification and characterization of Ixodes scapularis antigens that elicit immunity to ticks by visualizing the yeast surface. PLoS ONE 2011, 6, e15926.
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328.
- Yunker, C.; Keirans, J.; Cliffornd, C.; Easton, E. Dermacentor ticks (Acari: Ixodoidea: Ixodidae) of the new world: A scanning electron microscope atlas. Proc. Entomol. Soc. Wash. 1986, 88, 609–627.
- Eisen, R.; Kugeler, K.; Eisen, J.; Beard, C.; Paddock, C. Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 2017, 58, 319–335.
- Dergousoff, S.; Galloway, T.; Lindsay, L.; Curry, P.; Chilton, N. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 2013, 50, 510–520.
- Zhang, G.; Zheng, D.; Tian, Y.; Li, S. A dataset of distribution and diversity of ticks in China. Sci. Data 2019, 6, 105.
- Martin, J.; Fischhoff, I.; Castellanos, A.; Han, B. Ecological predictors of zoonotic vector status among Dermacentor ticks (Acari: Ixodidae): A trait-based approach. J. Med. Entomol. 2022, 59, 2158–2166.
- Huercha, R.; Min, L.; Xinli, F.; Zhengxiang, H.; Lijiang, W.; Yongchang, L.; Wei, Z.; Yang, Z.; Yuhui, M.; Chahan, B. Characterization of glutathione S-transferase of Dermacantor marginatus and effect of the recombinant antigen as a potential anti tick vaccine. Vet. Parasitol. 2020, 279, 109043.
- Jittapalapong, S.; Kaewhom, P.; Pumhom, P.; Canales, M.; de la Fuente, J.; Stich, R. Immunization of rabbits with recombinant serine protease inhibitor reduces the performance of adult female Rhipicephalus microplus. Transbound. Emerg. Dis. 2010, 57, 103–106.
- Imamura, S.; da Silva Vaz, I.; Sugino, M.; Ohashi, K.; Onuma, M. A serine protease inhibitor (Serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 2005, 23, 1301–1311.
- Zhou, J.; Gong, H.; Zhou, Y.; Xuan, X.; Fujisaki, K. Identification of a glycine-rich protein from the tick Rhipicephalus haemaphysaloides and evaluation of its vaccine potential against tick feeding. Parasitol. Res. 2006, 100, 77–84.
- Kemp, D.; Pearson, R.; Gough, J.; Willadsen, P. Vaccination against Boophilus microplus: Localization of antigens on the tick gut cells and their interaction with the host immune system. Exp. Appl. Acarol. 1989, 7, 43–58.
- Weiss, B.; Kaufman, W. Two feeding-induced proteins from the male gonad trigger engorgement of the female tick, Amblyomma hebraeum. Proc. Natl. Acad. Sci. USA 2004, 101, 5874–5879.
- Hajdusek, O.; Almazán, C.; Loosova, G.; Villar, M.; Canales, M.; Grubhoffer, L.; Kopacek, P.; de la Fuente, J. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010, 28, 2993–2998.
- Tsuda, A.; Mulenga, A.; Sugimoto, C.; Nakajima, M.; Ohashi, K.; Onuma, M. cDNA cloning, characterization and vaccine effect analysis of Haemaphysalis longicornis tick saliva proteins. Vaccine 2001, 19, 4287–4296.
- Francischetti, I. The role of saliva in tick feeding. Front. Biosci. 2009, 14, 2051–2088.
- Mudenda, L.; Pierlé, S.; Turse, J.; Scoles, G.; Purvine, S.; Nicora, C.; Clauss, T.; Ueti, M.; Brown, W.; Brayton, K. Proteomics informed by transcriptomics identifies novel secreted proteins in Dermacentor andersoni saliva. Int. J. Parasitol. 2014, 44, 1029–1037.
- Tirloni, L.; Reck, J.; Terra, R.; Martins, J.; Mulenga, A.; Sherman, N.; Fox, J.; Yates, J.; Termignoni, C.; Pinto, A.; et al. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS ONE 2014, 9, e94831.
- Tirloni, L.; Islam, M.; Kim, T.; Diedrich, J.; Yates, J.; Pinto, A.; Mulenga, A.; You, M.; da Silva, I. Saliva from nymph and adult females of Haemaphysalis longicornis: A proteomic study. Parasit. Vectors 2015, 8, 338.
- Kim, T.; Tirloni, L.; Pinto, A.; Moresco, J.; Yates, J.; da Silva Vaz, I.; Mulenga, A. Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS Negl. Trop. Dis. 2016, 10, e0004323.
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281.
- Leboulle, G.; Rochez, C.; Louahed, J.; Ruti, B.; Brossard, M. Isolation of Ixodes ricinus salivary gland mRNA encoding factors induced during blood feeding. Am. J. Trop. Med. Hyg. 2022, 66, 225–233.
- Mans, B. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J. Innate Immun. 2011, 3, 41–51.
- Ribeiro, J.; Francischetti, I. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88.
- Wikel, S. Host immunity to ticks. Annu. Rev. Entomol. 1996, 41, 1–22.
